The contemporary role of metastasectomy in the management of metastatic RCC
Zachary Feuer, Jacob I. Taylor, William C. Huang
Department of Urology, New York University Langone Health, New York, NY 10016, USA.
Abstract Metastasectomy was initially described in the 1970s as a therapeutic strategy for patients with metastatic renal cell carcinoma. Since that time, systemic therapy options have grown exponentially, most recently with the introduction of immunotherapy. We aimed to review the contemporary literature regarding the role of metastasectomy in the era of targeted therapy and immunotherapy. Historically, metastasectomy has benefited patients with small volume, single-organ metastases, with favorable outcomes amongst younger, healthier patients with metastases to specific sites. The interplay between the employment of metastasectomy and systemic therapy has been limited to small, retrospective series with significant patient selection bias. More recently, investigators have conducted randomized controlled trials exploring the use of targeted therapies in the adjuvant setting after metastasectomy. Initial randomized data suggested no benefit in using sorafenib in this setting, and a subsequent study demonstrated possible harm in using pazopanib after metastasectomy. However, the role of other novel systemic therapies, including immunotherapy, nor the timing of use, have been meaningfully explored.Metastasectomy appears to be a valuable therapeutic option in the properly selected patient, requiring a multidisciplinary management strategy and, pending future trials, a multimodal treatment approach.
Keywords: Clear-cell metastatic renal cell carcinoma, metastasectomy, immunotherapy
INTRODUCTION
Nearly 30% of patients with renal cell carcinoma (RCC) have metastatic disease at the time of diagnosis[1].Amongst men treated for localized RCC, approximately 25% progress in developing metastatic disease[2].Metastases commonly present in the lung (60%-75%), lymph nodes (60%-65%), bone (39%-40%), liver (19%-40%) and brain (5%-7%)[3]. Presently, treatment options for metastatic RCC (mRCC) include observation,clinical trial enrollment, systemic therapy, and metastasectomy (with or without SBRT and/or ablative techniques). There are no recommendations regarding which of these modalities is preferred[3].
With the rapid evolution of systemic therapy options in the era of targeted therapies and immuno-oncology,level I evidence for the role of surgery in metastatic disease, and specifically metastasectomy, remains sparse.This is due to study limitations associated with patient performance status, disease distribution, and the surgical accessibility of metastatic sites, all of which are associated with significant selection bias[4].Nevertheless, metastasectomy remains a recommended management option for mRCC in select patients[5].
We aim to review the literature pertaining to the historical role of metastasectomy, including a discussion of outcomes-based upon metastatic site, and determine the role of metastasectomy in the era of immunotherapy.
THE EARLY ROLE OF METASTASECTOMY
The role of metastasectomy has evolved over the past several decades as the landscape of treatments for mRCC has transitioned from the cytokine era to targeted therapies and immuno-oncology, as well as combinations of these therapies. The first large, retrospective series to examine the role of metastasectomy in the cytokine era was conducted in 278 patients and demonstrated that solitary metastasis, longer diseasefree interval (DFI), and younger age were associated with extended survival. In this study, 5-year survival was approximately 55%[6]. A subsequent study retrospectively evaluated 152 resections in 101 patients.Disease-free survival was noted to be low, ~7%, at 60 months, but resection was determined to be feasible with low morbidity. Overall survival was not assessed[7].
Another retrospective analysis, conducted by Altet al.[8]during the cytokine era, assessed 887 patients who underwent nephrectomy for RCC and subsequently developed metastases. After controlling for patient performance status, timing, location, and the number of metastases, they found that complete metastasectomy was associated with prolongation of cancer-specific survival (4.8 yearsvs.1.3 years). In patients with lung-only metastases, 5-year cancer-specific survival was 73.6% with complete resectionvs.19% amongst patients who were managed non-operatively[8]. These early studies demonstrated that in the absence of effective systemic therapy, complete metastasectomy was an effective treatment strategy for patients with mRCC.
