Novel standards and emerging therapies for systemically treatment-naïve clear cell renal cell carcinoma - a rapidly changing landscape
Ritesh R. Kotecha, Martin H. Voss
Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY 10065, USA.
Abstract The last decade has brought about major advances in systemic therapy for patients with advanced clear cell renal cell carcinoma. The introduction of angiogenesis targeting agents, immune checkpoint inhibitors, and combinations thereof has resulted in a multitude of therapeutic standards for patients with newly diagnosed advanced disease.With the rapid adoption and increasing number of available options for patients, selection amongst treatment regimens has become complex. Further, a new balance is also being sought to optimize treatment outcomes and limit treatment-related toxicities. With a rising bar against which novel therapeutics are being measured, the field looks toward an evolved understanding of tumor biology to help pave new ways forward for individualized therapy.Here, we review pivotal studies that led to regulatory approvals and ongoing clinical trials conducted in patients with systemically untreated clear cell renal cell carcinoma and provide perspective on how newly emerging data can be integrated into this rapidly changing landscape.
Keywords: Renal cell carcinoma, combination immunotherapy, clinical trials, first-line
INTRODUCTION
Over the last two decades, the clinical management of advanced clear cell renal cell carcinoma (ccRCC) has transformed significantly, and the serial introduction of novel classes of therapeutic agents has notably improved clinical outcomes over time. Earlier versions of immunotherapy in the form of recombinant cytokines constituted a standard in the 1990s, but their use was limited by poor tolerance, and only modest activity with sustained clinical benefit was achieved in a minority of patients[1]. Molecular targeting agents,including multi-tyrosine kinase inhibitors (TKIs) of the vascular endothelial growth factor (VEGF) receptor and mTOR pathway blockers, subsequently took their place as the standard of care for patients in the firstline setting and were typically given as single agents[2]. The introduction of contemporary immunotherapy in the form of immune checkpoint inhibitors (ICIs) initially occurred in pre-treated patients treated with PD-1 directed monotherapy[3], but this quickly moved into the frontline setting where combination therapy pairing agents targeting PD-1 and CTLA-4 were shown to improve patient survival, freedom from progression and quality of life compared to the prior gold standard, single-agent TKI therapy[4]. Soon after,the introduction of combinations pairing the old with the new, TKI plus ICI therapy again demonstrated superior patient response and survival outcomes when compared to TKI alone. The advent of several new regimens approved over 2018-2021 has now ushered in a new treatment era, with combination therapy as the frontline standard for all eligible advanced ccRCC patients[5-8]. As all these novel regimens have demonstrated superiority over TKI alone, but none have been compared to one another, selection of a preferred contemporary standard for clinical practice or as the comparator on new prospective trials remains complex. Lastly, while SURTIME[9]and CARMENA[10]investigated the role of cytoreductive nephrectomy with sunitinib, these studies provide complementary data and inform that many patients will start with immediate upfront systemic therapy with subsequent consideration of cytoreduction if clinical circumstances permit. Here, we review currently approved treatment regimens and overall treatment strategies for patients systemically treatment-naïve ccRCC.
COMBINATION THERAPY IS THE FRONTLINE STANDARD FOR ADVANCED CCRCC
For many years the routine assessment of patients with metastatic ccRCC initiating first-line systemic therapy integrated clinical prognostic tools like the Memorial Sloan Kettering Cancer Center (MSKCC) and the International Metastatic RCC Database Consortium (IMDC) risk classification system[11,12]. Developed during the cytokine and targeted therapy eras, respectively, these programs integrate clinical and laboratory data to group patients into favorable, intermediate, and poor-risk strata. However, for years, these tools were merely appreciated (and serially validated) for their usefulness in informing disease prognosis, and registration strategies in the metastatic setting rarely focused on specific risk categories[13,14]. More recent data highlight that these risk classifiers serve as clinical surrogates for underlying tumor biology, with RCC tumors characterized across different disease phenotypes, including enrichment of upregulation of tumor angiogenesis in favorable risk patientsvs.proliferative/inflammatory profiles in patients with intermediate/poor-risk disease[15-17]. This has enabled a more nuanced understanding of how risk status (and underlying biology) may be informative for choice of therapy in previously systemically untreated ccRCC patients.
