GLUD1在肝细胞癌患者中表达特点的研究
宋树娟 范琳 王振国

【摘要】 目的:探讨谷氨酸脱氢酶1(glutamate dehydrogenase 1,GLUD1)在肝细胞癌患者中的表达特点。方法:在TCGA数据库中下载343例肝细胞癌患者临床数据及RNA表达数据,比较不同年龄、不同种族及不同肿瘤分期患者的GLUD1表达水平;按照GLUD1表达水平将患者排序,并分为高表达组(n=171)和低表达组(n=172),比较两组患者生存时间。结果:GLUD1表达水平与年龄无相关性(r=-0.102,P>0.05)。不同种族间GLUD1表达水平比较,差异无统计学意义(P>0.05)。Ⅰ、Ⅱ、Ⅲ、Ⅳ期患者GLUD1表达水平分别为(13.51±0.38)、(13.29±0.54)、(13.16±0.79)、(13.65±0.51),四组比较差异有统计学意义(F=3.915,P=0.007);Ⅱ、Ⅲ期患者的GLUD1表达水平均低于Ⅰ期,差异均有统计学意义(P<0.05)。GLUD1高表达组患者的中位生存时间长于GLUD1低表达组,差异有统计学意义(P<0.05)。结论:GLUD1高表达患者的肿瘤级别较低、生存期较长。
【关键词】 肝细胞癌 谷氨酸脱氢酶1 富集分析
Research on Expression Characteristics of GLUD1 in Patients with Hepatocellular Carcinoma/SONG Shujuan, FAN Lin, WANG Zhenguo. //Medical Innovation of China, 2021, 18(15): -143
[Abstract] Objective: To investigate the expression characteristics of GLUD1 in patients with hepatocellular carcinoma (HCC). Method: The clinical data and RNA expression data of 343 patients with hepatocellular carcinoma were downloaded from TCGA database, and the GLUD1 expression levels of patients with different ages, races and tumor stages were compared, and the patients were sorted according to the expression level of GLUD1, and divided into high expression group (n=171) and low expression group (n=172), and the survival time of two groups was compared. Result: There was no correlation between GLUD1 expression and age (r=-0.102, P>0.05). There was no significant difference in the expression level of GLUD1 