IMAGING IN METASTATIC RENAL CELL CARCINOMA STAGING
The primary imaging techniques for the initial staging of a primary renal mass include computerized tomography (CT) and magnetic resonance imaging (MRI). Generally, CT is utilized as the primary modality, conserving MRI for situations in which iodinated contrast is contraindicated or when further soft tissue delineation is necessary for accurate staging[9]. The National Comprehensive Cancer Network recommends that chest imaging be obtained with a chest radiograph (CXR). Meanwhile, these guidelines suggest that CT of the chest, CT of the brain, and/or a bone scan are obtained if clinically indicated[3].However, the American College of Radiology Appropriateness Criteria recommends obtaining CT of the chest for stage ≥ T2 tumors, as small pulmonary metastases may be missed on CXR[10].
Nuclear medicine may play a role in mRCC diagnosis and staging. Positron emission tomography(PET)/CT represents a modality that may provide better sensitivity for the diagnosis of metastases[11].Fluorine 18-sodium fluoride ligand has been demonstrated to be sensitive for the detection of bone metastases[10]. More recently, PET/CT using Zr-89-girentuximab, a monoclonal antibody-based ligand, was demonstrated to be more sensitive than CT alone, than F-fluorodeoxyglucose (18F)-PET/CT for detecting bone and soft tissue lesions in patients with good and intermediate-risk mRCC[12].
THE ROLE OF METASTASECTOMY IN SPECIFIC ORGAN SITES
The role of metastasectomy has been reviewed extensively in the literature, often in series assessing the role of metastasectomy by specific organ site. Metastasectomy outcomes by organ site are summarized in Table 1.
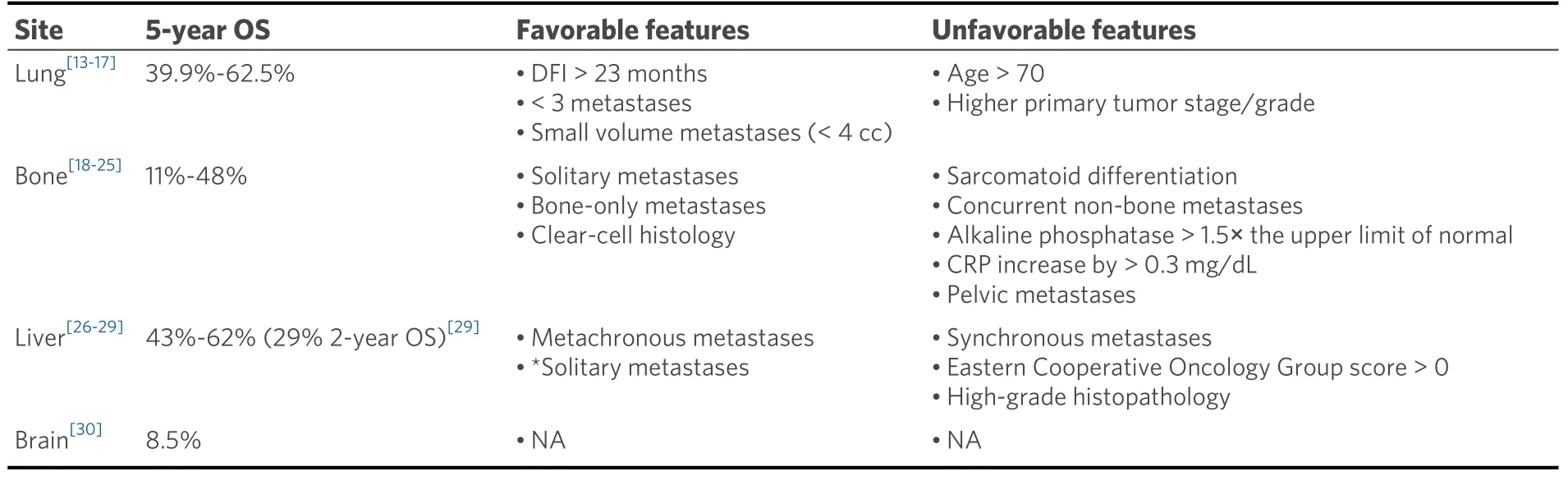
Table 1. Summary of metastasectomy outcomes by metastatic site
Lung
The role of metastasectomy in the management of pulmonary metastases in the setting of mRCC has been well-characterized. Pfannschmidtet al.[13]retrospectively assessed pulmonary metastasectomy in 191 patients, including patients with no primary tumor site recurrence and no extrapulmonary metastases.Complete resection was achieved in 145 cases. This study demonstrated an overall 5-year survival of 36.9%,41.5% in patients who underwent complete resectionvs.22.1% amongst those with partial resections. The authors dichotomized prognostic indicators based on values that demonstrated the most significant discrimination between good and poor outcomes. They noted a significant survival difference amongst patients with < 7 metastases as compared to ≥ 7 individual sites (46.8%vs.14.5%). Finally, they noted that DFI greater than 23 months was associated with an improvement in 5-year survival (47%vs.24.7%)[13].