COMBINATION IMMUNE CHECKPOINT BLOCKADE THERAPY
The first modern combination therapy proven to be superior to TKI monotherapy was ipilimumab plus nivolumab on the CheckMate-214 trial[4]. Extended follow-up analyses of this global phase 3 study with a dedicated focus on IMDC intermediate/poor-risk patients confirm superior clinical outcomes over singleagent sunitinib for the three co-primary endpoints: overall response rate (ORR), progression-free survival(PFS, HR = 0.73), and overall survival (OS, HR = 0.68) (see Table 1)[18,19]. In the updated 4-year and recently presented 5-year survival analyses, there is also a better appreciation for the long-term clinical benefit from therapy in those patients who achieve radiographic response to therapy. For instance, of those patients who achieved a complete response (CR), 32% of patients continue to remain on systemic therapy, and 46% of RCC tumors with sarcomatoid features, high response rates and survival outcomes can also be achieved with combination ipilimumab and nivolumab[20]. In this population, the confirmed ORR was 60.8% with notably 18.9% of patients achieving a complete response, highlighting a significant step forward for this patient subgroup with a historically poor prognosis.

CLEAR Lenvatinib + Pembrolizumab 26.6 months 1069 31/59.2/9.3 7.9 73.8 71 16.1 5.4 23.9 0.39 (0.32-0.49)0.66 (0.49-0.88)CheckMate 9ER Cabozantinib + Nivolumab 18.1 months 651 22.6/57.6/19.7 11.5 68.7 55.7 85.6 16.6 0.51 (0.41-0.64)0.6 (0.4-0.89)Javelin Renal-101 Axitinib + Avelumab 11.6 months 560 19.3/64.1/16.3 12.2 83 PD-L1+5.6 11.5 13.8 0.61 (0.47-0.79)0.82 (0.53-1.28)Table 1. Summary of approved combination therapytrials for systemically untreated ccRCC patients KEYNOTE-426★Axitinib + Pembrolizumab 42.8 months 861 31.9/55.1/13 17.9 82.6 60.455.2 10 11.3 15.7 0.68 (0.58-0.80)0.73 (0.60-0.88)CheckMate-214★Ipilimumab + Nivolumab ITT 39 12 17.6 12 0.86 (0.73-1.01)0.72 (0.62-0.85)67.7 months 1096 23/61/17 13.2 82 Int/Poor 42 11 19.3 12 0.73 (0.61-0.87)0.68 (0.58-0.81)Median follow-up Total patients IMDC Fav/Int/Poor Sarcomatoid features (%)Nephrectomy status (%)ORR (%)CR (%)PD (%)Median PFS (months)PFS HR (CI)OS HR (CI)*Extended follow-up. IMDC: International Metastatic RCC Database Consortium; ORR: objective response rate; CR: complete response; PD: progressive disease; PFS: progression-free survival; HR: hazard ratio; CI:confidence interval; OS: overall survival; ITT: intention to treat.patients have discontinued treatment without needing a subsequent therapy[18,19]. Early treatment failure was not uncommon with a landmark PFS at 18 months being 42% and progressive disease (PD) being the best response in 20% of patients. However, progression events became notably less likely beyond the 2-year mark with 30-month and 42-month landmark PFS rates nearly identical at 35% and 33%, respectively. For those patients who sustained response at 3 years, the probability of remaining in response for an additional 2 years was 89% (ITT population) and 90% (intermediate/poor risk population)[18,19]. This “tailof-the-curve” phenomenon, together with the observed response durability and the marvel of treatment-free survival intervals, highlight the draw to immunotherapy, particularly in contrast to the waning responses and enduring toxicities usually seen with chronic TKI therapy. In patients with IMDC favorable risk disease (not part of the primary efficacy analysis pre-specified in the trial’s statistical analysis plan), ORR, PFS, and even landmark OS analyses numerically favored the sunitinib arm on this trial; hence, the FDA label is limited to patients with intermediate/poor disease. Interestingly, complete responses were observed across all IMDC risk groups - 11% for intermediate-poor risk patients and 13% for favorable risk patients, and responses were more durable on the investigational arm, regardless of IMDC risk status[18]. The unique safety profile of an all-ICI regimen is not as well characterized by adverse event assessment (by CTCAE); instead, the fact that 29% of patients on the ipilimumab/nivolumab arm required high-dose corticosteroids for the treatment of adverse events, including 10% for 30+ days speaks to the unique challenges of managing the immune-mediated toxicities of this regimen. Notably, there remains no data to support that steroid use impacts the efficacy of this regimen. With attention to histologic variants, post-hoc analyses further show that in
COMBINATION ANTI-ANGIOGENESIS AND IMMUNE CHECKPOINT BLOCKADE THERAPY
