Cancer-directed surgery in malignant pleural mesothelioma: extrapleural pneumonectomy and pleurectomy/decortication
Brian Housman, Andrea S. Wolf
Department of Thoracic Surgery, Icahn School of Medicine at Mount Sinai, New York 10029, USA.
Abstract Malignant pleural mesothelioma (MPM) is a primary solid malignancy related to inhalational asbestos exposure.Despite advances in therapy, MPM remains challenging to treat with a post-treatment survival of only 15% at 5-year. In recent years, extra-pleural pneumonectomy has decreased in popularity due to a high morbidity rate and mortality compared to pleurectomy/decortication and other therapeutic alternatives. In this review, we will discuss both procedures, outcomes, ongoing studies, and the roles of surgery in the future treatment of this disease.
Keywords: Surgery for malignant pleural mesothelioma, extrapleural pneumonectomy, pleurectomy/decortication,recurrence perioperative mortality
INTRODUCTION
Malignant pleural mesothelioma (MPM) is a primary solid malignancy of pleural mesothelial cells related to inhalational asbestos exposure[1]. When left untreated, MPM carries a median survival of 6 to 9 months[2,3].Increasing global restrictions in the production and use of asbestos notwithstanding, the incidence of MPM has increased over the last several decades; the phenomenon is likely due to a lag time of 30-50 years between exposure and development of the disease[3,4].
Despite advances in chemotherapy, radiation, and immunotherapeutic agents, MPM remains challenging to treat with a post-treatment survival of only 15% at 5-year[5]. When possible, surgery remains a cornerstone of multimodal therapy and may improve survival[1,3]. The two most common techniques are extra-pleural pneumonectomy (EPP) and pleurectomy/decortication (P/D). In this review, we will discuss both procedures, their outcomes, and their roles in the future treatment of this disease.
DEFINITIONS OF TERMS
Due to variations in terminology, the International Association for the Study of Lung Cancer (IASLC)established a uniform set of definitions for each surgical procedure for MPM[3,6]. EPP refers to an en bloc resection of the parietal and visceral pleura with the ipsilateral lung, pericardium, and diaphragm. Extended P/D is a parietal and visceral pleurectomy to remove all gross tumors with resection of the diaphragm and/or pericardium. P/D is defined as a parietal and visceral pleurectomy to remove all gross tumors but without diaphragm or pericardial resection. Finally, partial pleurectomy is a partial removal of parietal and/or visceral pleura for diagnostic or palliative purposes but leaving gross tumor behind[6]. While these definitions are useful guidelines, many authors still refer to procedures differently without a universally accepted benchmark[6]. For example, the “radical P/D” remains a widely used term to describe full parietal and visceral pleurectomy with or without diaphragmatic and pericardial resection[6]. For this review, unless otherwise specified, P/D will refer to extended pleurectomy/decortication or the equivalent radical procedure.
SURGERY FOR MPM
Multiple studies have shown that cancer-directed procedures can contribute to longer survival[1,3,7,8].Unfortunately, the majority of patients with MPM do not undergo surgical resection[9]. According to a study of 5937 patients in the Surveillance, Epidemiology, and End Results database from 1990 to 2004, only 22% of patients with MPM undergo cancer-directed surgery, and only 40% of patients are offered surgery at tertiary referral centers[9]. While a variety of factors, including fitness for surgery, likely contribute to this finding, it underscores the reality that most patients with MPM are not receiving all available therapy.
Additionally, both EPP and P/D are often described as being performed with “curative-intent”. This term can be misleading and should only be used to differentiate therapeutic surgery from procedures that are purely diagnostic or palliative (e.g., exploration, pleural biopsy, or partial P/D). The goal of cancer-directed intervention should be the removal of all gross disease, which is referred to as an R1 or macroscopic complete resection (MCR)[10]. True R0 resection is theoretically impossible with either procedure due to the inability to achieve surgical margins or eliminate microscopic disease[3,10-14]. One study reported that following “macroscopic complete resection” with EPP, 70% of specimens were found to have positive margins on final pathlogy[15]. As a result, any cytoreductive procedure for MPM must be supplemented with chemotherapy and/or radiation to attempt to control residual microscopic disease[1,13,16].