A German study analyzed the impact of the histologic characteristics of the primary tumor on survival in 107 consecutive patients undergoing pulmonary metastasectomy. Complete resections were performed in 97.2% of patients, and 33.6% received systemic therapy prior to pulmonary metastasectomy. The mean survival was 63.4 months with 5- and 10-year survival rates of 47% and 9%, respectively. The authors found that node status, primary tumor histologic grade, and primary tumor stage were all associated with worse survival outcomes despite metastasectomy. The effect of systemic therapy was not evaluated.[14]
Kudelinet al.[15]reviewed 116 patients who underwent pulmonary metastasectomy with intrathoracic lymph node dissection. In this cohort, 34.5% of patients had systemic therapy prior to metastasectomy. Five- and ten-year survival was noted to be 49% and 21%, respectively. Age > 70, female gender and the number of metastases were noted to be poor prognostic factors. Only age > 70 remained significant in multivariate analysis[15].
More recently, Saricamet al.[16]evaluated the role of pulmonary metastasectomy in 48 patients,demonstrating a median survival of 56.2 months, with a 5-year survival of 62.5%. The study noted that DFI> 32 months, fewer, and smaller volume metastases (< 4 cc3) were factors associated with better prognosis[16].
Bone
Bone metastases are present in approximately 40% of patients with mRCC[3]. To assess prognostic indicators, Kumeet al.[17]reviewed 94 patients with bone metastases at multiple tertiary referral centers. The authors noted that sarcomatoid differentiation, vertebral bone involvement, concurrent non-bone metastases, alkaline phosphatase increases > 1.5 times the upper limit of normal, and C-reactive protein increases > 0.3 mg/dL were associated with poor prognosis[17].
A recent descriptive study demonstrated that men were four times more likely to harbor bone metastases.Lesions had a predilection for axial bone and the incidence of severe events, including pathologic fracture,was nearly 55%. Shorter time to bone metastasis, older age, multiorgan metastases, and carbonic anhydrase expression loss were associated with poor prognosis. Huanget al.[18]noted a median overall survival of 45 months for patients with favorable risk factors and 32 months for patients who were deemed unfavorable risk.
Kollenderet al.[19]retrospectively analyzed 45 patients with solitary bone metastases and either intractable pain or impending/current pathologic fracture who underwent surgical intervention. The authors noted significant pain relief and good functional outcomes in approximately 90% of patients. Overall, half of the patients survived more than 2 years, and 38% survived more than 3 years[19].
Higuchiet al.[20]evaluated survival in 58 patients who underwent surgical intervention for either solitary or multiple bone metastases at a single institution. Amongst the cohort, metastases were noted in the spine in 33 patients, in the appendicular skeleton in 10 patients, in the pelvis in 8 patients, in the thoracic bones in four patients, and in soft tissue in 3 patients. Forty-six patients underwent metastasectomy, and 12 patients underwent curettage. The authors noted an 89% overall survival at 1 year, 62% overall survival at 5 years,and 48% overall survival at 10 years. The median overall survival for patients undergoing metastasectomy was 127 months, and for curettage was 54 months. Of note, pelvic metastases were associated with shorter median overall survival[20].