among different races (P>0.05). The expression levels of GLUD1 in Ⅰ, Ⅱ, Ⅲ and Ⅳ stage patients were (13.51±0.38), (13.29±0.54), (13.16±0.79) and (13.65±0.51), respectively, and the differences among four groups was statistically significant (F=3.915, P=0.007); the expression levels of GLUD1 in both Ⅱ and Ⅲ stage patients were lower than that in Ⅰ stage patients, the differences were statistically significant (P<0.05). The median survival time of patients in the group with high GLUD1 expression was longer than that in the group with low GLUD1 expression, and the difference was statistically significant (P<0.05). Conclusion: Patients with high expression of GLUD1 have lower tumor grade and longer survival time.
[Key words] Hepatocellular carcinoma Glutamate dehydrogenase 1 Analysis of enrichment
First-author’s address: Qingdao Third People’s Hospital, Qingdao 266000, China
doi:10.3969/j.issn.1674-4985.2021.15.034
肝癌在全球范圍内每年导致超过70万人死亡,致死率在所有恶性肿瘤中排在第三位[1-3],可见肝癌的防治形势严峻[4]。但随着生物信息学的快速发展,研究发现肝细胞癌的发生发展涉及多条信号通路,发病机制复杂[5-6],因此寻找出能够提示肝癌患者预后的生物标志物,并针对该靶基因制定早期治疗方案对于提高肝癌的治疗效果意义显著。谷氨酸脱氢酶1(Glutamate dehydrogenase 1,GLUD1)可将L-谷氨酸转化为α-酮戊二酸,是谷氨酰胺代谢途径中的关键酶[7-8]。谷氨酰胺与自噬和炎症因子的分泌密切相关,研究表明抑制谷氨酰胺合成可使巨噬细胞诱导T细胞募集的能力增强,削弱癌细胞运动的能力[9]。Craze等[10]在乳腺癌相关研究中发现,GLUD1低表达与患者远期预后不良相关。但是在肝细胞癌中,GLUD1与患者预后的相关性尚未阐明,为此本研究通过文献[11]的癌症基因组图谱(the cancer genome atlas,TCGA)数据库分析GLUD1对肝细胞癌患者肿瘤分期及生存率的影响,从而为肝细胞癌的预后判断和治疗提供参考,现报道如下。
1 资料与方法