EXTRA-PLEURAL PNEUMONECTOMY
The EPP was first described by Sarot in 1949 as a treatment for collapse-therapy and thoracoplasty-resistant tuberculosis[17]. It was first reported as a treatment for MPM in 1976 when Butchartet al.[18]described their experience in 29 patients with diffuse disease. Ultimately, they recommended it be used only in early-stage disease with epithelioid histology as the “…pleuropneumonectomy does not appear materially to affect the course of the disease in cases of mixed epithelial and mesenchymal histologic type[18]”.
Years of subsequent studies have investigated the virtue of EPP for MPM. Treatment paradigms have long involved a combination of systemic and local therapy, and studies are often described in this multimodal context[16].
For example, a phase II trial conducted by Floreset al.[19]investigated a protocol of neoadjuvant gemcitabine/cisplatin chemotherapy, followed by EPP and radiotherapy. Median survival was 33.5 months in the patients who underwent surgeryvs. 9 months in patients determined to be unresectable[19].
Another study by Yanet al.[20]retrospectively examined 70 patients undergoing EPP followed by chemotherapy and/or radiation. They reported a morbidity rate of 37%, a mortality rate of 5.7%, and a median survival of 20 months. Survival in patients who received adjuvant radiation was 90 monthsvs. 14 months, and in the recipients of adjuvant chemotherapy was 60 monthsvs. 14 months[20].
Sugarbakeret al.[21]examined 183 patients who underwent EPP followed by adjuvant chemotherapy and radiation. The perioperative morbidity rate was 50%, and the mortality rate was 3.8%. Overall median survival was 19 months, and among 31 patients with a combination of positive prognostic factors - epithelial histology, negative margins, and uninvolved mediastinal nodes - this increased to 51 months[21].
Wederet al.[22]also investigated the use of neoadjuvant platinum-based chemotherapy and EPP followed by adjuvant radiation. A total of 18 patients received preoperative chemotherapy, and 16 patients underwent EPP with a 31.5% morbidity rate and 15.7% mortality rate. Of the original cohort, only 13 received postoperative radiation and completed the treatment protocol. Overall median survival was 23 months, and two patients remained free of disease 41- and 38-month following therapy. The authors note that the plane of dissection was obliterated by dense fibrosis following induction chemotherapy compared with primary surgery, and recommended caution during post-induction procedures[22].
The same author conducted a similar study on 61 patients in a multicenter prospective trial[23]. A total of 58(95%) completed induction chemotherapy, 45 (74%) underwent EPP, and 36 (59%) completed at least part of planned radiotherapy[23]. Median survival was 19.8 months and 23 months for the 45 patients who received EPP[23].
More recently, a phase II study by Choet al.[24], the “SMART” trial, investigated patients undergoing EPP following induction radiation and receiving adjuvant chemotherapy for node-positive disease. A total of 96 patients underwent surgery 2-12 days following completion of radiation (median 5 days) to avoid radiationinduced pulmonary toxicity before surgery[24]. Median overall survival was 24.4 months. Disease-free survival was 18 months. The 5-year incidence of local recurrence was 17 (20.1%), distant recurrence was 62(63.3%), and at the time of publication, 24 patients had no disease recurrence. There were 4 deaths (4.2%); 1 in hospital from pneumonia, and 3 after discharge[24].