A study by Linet al.[21]retrospectively assessed 295 consecutive patients with bone metastases treated at a single institution. Surgical interventions included curettage, en bloc resection, closed nailing, or amputation.The authors noted an overall survival of 47% and 11% at 1 and 5 years, respectively. They reported that patients with solitary metastases had the most favorable outcomes, and that patients with bone-only metastases had a favorable prognosis relative to patients with additional extraosseous metastases.Furthermore, clear-cell histology was associated with an improved prognosis. Notably, 5% of patients died within 30 days of surgery[21].
Ptashnikovet al.[22]recently conducted a retrospective review of 100 patients with spinal mRCC.Metastasectomy was performed in 39 cases while 61 patients underwent decompression procedures with stabilization-only, analyzing pain, neurologic status, overall and progression-free survival. Of these patients,26 had adjuvant targeted therapy (7 of whom had undergone metastasectomy). The study noted improvement in neurologic function and pain relief, while the authors found no improvement in overall survival amongst patients managed with each modality. However, amongst all patients, there was an overall survival benefit for those who received targeted therapy[22].
Stereotactic body radiation therapy (SBRT) has been employed in the management of osseous metastases from RCC, both for the treatment of metastases and for palliation. In a study assessing local control of bone metastases after SBRT or external beam radiation therapy, authors assessed response to 95 bone lesions in 46 patients. They noted significantly improved pain control with SBRTvs.EBRT, 74.9%vs.39.9%, 74.9%,and 35.7% experiencing improvement at 12 and 24 months, respectively (P= 0.02). The median time to radiolographic failure in both groups was similar (7 months)[23]. Jhaveriet al.[24]demonstrated a dosedependent effect of SBRT on the efficacy (83%vs.73%) and durability (46%vs.12% symptom recurrence) of pain control for patients receiving > 85 Gyvs.< 85 Gy doses.
Liver
Renal cell carcinoma metastatic to the liver occurs in approximately 20%-40% of patients[3]. These metastases are difficult to manage as they typically present concurrently with multiple extrahepatic metastases. Furthermore, only 25% of patients have solitary liver lesions[25]. Ruyset al.[26]reviewed 33 patients who underwent intervention for hepatic metastases, with metastasectomy performed in 29 patients.Overall survival was 79% and 43% at 1 and 5 years after resection. The authors noted that prognostic factors for improved survival included metachronous metastases and radical resection. Meanwhile, size, solitary metastases, and the presence of extrahepatic metastases were not associated with an impact on overall survival[26].
Staehleret al.[27]retrospectively compared 48 patients who underwent liver metastasectomy and 20 patients who were denied surgery to evaluate overall survival. In this study, nearly 80% of patients received adjuvant systemic therapy with interleukin therapy. Overall survival at 5 years was 62% in the group who underwent resectionvs.29% in those who were observed. The survival difference was most substantial amongst patients with primary tumor low-grade histology (155 monthsvs.29 months) in the metastasectomy and observation cohorts, respectively. Notably, patients with synchronous metastases, ECOG scores > 0 and patients with high-grade histology did not benefit from surgical intervention[27]. Although differences were not statistically significant between cohorts, patients who were denied surgery tended to have higher grade disease and higher volume metastatic burden.
In a recent study, Joyceet al.[28]analyzed 34 cases of hepatic metastasectomy. In 17 patients, hepatic resection was performed for a direct invasion of the liver, while 21 patients underwent simultaneous nephrectomy and hepatic metastasectomy. Four patients had both locally invasive and separate hepatic metastases. Amongst these patients, 2-year cancer-specific survival and overall survival were 40% and 29%,respectively. In a matched cohort of 68 patients undergoing metastasectomy for mRCC to non-hepatic sites,2-year cancer-specific survival and overall survival were 40% and 28%, respectively[28].