1.1 一般资料 从TCGA数据库下载肝细胞癌患者数据集RNASeq和Clinical,共343例,平均年龄(67.12±8.36)岁;其中男196例,女147例;白种人176例,黄种人102例,黑种人64例,印第安人1例;Ⅰ期139例,Ⅱ期106例,Ⅲ期95例,Ⅳ期3例。
1.2 方法
1.2.1 比较GLUD1表达水平与种族、年龄的相关性 将每例患者GLUD1表达水平作为横坐标,年龄作为纵坐标绘制散点图,比较GLUD1表达水平与年龄的相关性。将患者分为亚洲人、黑种人和白种人三组,比较不同种族之间GLUD1表达差异。
1.2.2 不同肿瘤分期患者GLUD1中表达差异 将患者根据肿瘤分期归入不同组别,比较不同分期患者GLUD1表达差异。
1.2.3 生存曲线分析 按照GLUD1表达水平由高到低排序,将排在前50%的患者设为高表达组(n=171),剩余患者设为低表达组(n=172),绘制生存曲线。
1.2.4 富集分析 将GLUD1基因导入STRING数据库,找到与其相关性最高的10个上下游基因,并进行GO富集分析和KEGG通路富集分析。
1.3 统计学处理 使用SPSS 17.0软件进行统计学分析。计量数据采用(x±s)表示,组间比较采用单因素方差分析,相关性分析采用Pearson相关性检验,生存时间的比较使用Breslow分析。以P<0.05为差异有统计学意义。
2 结果
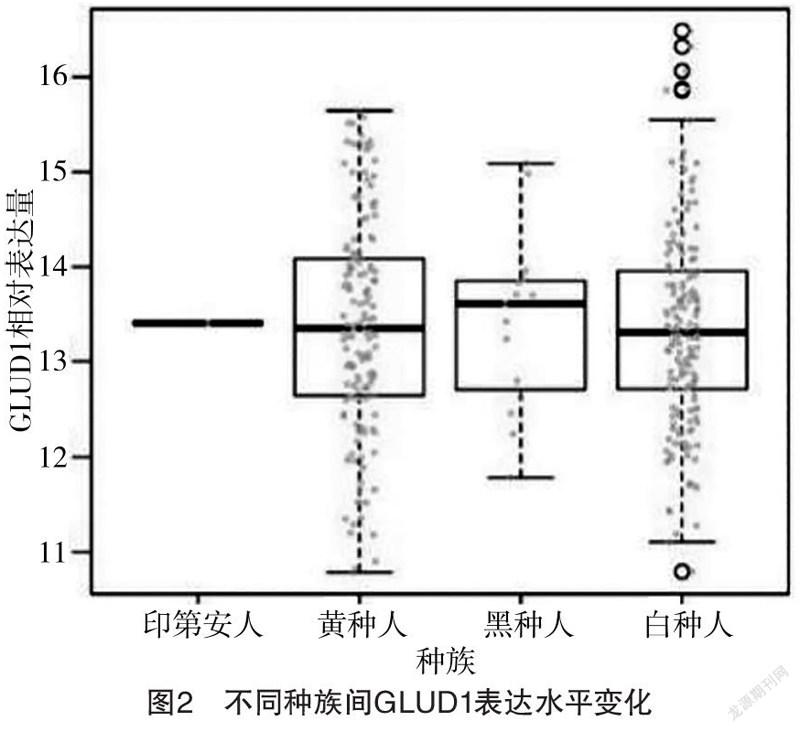
2.1 GLUD1表达水平与年龄的相关性及不同种族间GLUD1表达水平变化 GLUD1表达水平与年龄无相关性(r=-0.102,P=0.927),见图1;印第安人、黄种人、黑种人、白种人的GLUD1表达水平分别为13.41、(13.35±2.98)、(13.70±1.55)、(13.24±3.60),不同种族间GLUD1表达水平比较,差异无统计学意义(F=1.720,P>0.05),见图2。
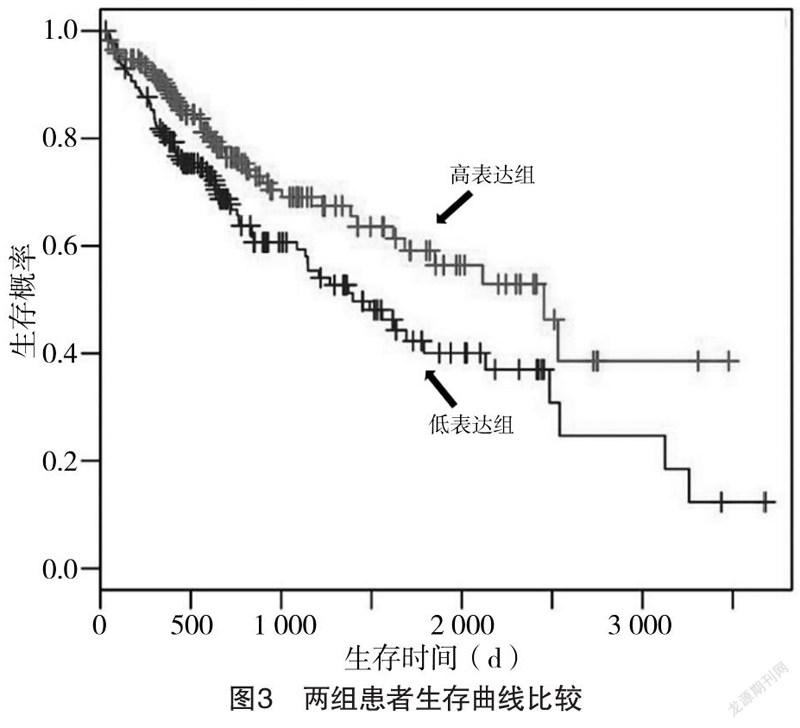
2.2 GLUD1表达水平对生存时间的影响 GLUD1高表达组患者的中位生存时间为1 014 d,长于GLUD1低表达组的872 d,差异有统计学意义(χ=43.64,P<0.001),见图3。
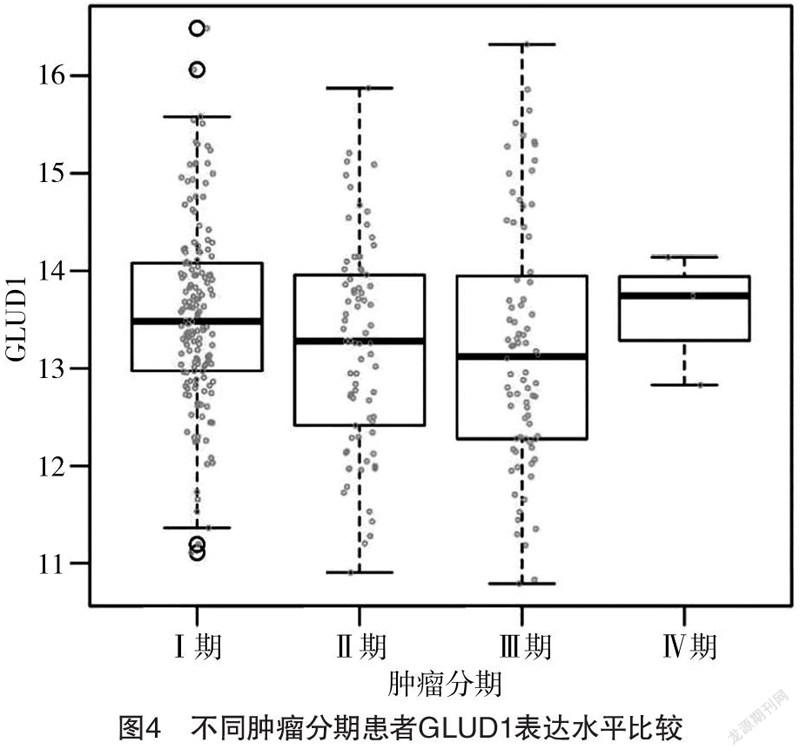
2.3 不同肿瘤分期患者GLUD1表达水平比较 Ⅰ、
Ⅱ、Ⅲ、Ⅳ期患者GLUD1表达水平分别为(13.51±0.38)、(13.29±0.54)、(13.16±0.79)、(13.65±0.51),四者比较差异有统计学意义(F=3.915,P=0.007);Ⅱ、Ⅲ期患者GLUD1表达水平均低于Ⅰ期,差异均有统计学意义(P<0.05),其余组间比较差异均无统计学意义(P>0.05)。见图4。
2.4 富集分析 与GLUD1最为密切相关的10个上下游基因分别是丙氨酸转氨酶2、天冬氨酸转氨酶1、异柠檬酸脱氢酶、天冬氨酸转氨酶2、谷氨酰胺合成酶、谷氨酸脱氢酶2、NAD依赖蛋白脂酰胺酶sirtuin-4、谷氨酰胺酶肾脏异构体、谷氨酰胺酶肝脏异构体、δ-1-吡咯啉-5-羧酸脱氢酶。其主要富集的生物学进程为二羧酸代谢过程,主要富集的分子功能为辅酶结合,主要富集的细胞组分为线粒体基质。主要富集的信号通路为丙氨酸、天冬氨酸和谷氨酸代谢以及精氨酸生物合成。
3 讨论