These examples also illustrate the problem with MPM research: heterogeneity in study design, the number of subjects, and reported outcomes. All despite purported similarities in chemotherapy, radiation treatment,and surgical approach. This may be influenced by variation in chosen time to progression, reported patterns of recurrence, and chosen endpoints which influence statistical evaluation and may compensate for small numbers of subjects[13]. Combined with differences in follow-up practices, diagnostic modalities, and the definitions of progression and recurrence, research into mesothelioma outcomes is sometimes limited by a lack of standarization[13].
PLEURECTOMY/DECORTICATION
The pleurectomy was first reported as a palliative intervention for MPM in 1975 by Martiniet al.[25]in a series of malignant pleural effusions. A year later, in 1976, Waneboet al.[26]published that patients undergoing pleurectomy with chemotherapy and radiation for the epithelioid disease had a median survival of 21 months.
In the years that followed, the P/D was viewed only as a cytoreductive alternative to the EPP in patients who were not candidates for pneumonectomy[27]. In the last several decades, P/D has been increasingly investigated as the procedure of choice for mesothelioma. While many studies are conducted in the setting of comparison with EPP, several noteworthy groups investigated P/D on their own.
A study by Bölükbaset al.[28]studied 35 patients, 19 of whom had stage III and IV disease, who underwent P/D followed by chemotherapy and radiation. A total of 33 patients (94.3%) completed trimodality therapy.Overall median survival was 30.0 months with 1-year, 2-year, and 3-year survivals of 69%, 50%, and 31%,respectively. The authors concluded that since the P/D better maintains postoperative physiologic reserve,there is greater potential for multimodal options in the long term[28].
Lapidotet al.[29]conducted a retrospective study of 355 consecutive patients undergoing P/D. MCR was achieved in 304 patients (85.6%) with a median survival of 23.2 monthsvs. 11.6 months in the non-MCR group. Median progression-free survival was 11.7 months. The 5-year survival in patients who had MCR was 21.2%vs. 17.9%. The 30- and 90-day mortalities were 3.0% and 4.6%. The most common complications were prolonged air leak (39.7%), deep vein thrombosis (18.0%), atrial fibrillation (11.8%), chylothorax(6.8%), and empyema (6.5%)[29].
Marulliet al.[27]conducted a review of 314 patients undergoing different forms of P/D in 11 Italian hospitals.Of the total, 162 patients underwent extended P/D, 115 received P/D without pericardial or diaphragmatic resection, and 37 underwent partial pleurectomy[27].
Neoadjuvant chemotherapy was given to 57% of patients, and adjuvant radiation was given to 39.2%.Median overall survival was 23.0 months. The hazard ratios for extended P/D and P/D were similar at 0.46 and 0.52, respectively, and were both independent predictors of survival. Additionally, the authors also noted that while partial pleurectomy was associated with a poor prognosis, R2 resection in the setting of P/D had no impact on survival[27].
A recent study by Nakamuraet al.[30]investigated 90 patients who underwent neoadjuvant chemotherapy followed by P/D. The 1-year and 3-year overall survival rates were 93.3% and 65.3%, with recurrence-free survival of 19.0 months[30].
Sharkeyet al.[31]examined 300 patients who underwent P/D, 82 of whom were > 70 years old. Median overall survival was similar for patients younger and older than 70 years, at 14 months and 10.3,respectively. Older patients with positive nodes had poorer survival rates, but on multivariable analysis, age was not independently associated with poorer outcomes[31].
In 2011, Tehet al.[32]conducted a comprehensive review of lung-sparing extirpative surgery. Unsurprisingly,substantial heterogeneity was found in both the nature of surgery, and the degree to which it was described.From 26 papers and including 1270 patients, the authors present almost every conceivable variation on the pleurectomy; including partial pleurectomy, total pleurectomy, complete pleurectomy, debulking, debulking to nodules < 1 cm, cytoreduction, macroscopic complete resection, en bloc chest wall resection,diaphragmatic and pericardial resection “when indicated”, and organ sparing when frank invasion was identified[32]. Some studies had multiple forms of extirpation being performed in the same population, which made reporting of outcomes even more challenging. Though some papers reported promising outcomes,Tehet al.[32]ultimately drew no conclusions with regard to overall survival or symptomatic benefits. It was,however, one of the earliest studies to codify the descriptive difficulties in mesothelioma research. Later that same year, Riceet al.[6]published their paper with the IASLC calling for formal definitions of cancerdirected mesothelioma procedures.