The increasing number of different VEGF and ICI therapy permutations has provided valuable lessons on careful drug selection and dosing of paired agents. Conceptually, many VEGF targeting therapies with established roles in the monotherapy setting have favorable downstream immunomodulatory effects on the RCC suppressive tumor microenvironment. Furthermore, with toxicity profiles that are mechanistically non-overlapping with ICI therapy, these should, in principle, all lend themselves well to combination strategy[21]. However, a steadily increasing number of trials studying various TKI/ICI combinations have revealed stark differences in efficacy and toxicity patterns, which emphasizes the importance of agent selection. For instance, the pairing of sunitinib or pazopanib with ICI therapy was met with unacceptably high rates of toxicity, with 39.4% and 80% of patients discontinued nivolumab plus sunitinib and pembrolizumab plus pazopanib, respectively[22,23]. In addition, certain regimens, including axitinib with avelumab (JAVELIN-Renal-101) and bevacizumab with atezolizumab (IMmotion-151), improved upon radiographic responses achieved with TKI therapy alone but did not appear to carry an OS advantage[8,24].Amongst these reported combinations, the three that have demonstrated level 1 evidence of unequivocal superiority over TKI monotherapy include axitinib with pembrolizumab, cabozantinib with nivolumab, and lenvatinib with pembrolizumab.
Reported extended follow-up of KeyNote-426, the global phase III study of axitinib and pembrolizumabvs.sunitinib, continues to confirm the significant clinical benefit for patients with systemically untreated ccRCC with respect to dual primary endpoints PFS and OS, with HR of 0.73 and 0.68, respectively (see Table 1)[25,26]. ORR as a key secondary endpoint also favored the combination, and patients treated with combination axitinib and pembrolizumab achieved an ORR of 60%, of which 10% sustained a CR. The superiority of the investigational arm was apparent early on this trial, and in the extended follow-up, 83% of patients sustained some measure of tumor reduction, and the rate of PD as the best response was only 11%with combination therapy. The primary analysis plan was neither specific to any IMDC risk category nor tumor PD-L1 status, and superiority was apparent across all such groups on secondary analyses. In a recent update, 29.9% of enrolled participants have even completed at least 2 years of systemic therapy, and of those,14% achieved a CR[27]. In the CheckMate 9ER study, cabozantinib combined with nivolumab was compared to sunitinib monotherapy and demonstrated superior PFS, the primary endpoint of the trial (see Table 1).Secondary endpoints ORR and OS were also notably improved for cabozantinib/nivolumab[6]. At a median follow-up of approximately 18 months, combination therapy had an ORR of 55.7%, with 8% of patients achieving a complete response. Further, superior OS (HR = 0.6) was found regardless of PD-L1 expression and across all IMDC risk groups, and emerging data also confirms that this clinical benefit is extended for patients with sarcomatoid RCC[28]. Based on the data from these pivotal studies, both axitinib/pembrolizumab and cabozantinib/nivolumab have received FDA approval and remain standard options in the first-line setting for all eligible advanced ccRCC patients. In contrast to ipilimumab/nivolumab, their registration is not tied to a specific IMDC/MSKCC risk status.
The most recently reported TKI/ICI combination to gain FDA approval was gathered on the CLEAR study,a three-arm trial which randomized patients 1:1:1 to the TKI/ICI combination of lenvatinib plus pembrolizumabvs.the non-ICI-based combination of lenvatinib plus everolimusvs.TKI monotherapy with standard sunitinib[7]. In this phase III global clinical trial, which tested PFS with each combinationvs.sunitinib as the primary endpoint, the study indeed confirmed that patients treated with either lenvatinib combination (pembrolizumab or everolimus) achieved significantly prolonged PFS compared to sunitinib alone (HR = 0.39 for pembrolizumab combination and HR = 0.65 for everolimus combination, respectively,see also Table 1). In addition, patients treated with lenvatinib/pembrolizumab demonstrated superior OS compared to sunitinib (HR = 0.66), with a high overall response rate (71%), notable CR rate (16.1%), and low rate of primary PD (5.4%). The OS for patients treated with lenvatinib/everolimus was not found to be superior to sunitinib (HR = 1.15). Subgroup analyses support that the OS benefit seen for combination lenvatinib and pembrolizumab over sunitinib likely extends to those patients with PD-L1 negative tumors,but this could not be confirmed in patients with favorable risk disease.