GLUD1是一种线粒体酶,主要存在于肝脏、肾脏及心肌线粒体中,在谷氨酰胺代谢中起关键作用。GLUD1高表达可促进谷氨酰胺的分解,降低细胞内谷氨酰胺的含量[12]。谷氨酰胺与炎症因子的分泌和自噬密切相关[13],抑制谷氨酰胺合成可使M2极化的巨噬细胞向M1样表型倾斜,增强巨噬细胞诱导T细胞募集的能力,抑制癌细胞运动能力[14]。已有研究表明GLUD1在急性髓系白血病、乳腺癌等肿瘤中表达异常[10],但GLUD1在肝细胞癌中的作用,特别是与患者远期生存率的相关性研究鲜有报道。本研究通过分析TCGA数据库中肝细胞癌患者的临床数据,探讨GLUD1对肝细胞癌患者预后的影响,从而为肝细胞癌的预后判断及临床治疗策略的制定提供一定参考。
本研究发现,Ⅰ期患者GLUD1表达水平高于Ⅱ、Ⅲ期患者(P<0.05),且GLUD1高表达组患者的中位生存时间长于GLUD1低表达组(P<0.05),表明在肝细胞癌中GLUD1高表达可提示患者预后相对良好。这可能由于在肿瘤早期,细胞对能量需求增加,谷氨酰胺作为能量代谢中的重要底物可出现代偿性表达升高,在正反馈的作用下GLUD1随之升高。但随着肿瘤进展,肿瘤细胞能量需求进一步升高,但线粒体功能逐渐退化,能量代谢失代偿,从而出现GLUD1表达水平降低。
为了进一步探讨GLUD1具体的作用机制,本研究检索了与GLUD1最为密切相关的10个上下游基因,及其富集水平。结果发现10个基因均为能量代谢中重要的酶或辅酶,其中丙氨酸转氨酶2催化丙氨酸和2-氧戊二酸之间的可逆转氨反应,生成丙酮酸和谷氨酸,属于Ⅰ类磷酸吡哆醛依赖的氨基转移酶家族[15]。天冬氨酸转氨酶参与了发育过程中肝脏葡萄糖的合成和脂肪细胞甘油的合成,以L-半胱氨酸为底物,调节硫化氢的重要来源—巯基丙酮酸的水平[16]。异柠檬酸脱氢酶能与丙酮酸脱氢酶复合物紧密結合或相互作用,属于异柠檬酸和异丙基苹果酸脱氢酶家族[17-18]。谷氨酰胺合成酶可催化谷氨酰胺和4-氨基丁酸的产生[19]。NAD依赖蛋白脂酰胺酶sirtuin-4可催化ADP-核糖基转移到靶蛋白上,抑制GLUD1酶活性[20]。δ-1-吡咯烷-5-羧酸脱氢酶可催化从脯氨酸或鸟氨酸衍生的δ-1-吡咯烷-5-羧酸到谷氨酸的不可逆转化,这是连接尿素和三羧酸循环的途径中必要的一步[21]。富集分析可见GLUD1相关上下游基因主要参与线粒体中的能量代谢通路。线粒体在调控细胞能量代谢的过程中发挥重要作用,既往研究可见由于肿瘤细胞快速增殖需要大量的蛋白质、脂类等生物大分子,其自身的能量代谢显著高于正常的细胞[22],在其肿瘤进展的过程中亦可观察到广泛的脂肪酸代谢、糖代谢和氨基酸代谢异常[23]。与此同时,能量代谢过程中产生的活性氧族和钙离子稳态异常同样可作用于肿瘤细胞,影响肿瘤细胞的侵袭和迁移[24]。这提示线粒体内的物质能量代谢通路是GLUD1影响肝细胞癌进展的潜在机制。
綜上所述,本研究通过数据挖掘,发现GLUD1高表达患者的肿瘤级别较低、生存期较长,这为肝细胞癌的早期预后判断与治疗提供了参考。
参考文献
[1] Bray F,Ferlay J,Soerjomataram I,et al.Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J].CA: A Cancer Journal for Clinicians,2018,68(6):394-424.