EPP vs. P/D
The EPP remained the gold standard of mesothelioma surgery for decades[33]. Unfortunately, despite improvements in technique and supportive care, a considerable volume of research has revealed a high morbidity rate and mortality associated with the EPP[13,16]. In some studies, the outcomes are so poor that undergoing surgery with curative intent actually reduces overall survival[10,13,34].
In one of the most widely referenced studies on the EPP, the original MARS trial - now referred to as MARS 1 - randomized 50 patients to EPP and no EPP treatment arms[34]. Median survival was 14.4 months in the EPP group and 19.5 months in the no EPP group. The authors found that the hazard ratio for overall survival - when adjusted for sex, histologic subtype, stage, and age at randomization was 2.75 (1.21-6.26,P=0.016). There were also five times the number of adverse events in the EPP group (10vs. 2), which was likely reflected in a lower median quality of life scores in the survivors[34].
Arguably the decision to continue to perform the EPP over P/D has been based on surgeon preference,historical inertia, and the intuition that more tissue resection confers better oncologic outcomes[10,14,16]. But despite the greater radicality of the procedure, and possibly because of it, numerous studies since the 2000s have shown equal or better survival after P/D than EPP[10,13,35-37].
Floreset al.[13], in what remains one of the largest retrospective studies of MPM, investigated outcomes of 663 patients undergoing EPPvs. P/D. The EPP group was more likely to receive multimodality therapy and had a higher proportion of epithelioid histology. The patients undergoing P/D had earlier stage tumors, but also significantly greater age. These represent a constellation of factors that should have favored outcomes in the EPP group. They nevertheless found that operative mortality was 7% for the EPP group and 4%following P/D. Additionally, serious respiratory complications occurred in 10% of EPP patients and 6.4% of P/D patients. In a cox proportional hazard model controlled for histology, stage, gender, and multimodal therapy, EPP had a hazard ratio of 1.4 compared to P/D.
A meta-analysis performed by Taioliet al.[38]examined 1391 patients undergoing EPP compared to 1512 undergoing P/D. They found 2.65 times higher 30-day mortality rate associated with EPP (4.5%vs.1.7%).Median survival appeared to be equivocal, with 53% of studies reporting longer survival after PDvs. 47%after EPP (see Figures 1 and 2)[38]. However, of the 7 studies reporting at least 2-year survival, there were no significant differences between the two procedures[38].

Figure 1. Meta-analysis of reported short-term (perioperative and 30-day) mortality[38].
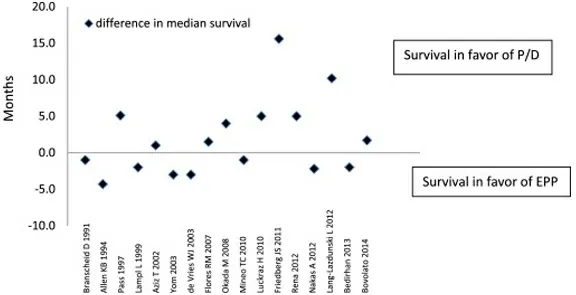
Figure 2. Difference in median survival between extrapleural pneumonectomy and pleurectomy decortication among 17 studies[38].
A combination of higher rates of complications - including acute respiratory distress syndrome,reintubation, bleeding, bronchopleural fistula, unexpected reoperation, sepsis, and mortality - all contribute to hazard ratios in EPP of up to 10-times the surgical alternatives, and have forced many practitioners to advocate against it as the procedure of choice[10,13,34,38,39].