In all ICI/TKI trials, safety and tolerance observed for these above regimens listed here were largely in keeping with the adverse event profiles typically seen with each of the individual agents without the sense that an amplified toxicity signal emerged as being dose-limiting. Individual regimens varied in the reported incidence of hepatic and GI toxicity, which may be related to mechanisms of action, drug interaction,differences in the starting dose, or all the above.
All combinations described above have expanded the number of combination options available to patients with advanced disease and increased the complexity of individualizing care. The choice between an ICI doublet (ipilimumab/nivolumab) and one of the various TKI/ICI combinations may be driven by: (1)IMDC risk status (as ipilimumab/nivolumab being solely approved in intermediate/poor risk patients); (2) a notably lower upfront failure rate for all TKI/ICI combinations (explained by dual mechanism of action and relevant in patients with rapidly progressive, symptomatic disease who may not reach second-line therapy for which the risk remains higher with ipilimumab/nivolumab)[29]; (3) the above mentioned PFS “tail of the curve” for ipilimumab/nivolumab (which should motivate consideration of this regimen in patients with no/little concern for rapid progression and deterioration, until TKI/IO datasets are more mature and can speak to the presence of absence of a tail of the curve for those regimens); and (4) co-morbidities(uncontrolled cardiovascular disease arguing against TKI-containing regimen; conditions limiting tolerability of high-dose corticosteroids causing concern for use of the ICI doublet, which comes with a notably higher risk of requiring steroids for high-risk immune related toxicity). Once the determination is made to proceed with TKI/ICI, treating physicians are left with several regimens that have met all primary endpoints during the registration process. Study populations for KeyNote-426, CheckMate 9ER and CLEAR varied notably, and a clear “winner” cannot be chosen while follow-up is comparatively short for all of these, and the “tail-of-the-curve” question is left unanswered still. Instead, the decision will be driven by physician experience with and their preference for individual agents, reported starting doses (e.g.,cabozantinib is started at an attenuated dose with nivolumab; axitinib is started at a standard dose with pembrolizumab; lenvatinib is started at a higher dose with pembrolizumab compared to the previously approved combination with everolimus) and the individual nuances of toxicity that may be particularly enticing with each (e.g., low rate of hepatic events with lenvatinib and pembrolizumab or low rate of dose reductions needed for cabozantinib with nivolumab).
OPTIMIZING ICI THERAPY - CAN WE ACHIEVE MORE WITH LESS?
Deep responses and durable anticancer effects have been observed in those patients receiving ICI monotherapy - albeit in a lesser proportion of patients[30]. Results from KeyNote-427 Cohort A, a phase II study of pembrolizumab monotherapy in systemically untreated ccRCC patients, demonstrated an ORR of 36.4%, of which 3% of patients also achieved a complete response and 15% of patients achieved a high depth of response with > 80% tumor reduction[30]. Such ICI monotherapy comes at a notably lower risk for highgrade toxicity than ICI-containing combination therapy. Several groups have investigated adaptive designed trials which escalate and de-escalate immunotherapy based upon an individualized response to minimize risk while attempting to achieve durable benefit.