Brain
The role of surgical metastasectomy of brain metastases in mRCC remains limited. The surgical approach typically includes either SBRT, whole-brain radiotherapy, or surgical resection. Analyses of the role of surgical resection remain limited to case reports and small series. The largest series analyzing the role of surgical resection for brain metastases in mRCC retrospectively analyzed 50 patients with metachronous brain metastases after primary treatment. The median overall survival from the time of craniotomy was approximately 13 months, with a 10% postoperative mortality rate. Twenty-two patients received additional whole-brain radiotherapy, while 18 patients did not. Overall survival at 1 and 5 years was 51% and 8.5%,respectively[29].
Lymph nodes
Lymph nodes are involved in 60%-65% of mRCC cases[3]. These are typically identified as synchronous metastases, as local nodal recurrence is rare[30]. The initial management of lymph node dissection at the time of nephrectomy was assessed when Blomet al.[31]randomized 732 patients, 1:1, to undergo lymph node dissection at the time of nephrectomy or nephrectomy alone. Lymph node dissection did not improve overall survival (HR = 1.02, 95%CI: 0.80-1.29), local regional progression (HR = 0.77, 95%CI: 0.46-1.28) or distant progression (HR = 1.05, 95%CI: 0.73-1.50)[31]. However, the major criticism of this study was that investigators included many low-risk patients with T1 and T2 tumors less likely to harbor nodal metastasis.
The role of lymph node dissection at the time of radical nephrectomy for patients with suspected nodal metastases was studied by Pantucket al.[32]. There was no increase in median survival for patients without preoperative evidence of nodal metastases undergoing lymph node dissectionvs.those who underwent radical nephrectomy alone. However, there was a survival benefit noted amongst patients with concern for nodal metastases who underwent lymphadenectomy as compared to those that did not. The authors reported a 5-month improvement in median overall survival in this cohort[32]. Subsequent studies noted no benefit in patients with preoperatively diagnosed mRCC[33,34]. To date, the role of lymph node dissection in high-risk patients has not been prospectively studied[35].
INFLUENCE OF PRIMARY TUMOR HISTOLOGY
Primary tumor histology has been demonstrated to impact prognosis in patients undergoing metastasectomy significantly. A recent systematic review demonstrated that primary tumor features,including findings of Fuhrman high-grade histology and sarcomatoid features, were associated with worse outcomes[36]. Takagiet al.[37]noted similar findings with regard to the presence of sarcomatoid features (HR= 8.89,P= 0.028), however, found that Fuhrman grade was not independently associated (HR = 0.83,P=0.757). Another study by Ishiharaet al.[38]found that the risk of death amongst patients with non-clear cell histology was double that of patients with clear cell histology.
The impact of primary tumor histology on outcomes has not been directly explored as a primary outcome,and stratification by cell types has been limited given the proportionally low number of patients with nonclear cell findings. However, given the lack of effective systemic therapies for patients with non-clear cell histology, the role of surgical management should be further explored.
COMPLICATIONS OF METASTASECTOMY
Despite the potential for successful management of mRCC, metastasectomy carries the risk of surgical complications. Palumboet al.[39]reviewed 351 patients who underwent metastasectomy, noting a complication rate of 55%, including 22% pulmonary complications, which were not further defined, and a need for transfusion in 15.7% of patients. A recent retrospective review of 1102 patients who underwent metastasectomy noted an overall complication rate of 45.7%, with a major complication rate Clavien III-IV of 27.5%. The investigators reported that older age, Charlson-Deyo score ≥ 2, and resection of hepatic metastases were associated with an increased risk of major complications (OR = 2.59,P< 0.001), while resection of pulmonary metastases was associated with a decreased risk (OR = 0.63,P< 0.001)[40]. Despite these complications, resection of metastases has a range of value and chance for intermediate-term survival depending on metastatic disease site.
ROLE OF SYSTEMIC THERAPY AND METASTASECTOMY
Karamet al.[41]were amongst the first to assess the combined role of metastasectomy and systemic therapy in a retrospective analysis of 22 patients with mRCC, all of whom were treated with targeted therapy followed by consolidative metastasectomy. Twenty-one patients were alive at the time of the study at a median follow-up of approximately 2 years, while surgical-associated morbidity was low. Amongst these patients, 11 patients developed a recurrence at a median 42 weeks from the time of metastasectomy, while the other 11 patients had not recurred at a similar median interval. Of note, 9 patients received additional systemic therapy after metastasectomy[41].