A recent meta-analysis performed by Magouliotiset al.[40]compiled a total of 1672 patients treated with EPP and 2236 treated with P/D from 1990 to 2018. Compared to the 2.5 fold increase in short-term mortality found by Taioli, they identified an OR of 3.24 in 30-day mortality among the EPP group. They also note a significantly higher rate of postoperative atrial fibrillation, hemorrhage, empyema, bronchopleural fistula and, - interestingly - air leak in the EPP group[40]. While there were no significant differences in survival at 90 days or from 1-5 postoperative years, they found that overall median survival was significantly higher in the P/D group[40]. This finding, in particular, appears to suggest that the higher radicality of EPP does not confer an oncologic advantage over P/D. The procedure not only carries a higher initial risk of major complications and death, but life expectancy is also not greater in long-term survivors.
These findings were mirrored in an earlier meta-analysis by Caoet al.[37]. In a systematic review encompassing 632 EPP, and 513 P/D patients, the morbidity rate was 62.0% for EPPvs. 27.9% for P/D, and the mortality rate was 6.8% for EPPvs. 2.9% for P/D[37]. Overall median survival for EPP was 12-22 months,while P/D was 13-29 months.
A single-center, 10-year experience published by Azizet al.[39]examined 302 patients with MPM. Of the total, 191 received palliative care, 47 patients were treated with P/D, and 64 patients underwent EPP, the last 51 of which also received intrapleural and adjuvant systemic chemotherapy. Median survival was 35 months[39]. Survival was 8.9 months for patients receiving chemotherapy alone, 13 months for EPP, and 14 months for P/D. Patients treated with EPP and adjuvant chemotherapy had a median survival of 35 months.Unfortunately, EPP was also associated with a 9% mortality rate compared to 0 following P/D for an OR of 10.56, one of the largest in the literature[39,40].
Any possible, and yet unproven, benefit that the greater radicality of EPP may offer is not balanced by a clear survival benefit, even when surgery is successful[13,38,40]. There is insufficient evidence even to prove that the resection of the diaphragm or pericardium improves outcomes[6].
The advantage of P/D over EPP has been shown even when performed in less healthy subjects[36].Luckrazet al.[36]performed a retrospective study of 139 patients undergoing surgery for mesothelioma. They showed that despite more advanced disease and less fit patients, P/D with adjuvant chemoradiotherapy was the strongest predictor of longer survival (HR = 3.6). Worse, controlling for other factors, EPP was found to be an independent risk factor for decreased survival[36].
This pattern is at least partially explained by postoperative lung volumes and ventilatory dynamics.Bölükbaset al.[41]found that there was a significant improvement in both FVC and FEV1following P/D,even in patients who underwent diaphragmatic resection. The average increase in FEV1was 23.9%, with a higher relative increase in patients with poorer preoperative pulmonary function. While this observation codifies the virtue of P/D as a palliative procedure, it also helps explain post-surgical resilience. Preserved cardiopulmonary function following P/D permits both more opportunities for adjuvant therapy and confers greater resistance to postoperative complications[10,28,41]. In fact, additional chemotherapy is administered in 64% of patients after P/D and only 25% of patients after EPP[28,41].
Other studies have observed that surgical intervention does not provide any meaningful benefit. In a retrospective multicenter Italian study of 1365 patients, the median survival was not different between EPP,P/D, and chemotherapy alone (20.9, 24.6, 18.6,P= 0.596)[12].
Batirelet al.[42]in 2018 published that MCR is not associated with improved survival. A group of 154 patients was examined; 90 underwent P/D, 42 EPP, and 22 partial pleurectomy. MCR was achieved in 75 patients (49%), 19 in the P/D group, 38 after EPP, and 18 after partial pleurectomy. A total of 133 patients received adjuvant therapy, 45 of whom had chemotherapy and radiation, 33 after MCR, and 12 without MCR. The study found no statistically significant difference in survival between those who had surgical MCR and those who did not (21.4 monthsvs. 16.3 months).