Three previously reported adaptive trials (TITAN, OMNIVORE, HCRN-GU-260) had explored a novel stepwise scheme of adjusting the intensity of ICI therapy per radiographic response. All three studies enrolled ICI-naïve patients with clear cell histology and initially started with the anti-PD1 monotherapy nivolumab. Subsequently, per response assessment during the initial months of therapy, treatment was matched to the degree of therapeutic benefit observed, incorporating varying strategies with de-escalation(to active surveillance)vs.intensification (with the addition of ipilimumab)vs.continuation of nivolumab alone. The three studies varied in study population (distribution of risk status and prior treatment exposure), length of nivolumab induction and the type of risk adaptation escalation; hence, the results must be viewed within the context of the individual study design. For instance, nearly half the patients on TITAN had received prior therapy, and radiographic responses in the first 4 months were achieved in 22.7% of patients. At that point, those with SD or PD received up to 4 cycles of ipilimumab/nivolumab with additional responses observed in 12% of previous non-responders (n= 12/104 patients)[31]. In OMNIVORE,where half the patients had received TKI therapy, patients were initially treated with nivolumab monotherapy for up to 6 months; those who achieved a response had treatment discontinued altogether,while those who did not were treated with 2 cycles of ipilimumab[32]. The 6-month ORR was 14%, and 5 patients sustained a treatment-free interval for more than a year. For non-responders, the addition of 2 cycles of ipilimumab for salvage therapy applied after 6 months led to a response rate of only 4% (n= 2/57 patients). Lastly, in the HCRN-GU-260 study, all patients were treatment-naïve and underwent therapy with escalating doses of nivolumab (240 mg × 6 doses, 360 mg × 4 doses, and 480 mg monthly). Patients who experienced disease progression prior to or stable disease at 48 weeks were then offered salvage ipilimumab and nivolumab for 4 cycles[33]. In this schema, induction nivolumab was associated with PRs and CRs of 26% and 5.7% of patients, respectively (ORR = 31.7%), and the addition of salvage ipilimumab led to an added response in 4/30 patients (13.3%). Expectedly, high-grade immune-mediated adverse events were more frequent during the salvage ipilimumab portion of each study, but data in terms of escalating toxicities with the additional ipilimumab for those who sustained minor adverse events has not been reported. The relatively small number of patients whose treatment was escalated/de-escalated on these trials make it challenging to appreciate the nuances of how patients fair with individualized treatment intensity.Nonetheless, one can make valuable observations across all three studies. First, the addition of ipilimumab to nivolumab monotherapy could convert non-response to at least PR in all three trials, albeit in only a small proportion of patients (4%-13% of patients); and second, the composite ORR across all treated patients receiving individualized treatment on these three trials, and particularly the reported CR rates raise concern that the sequence of PD-1 to combination CTLA-4 directed combination therapy comes at the price of lost efficacy. As we await ongoing prospective efforts to formally assess the differences between ipilimumab plus nivolumabvs.nivolumab alone in the systemically untreated setting (NCT03873402), the low conversion and CR rates on OMNIVORE, TITAN and HCRN-GU-260 argue against the broad use of sequenced nivolumab/ipilimumab and support the use of combination therapy in the upfront setting.
Another effort is accepting upfront ipilimumab/nivolumab as the standard frontline regimen but tailoring treatment beyond the initial imaging assessments. For example, in the pivotal phase III PDIGREE study(NCT03793166), systemically untreated IMDC intermediate-poor risk ccRCC patients undergo treatment with induction ipilimumab and nivolumab therapy per standard schedule. Those who sustain PD are shifted towards cabozantinib monotherapy. Patients who achieve a CR to induction ipilimumab/nivolumab continue standard nivolumab maintenance up to 1 year. Patients who achieve a partial response or stable disease are randomized to receive nivolumab monotherapy or combination cabozantinib/nivolumab therapy. As these data remain forthcoming, understanding the optimal sequence of these combinations and other ongoing efforts will be critical to put this data within the context of cabozantinib/nivolumab in the first-line setting and increasing data supportive of combination therapy in the post-ICI space[34]. Lastly, in a similar vein in favor of de-escalation, a discontinuation design study where patients treated with axitinib and avelumab discontinue axitinib if sustaining a response within the first 36 weeks of therapy also may provide perspective on these types of adaptive strategies (TIDE-A, NCT04698213).
Towards an individualized designed study using a pre-therapy biomarker for therapy selection, the BIONIKK study (NCT02960906) was recently presented[35]. In this innovative clinical trial, tumor tissue submitted at study entry was analyzed to distinguish participating systemically untreated ccRCC patients into four molecularly defined groups (ccRCC1-4) based on previously defined transcriptomic features[36].Molecular classification then determined treatment allocation - patients with ccRCC1 (immune-low) and ccRCC4 (immune-high) were randomized between nivolumab or ipilimumab and nivolumab, and patients with ccRCC2 (angiogenesis-high) and ccRCC3 (normal-like) tumor signatures were randomized between ipilimumab and nivolumab or TKI therapy with sunitinib or pazopanib. In these groups, no significant differences were observed in terms of the primary endpoint of treatment response, but combination ipilimumab and nivolumab was numerically higher in the ccRCC1 group (immune-low)[35]. While the results remain preliminary, this study notably provides a proof of concept that molecular stratification to target individual tumor biology rationally is feasible and provides initial foundational steps toward precision medicine approaches integrating angiogenesis targeting, immune therapy, and other novel treatment approaches.
ESCALATING BEYOND TKI/ICI THERAPY - CAN WE ACHIEVE MORE WITH MORE?