The use of neoadjuvant therapy followed by metastasectomy was further studied in 124 patients. In this retrospective review, 75 patients received targeted therapy only, 26 underwent targeted therapy followed by complete metastasectomy, and 23 underwent partial resection. In these patients, only complete resection was associated with improved overall survival (HR = 0.50). Of note, this study is had significant selection bias as there was an uneven distribution of poor-risk patients in the study groups. Nearly 23% of patients in the targeted therapy group were considered poor-risk, as compared to 3.8% and 0% in the complete and partial metastasectomy cohorts.[42]
ADJUVANT THERAPY AFTER METASTASECTOMY
Recently, the first RCT in a metastasectomy cohort randomized patients to sorafenib or observation after undergoing complete resection. Sixty-eight patients were randomized, with approximately 80% in each group harboring solitary metastases. They included patients who underwent cytoreductive nephrectomy with ECOG ≤ 2, less than 3 metastases, and predominant clear cell histology. Recurrence-free survival was 37 months in the observation arm and 21 months in the sorafenib arm, with significantly more adverse events in the treatment arm. Procopioet al.[43]concluded that sorafenib after metastasectomy was not recommended.
ECOG 2810 is a double-blind RCT, which sought to demonstrate that pazopanib improved disease-free survival in patients who underwent complete metastasectomy as compared to placebo. The study included 129 patients randomized 1:1 for 52 weeks of therapy, seeking to observe a 42% improvement in disease-free survival at 3 years. The study did not meet the primary endpoint (HR = 0.85,P= 0.47). On the contrary,overall survival favored patients receiving placebo (HR for OS = 2.65,P= 0.05)[44].
Beyond the use of vascular endothelial growth factor inhibitors and immune checkpoint inhibitors, a German group assessed the use of an adjuvant multi-peptide vaccine after metastasectomy. The vaccine was distributed to 19 patients after complete metastasectomy, and this group was compared with a separate contemporary cohort of 44 patients who underwent metastasectomy only. The vaccine was well-tolerated and demonstrated an improvement in overall survival (HR of death = 0.19,P= 0.012) compared to patients undergoing resection alone[45].
CURRENT ROLE OF METASTASECTOMY
The evidence supporting the role of metastasectomy, historically, is mostly retrospective and observational.However, despite the limited prospective evidence, available studies suggest a benefit of complete resection in the properly selected patient[38]. A recent systematic review of 56 studies demonstrated that overall survival ranges from 36-142 months for those undergoing metastasectomyvs.8-27 months for those subject to nonoperative management. Prognostic factors associated with overall survival included disease-free survival from time of nephrectomy, number of metastases, patient performance status, and primary tumor features, specifically grade, stage, presence of sarcomatoid features, and nodal status. The most important prognostic indicator was complete resection[36].
As previously discussed, the use of pazopanib in the adjuvant setting failed to demonstrate an improvement in survival and, further, may have worsened outcomes in patients receiving the immunotherapy compared to placebo[44]. However, the combined role of metastasectomy and many immunotherapeutic agents remains unknown. One recent editorial proposed the possibility that metastasectomy may be useful in the setting of treatment beyond progression due to the intratumor heterogeneity of disease in mRCC[46].
Investigators queried the Canadian Kidney Cancer information system database to review the records of 229 patients who underwent complete metastasectomy, matched with 803 patients who did not. Patients who did not undergo metastasectomy were more likely to receive targeted therapy in this cohort (74%vs.47%).The 1- and 5-year overall survival rates were 96% and 63% in the metastasectomy group, as compared to 90% and 51% in the group that did not undergo surgical resection, respectively. The effect of systemic therapy on these rates was not assessed. Risk of mortality was increased in patients who did not undergo metastasectomy (HR = 0.41,P< 0.001). Dragomiret al.[47]noted that age > 65 and the presence of brain metastases were factors associated with a worse prognosis.