The MARS 2 study, a phase II randomized control trail of P/Dvs. no surgery for patients undergoing platinum and pemetrexed-based chemotherapy, is ongoing[43].
RECURRENCE
Even following radical surgery and multimodal therapy, recurrence is unfortunately expected in patients with MPM[13]. Early studies estimated that up to 85% of patients died within the first 3 months of recurrence[44]. However, the addition of second-line chemotherapy and repeat surgical intervention increases survival with recurrent disease[41,45,46].
While the reporting of recurrence varies greatly between studies, the pattern of recurrence is consistently related to the index procedure[13]. Disease predominantly recurs in distal sites following EPP and the ipsilateral hemithorax after P/D[13,22].
In their retrospective study, Floreset al.[13]describe a 56.9% recurrence rate in EPP and 47.8% in P/D with a median follow-up of 17 months. Local recurrence was found in 33% of patients undergoing EPPvs. 65%after P/D. Distal recurrence - including the contralateral hemithorax, peritoneum, abdomen, bone, brain,skin, and “other” - occurred in 66% of EPP patientsvs. 35% in P/D[13]. In one series, the ipsilateral hemithorax and/or mediastinum was the site of the first recurrence in 95% of patients undergoing P/D[47].
In the previously mentioned study by Nakamuraet al.[30], the overall recurrence rate following P/D was 63.3%, with a 1- and 3-year recurrence-free survival of 69.7% and 34.0%. Median recurrence-free survival was 19.0 months. The 1-year post-recurrence survival was 59.5%, and the median post-recurrence survival time was 14.4 months[30]. Interestingly, the hazard ratio of treatment post-recurrence was 0.2, a finding that reinforces the observation that survival after recurrence is better in patients with P/D than EPP[30,48].
One exception may be external beam radiation. In some guidelines, radiation has been contraindicated following P/D due to the concern for postoperative pneumonitis in the preserved lung[10,49,50]. Surgeons who utilize intensity-modulated pleural radiation therapy (IMPRINT/IMRT) or intraoperative radiotherapy may be more likely to perform P/D due to the lower risk of pneumonitis[16]. On the other hand, some surgeons elect to perform EPP in anticipation of radiation due to the greater pleural exposure permitted by an empty hemithorax[16]. Other studies have reported the opposite concern; high rates of fatal pneumonitis with radiation therapy following EPP in the remaining lung[51].
A landmark study by Rimneret al.[52]evaluated the safety of IMPRINT after neoadjuvant platinum-based chemotherapy and P/D. They found that after a total of 27 underwent a protocol with a median dose of 46.8 Gy (range 28.8 to 50.4 Gy), only six patients experienced grade 2 radiation pneumonitis, and two patients experienced grade 3. No patients had grade 4 or 5 toxicities, and all 8 patients recovered with corticosteroids[52].
Unfortunately, it is unclear whether adjuvant radiation even provides appreciable local control. In a study by Guptaet al.[50], 123 patients underwent P/D and received adjuvant external beam radiotherapy. An additional 54 patients received brachytherapy for residual tumor. The 1-year local control rate was 42%,with a median survival of 13.5 months[50]. Overall survival was negatively affected by radiation dose < 40 Gy,non-epithelioid histology, left-sided disease, and use of implants. The investigators concluded that residual disease could not be eradicated with radiation with or without brachytherapy, and local control may require further surgery. It should be noted that the study investigated patients treated from 1974 to 2003, which may have included less sophisticated therapy than modern techniques[50].
The SAKK 17/04 study, a multicenter phase II trial, explored the utility of hemi-thoracic radiation after neoadjuvant chemotherapy and EPP[53]. A total of 54 patients were randomized into radiation and no radiation. Median survival was 20 months in both groups. The authors concluded the while a sicker patient population may be responsible for their outcomes, the use of radiation could not be supported. They did appear to question the contribution of the surgery as they noted the “role of extrapleural pneumonectomy has been called into question by the development of lung-sparing procedures with less morbidity”[53].