With recent advances having newly yet firmly established doublet combinations as the current standard of care, multiple ongoing investigations are taking combination therapy one step further and testing triplet regimens for the first-line treatment of ccRCC [Table 2]. One of the most notable ongoing studies is the pivotal global phase III COSMIC-313 study (NCT03937219), which randomizes patients with IMDC intermediate-poor risk disease to receive standard ipilimumab/nivolumab with either cabozantinib or placebo[37]. After the initial induction phase, patients receive nivolumab (for a maximum of 2 years) and cabozantinib or placebo. While encouraging anti-tumor efficacy and tolerability has been reported in phase I studies[38], it will be important to note specific and chronic toxicities, particularly in the context of the reported cabozantinib/nivolumab therapy data.
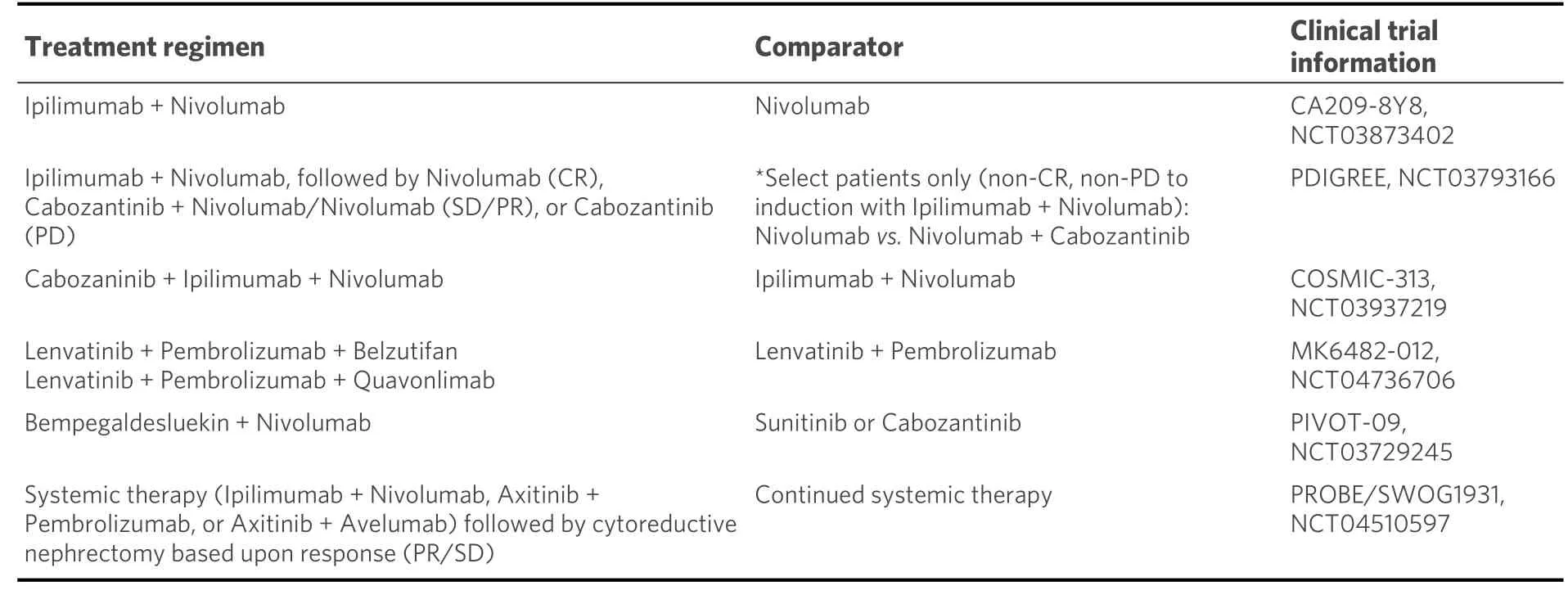
Table 2. Select phase III ongoing clinical trials for systemically treatment-naïve RCC
Novel agents that have also shown efficacy in the first-line setting are also being positioned in the triplicate space, with the incorporation of a VEGFR TKI and ICI backbone. For example, belzutifan (MK-6482) is a potent and selective orally available HIF-2α inhibitor that has received breakthrough designation for the treatment ofVHLassociated RCC in patients with non-metastatic tumors. In the phase II setting,encouraging therapeutic responses have been reported with monotherapy (NCT04195750) and in combination with cabozantinib (NCT03634540)[39]. In line with this, evaluation in the triplicate setting is now being pursued on a global phase III study (NCT04736706). Here, systemically untreated patients are randomized to receive lenvatinib/pembrolizumab, lenvatinib/pembrolizumab/quavonlimab (an anti-CTLA-4 antibody), or lenvatinib/pembrolizumab/belzutifan. This study will relevantly shed light on the combination of VEGF, ICI, and HIF-2α inhibition and will also notably provide data on a competing triplicate combination given the arm, including lenvatinib with dual ICI approach (similar to COSMIC-313).Building upon this framework, other promising therapeutic agents with strong clinical activity in the treatment-refractory setting are also being poised for a position in the triplicate space with combinations still under early investigation. For example, bempegaldesleukin (NKTR-214) is a pegylated first-in-class IL-2 receptor that potently binds to the CD122 surface to stimulate proliferation and mobilization into the tumor microenvironment[40]. Bempegaldesleukin was previously tested in a phase I/II study in patients with systemically treatment-naïve ccRCC and was found to have an ORR of 64% (7/11 patients) with responses seen regardless of PD-L1 expression[41]. Based upon these encouraging results, a global phase III study of bempegaldesleukin in combination with nivolumabvs.investigator’s choice TKI (sunitinib or cabozantinib)remains ongoing with results forthcoming (NCT03729245)[42]. In addition to this, an early phase I/II study(NCT04540705) tests this novel agent in combination with ICI and VEGFR TKI therapy (axitinib and cabozantinib, on separate arms) in the treatment-naïve setting. Other agents, including MEDI5752, a monovalent bispecific antibody co-targeting PD-1 and CTLA-4, paired with axitinib, similarly explore the triplicate combination of the mechanism of action for therapy of TKI and PD-1/CTLA-4 inhibition(NCT04522323)[43]. As more triplicate combinations are developed, it will be helpful to discriminate these additional strategies within the known context of angiogenesis and immune activation to optimize synergism and limit overlapping toxicities.