Lyonet al.[48]retrospectively assessed the role of complete metastasectomy in the post-cytokine era,including 586 patients who underwent primary treatment for renal cell carcinoma with subsequent development of metastatic disease between 2006 and 2017. One hundred-fifty-eight patients were treated with complete metastasectomy, 93% of which did not receive systemic therapy. The authors noted that cancer-specific survival was improved significantly amongst patients who underwent complete metastasectomy compared to patients who did not (84%vs.54%). In a multivariate analysis, the hazard ratio for death from mRCC was reduced significantly in patients who underwent complete metastasectomy (HR= 0.47,P< 0.001), regardless of age, gender, timing, number, and location of metastases[48].
In a retrospective review of 314 patients with mRCC, 98 patients underwent metastasectomy. Amongst these 98 patients, 45 patients underwent complete resection, while the remaining 53 were incompletely resected. The authors reported that metastasectomy status was an independent predictor of overall survival.The group further analyzed the survival for patients undergoing metastasectomyvs.no resection in the various systemic therapy eras. The Kaplan-Meier curve demonstrated similar results for metastasectomy in each era, including the early targeted therapy (2008-2011), late targeted therapy (2012-2016), and immunotherapy eras (2016-2018). However, the Kaplan-Meier curves for each systemic therapy demonstrated improving survival in each era, persistently inferior to survival demonstrated in patients undergoing metastasectomy[38].
ROLE OF SURGICAL MANAGEMENT IN THE ERA OF IMMUNOTHERAPY
Given the rapidly evolving landscape of systemic therapy for mRCC, and the sparsity of high-quality studies specifically addressing the role of systematic therapy in patients undergoing metastasectomy, the role of surgical management should be considered more broadly.
The role of cytoreductive nephrectomy in early studies demonstrated a survival benefit in men concurrently treated with interferons[49,50]. In the targeted therapy era, the CARMENA trial randomized 450 intermediate and poor-risk patients to cytoreductive nephrectomy followed by sunitinib or sunitinib alone. In this study,the hazard ratio for death amongst patients receiving sunitinib-alone was 0.89 (95%CI: 0.71-1.10),demonstrating noninferiority compared to patients undergoing nephrectomy followed by sunitinib. Overall survival was 13.9 and 18.4 months in patients in the nephrectomy-sunitinib and sunitinib cohorts,respectively[51]. However, a criticism of the CARMENA trial was the significant proportion of patients with poor performance status and poor-risk disease, which was subsequently associated with worse survival outcomes in a meta-analysis[52].
The SURTIME trial randomized 99 patients to immediate cytoreductive nephrectomy with subsequent sunitinib therapy or sunitinib therapy followed by deferred cytoreductive nephrectomy. Both arms demonstrated similar progression-free survival while deferred cytoreductive nephrectomy was associated with improved overall survival (overall survival HR = 0.57, 95%CI: 0.34-0.95,P= 0.03). Median overall survival was 15.0 and 32.4 months in the immediate and the deferred cytoreductive nephrectomy arms,respectively[53]. A subsequent systematic review concluded that upfront cytoreductive nephrectomy is not associated with survival benefit in patients with the intermediate and poor-risk disease. However, evidence in patients with good performance status and good or intermediate-risk disease suggest that intervention may be beneficial[54], paralleling the findings of observational studies in the metastasectomy literature.
The introduction of immunotherapy proposed a new set of questions regarding the respective roles of systemic therapy and cytoreductive nephrectomy in the management of mRCC. A study randomizing 104 patients to receive three combinations of immune checkpoint inhibitors with subsequent cytoreductive nephrectomy and adjuvant nivolumab was noted to be safe, but survival outcomes were not assessed[55].