Minatelet al.[54]report successful radiation therapy after P/D. Minatelet al.[54]published a series of 20 patients with overall median survival of 33 months and progression-free survival of 29 months with a 2-year survival rate of 70% and a 3-year survival rate of 49%. There was no reported fatal toxicity. Locoregional control at 2 years was 68% and 3 years was 59%. The predominant pattern of failure was distant, and only 3 of the 20 patients developed isolated locoregional recurrence[54].
No therapy for MPM recurrence has been universally accepted, and the elements of multimodal treatment continue to be studied, including radiation, IMPRINT, chemotherapy, immunotherapy, iodine,photodynamic therapy, and hyperthermic intraoperative chemotherapy[19,22].
ONGOING TRIALS
There are several notable ongoing trials evaluating multimodal therapy for MPM. Referenced earlier, the MARS 2 study will evaluate outcomes of platinum-based chemotherapy plus P/Dvs. chemotherapy alone[43]. EORTC 1205 is a randomized phase II trial that compares neo-adjuvant and adjuvant courses of cisplatin and pemetrexed with a standardized protocol of P/D[14]. NCT02707666 is a single-arm study at the University of Chicago exploring pembrolizumab, P/D, and postoperative chemotherapy that is currently recruiting. Another study, NCT02592551, out of Baylor St. Luke’s in Houston, seeks to investigate durvalumab and durvalumab plus tremelimumab before surgical resection (EPP or P/D) which is no longer recruiting. NCT04897022 Memorial Sloan Kettering in New York is one of many trials investigating the use of immunotherapeutic agents with the use of IMPRINT. A trial out of John’s Hopkins, NCT03918252, is investigating neoadjuvant immune checkpoint blockade with nivolumab in resectable MPM. Another promising trial, NCT04158141, is a Phase III randomized trial of P/D plus chemotherapy with or without Adjuvant IMPRINT.
Finally, NCT04525859 at the Mount Sinai Hospital in New York is exploring the safety and efficacy of preoperative injection of Poly-ICLC on recurrence-free survival. The study will also evaluate induced serologic changes in circulating immune cells, including regulatory T-cells and natural killer cells.
CONCLUSION
While many aspects of treatment for MPM remain controversial, the literature shows that no benefit of EPP over P/D can be consistently demonstrated. Whatever theoretical benefits of EPP as a more radical procedure - if they exist - appear to be counterbalanced by a high frequency of complications, lower quality of life, and perioperative death. Recurrence remains a problem in the treatment of MPM with both techniques. To date, no study convincingly proves that EPP is more effective at preventing recurrence than P/D.
Despite the absence of a randomized trial of surgical technique, P/D consistently displays lower morbidity and mortality with similar, if not superior, long-term survival[10,14,37]. Whether this is because it confers an oncologic benefit or simply avoids the hazards of EPP is unclear. Though further study is certainly warranted, EPP is a procedure that should be considered in select patients and experienced centers.
DECLARATIONS
Authors’ contributions
Performed review of existing literature, evaluated existing studies, wrote and edited manuscript:Housman B, Wolf AS
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
Both authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2021.
 Journal of Cancer Metastasis and Treatment2021年12期
Journal of Cancer Metastasis and Treatment2021年12期
- Journal of Cancer Metastasis and Treatment的其它文章
- Progress in the treatment of NK-cell lymphoma/leukemia
- Novel standards and emerging therapies for systemically treatment-naïve clear cell renal cell carcinoma - a rapidly changing landscape
- The contemporary role of metastasectomy in the management of metastatic RCC
- Intraoperative monitoring of the recurrent laryngeal nerve in surgeries for thyroid cancer: a review
- AUTHOR INSTRUCTIONS