CONCLUSION
The treatment paradigm for patients with systemically untreated ccRCC has significantly evolved, and multiple ICI-based combination therapies are the new standard for all eligible patients. Current first-line combinations have entered the arena in rapid succession, and while it is increasingly difficult to select amongst these available options, certain clinical situations and the clinician’s treating experience may guide the selection of one regimen over another. Doublet therapy has become the standard comparator for clinical trials, but regimen choice will likely remain investigator-dependent and within the context of prognostic risk stratification. As triplicate regimens become a new reality, management of treatment-related adverse events will also become increasingly complex, and a key question is whether an achieved gain in efficacy justifies added toxicity. All this constitutes an urgent call for biomarker-driven studies and innovative trial designs with an overarching goal to incorporate molecular profiling and identify determinants for response,resistance, and ideally adverse effects, with adaptive study designs that offer the ability to intensify and de-
escalate treatment for optimized antitumor effect and tolerance. As contemporary studies feed into this framework through exploration of additional biological axes pertinent for RCC biology and therapeutic effects, a new era for enhancing these combinations even further may soon come within reach.
DECLARATIONS
Authors’ contributions
Writing, reviewing, and editing: Kotecha RR, Voss MH
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
Kotecha RR has served as a consultant/advisory board member for Eisai, and reports receiving institutional research support from Pfizer and Takeda. Voss MH reports receiving commercial research support from Bristol-Myers Squibb, Pfizer and Genentech/Roche; honoraria from Novartis and Bristol-Myers Squibb;travel/accommodation from Astra Zeneca, Eisai, Novartis and Takeda; consultant/advisory board member for Aveo, Calithera Biosciences, Corvus Pharmaceuticals, Exelixis, Eisai, Merck, Onquality Pharmaceuticals,Novartis and Pfizer.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2021.
 Journal of Cancer Metastasis and Treatment2021年12期
Journal of Cancer Metastasis and Treatment2021年12期
- Journal of Cancer Metastasis and Treatment的其它文章
- Progress in the treatment of NK-cell lymphoma/leukemia
- The contemporary role of metastasectomy in the management of metastatic RCC
- Cancer-directed surgery in malignant pleural mesothelioma: extrapleural pneumonectomy and pleurectomy/decortication
- Intraoperative monitoring of the recurrent laryngeal nerve in surgeries for thyroid cancer: a review
- AUTHOR INSTRUCTIONS