A retrospective analysis of patients undergoing cytoreductive nephrectomy with neoadjuvant/adjuvant immunotherapyvs.immunotherapy-alone demonstrated improved overall survival amongst patients who underwent CN in combination with immunotherapy (HR = 0.23, 95%CI: 0.15-0.37,P< 0.001). In a subanalysis exploring differences in the timing of systemic therapy and CN, those undergoing systemic therapy followed by CN (n= 24) did not reach median overall survival, while median overall survival was 30 months for patients undergoing upfront CN (n= 197). These findings were not significant, likely due to the small number of patients receiving neoadjuvant treatment[56].
PROSPECTIVE ROLE OF METASTASECTOMY IN THE ERA OF IMMUNOTHERAPY
Review of the available literature suggests that one of the primary goals of metastasectomy should be complete resection, which is associated with significant survival benefits. However, evidence exploring the utility of metastasectomy in the era of immunotherapy has been limited to the use of immunotherapeutic agents in the adjuvant setting[44,45]. A recent animal study evaluating the timing of immunotherapy delivery strongly supports neoadjuvant, rather than adjuvant, use of these therapies[57].
It is well-established that renal cell carcinoma exhibits significant intratumoral heterogeneity[58,59]. Further,these genomic differences have been associated with differences in therapeutic response to PD-L1 inhibitors[60]. These findings suggest that the use of neoadjuvant, therapy to decrease metastatic burden prior to metastasectomy may increase the likelihood of complete resection, thereby conferring a survival benefit in these patients. However, any associated effect may be modulated by the location, extensiveness, or genomics of these metastases[61].
PROSPER RCC is a phase III RCT, randomizing patients with clinical stage ≥ T2, in a 1:1 fashion, to either surgery (partial or radical nephrectomy) alone or neoadjuvant PD-L1 blockade with nivolumab, followed by surgery and adjuvant nivolumab. The planned primary endpoint is 5-year recurrence-free survival. This trial may have significant implications on the treatment paradigm in the primary management of RCC and,pending findings, may also impact mRCC treatment strategies[62].
CONCLUSION
The role of metastasectomy in the era of immunotherapy remains underexplored and requires prospective study to be fully ascertained. Initial evidence suggests that adjuvant systemic therapy, including immunotherapy, may not improve outcomes. Nevertheless, the role of neoadjuvant systemic therapy has not been elucidated, nor have all contemporary treatment options been explored.
Historically, retrospective evidence has demonstrated that metastasectomy may be a valuable management option in the properly selected patient, dependent on factors related to (1) metastatic characteristics such as the site, number of organs involved and number of metastases per site; (2) primary tumor factors such as histologic grade, and stage; and (3) patient factors including age, and medical co-morbidities. Generally,complete resection of solitary, single-organ metastases, with favorable primary tumor histology, in patients with better performance status are associated with survival benefits after metastasectomy. Further, despite the evolving landscape of therapeutic agents, metastasectomy remains the only means of a definitive cure for mRCC. Nevertheless, it must be highlighted that a significant portion of the literature is retrospective in nature and, as such, is limited by associated biases.
The decision to pursue metastasectomy should be considered within the framework of a multi-disciplinary management discussion and, pending the results of future studies, may be a valuable tool in a multimodal treatment approach.
DECLARATIONS
Authors’ contributions
Made substantial contributions to conception and design of the study and performed data analysis and interpretation: Feuer Z, Taylor J, Huang WC
Performed data acquisition, as well as provided administrative, technical, and material support: Feuer Z,Taylor J, Huang WC
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2021.
 Journal of Cancer Metastasis and Treatment2021年12期
Journal of Cancer Metastasis and Treatment2021年12期
- Journal of Cancer Metastasis and Treatment的其它文章
- Progress in the treatment of NK-cell lymphoma/leukemia
- Novel standards and emerging therapies for systemically treatment-naïve clear cell renal cell carcinoma - a rapidly changing landscape
- Cancer-directed surgery in malignant pleural mesothelioma: extrapleural pneumonectomy and pleurectomy/decortication
- Intraoperative monitoring of the recurrent laryngeal nerve in surgeries for thyroid cancer: a review
- AUTHOR INSTRUCTIONS