[2] Yilmaz S,Sahin T,Saglam K.What Are the Immune Obstacles to Liver Xenotransplantation Which Is Promising for Patients with Hepatocellular Carcinoma?[J].Journal of Gastrointestinal Cancer,2020,51(4).
[3] Chang C,Huang C H.Poor dietary intake improved by total excision of oral cavity metastases in a patient with hepatocellular carcinoma and elevated myeloid-derived suppressor cells[J].Hepato Biliary Surgery and Nutrition,2020,9(4):558-561.
[4] Feng R M,Zong Y N,Cao S M,et al.Current cancer situation in China: good or bad news from the 2018 Global Cancer Statistics?[J].Cancer Communications,2019,39(1):22.
[5]李洪东,朱晓姝,王建新.生物信息学研究进展[J].玉林师范学院学报,2018,39(5):2-6.
[6]王欣凯,王硕.探究生物信息学的研究进展[J].科技资讯,2020,18(14):234-235.
[7] Luczkowska K,Stekelenburg C,Sloan-Béna F,et al.
Hyperinsulinism associated with GLUD1 mutation: allosteric regulation and functional characterization of p.G446V glutamate dehydrogenase[J].Human Genomics,2020,14(1):9.
[8] Spanaki C,Kotzamani D,Plaitakis A.Widening Spectrum of Cellular and Subcellular Expression of Human GLUD1 and GLUD2 Glutamate Dehydrogenases Suggests Novel Functions[J].Neurochem Res,2017,42(1):92-107.
[9] Roy K,Satapathy A K,Houhton J A L,et al.Congenital Hyperinsulinemic Hypoglycemia and Hyperammonemia due to Pathogenic Variants in GLUD1[J].Indian J Pediatr,2019,86(11):1051-1053.
[10] Craze M L,El-Ansari R,Aleskandarany M A,et al.Glutamate dehydrogenase (GLUD1) expression in breast cancer[J].Breast Cancer Res Treat,2019,174(1):79-91.
[11]林承杰,袁观斗,何松青.大数据促进肝脏外科实验研究进展[J].中华实验外科杂志,2018,35(3):393-395.
[12] Brandt A,Agarwal N,Giri D,et al.Hyperinsulinism hyperammonaemia (HI/HA) syndrome due to GLUD1 mutation: phenotypic variations ranging from late presentation to spontaneous resolution[J].Journal of Pediatric Endocrinology and Metabolism,2020,33(5):675-679.
[13] Dong X,Zhai R,Liu Z,et al.The Effect of Intravenous Infusions of Glutamine on Duodenal Cell Autophagy and Apoptosis in Early-Weaned Calves[J].Animals (Basel),2019,9(7):404.
[14] Saladini S,Aventaggiato M,Barreca F,et al.Metformin Impairs Glutamine Metabolism and Autophagy in Tumour Cells[J].Cells,2019,8(1):49.
[15] Wang G,Lu X,Du Q,et al.Diagnostic value of the γ-glutamyltransferase and alanine transaminase ratio, alpha-fetoprotein, and protein induced by vitamin K absence or antagonist Ⅱ in hepatitis B virus-related hepatocellular carcinoma[J].Sci Rep,2020,10(1):13519.
[16]马雪松,高志强,唐哲,等.天冬氨酸转氨酶血小板比值联合吲哚氰绿试验对巴塞罗那B期肝细胞肝癌患者肝切术后肝衰竭的指导价值[J].中华实验外科杂志,2019,36(1):123-126.
[17] Tunthanathip T,Sangkhathat S.Temozolomide for patients with wild-type isocitrate dehydrogenase (IDH) 1 glioblastoma using propensity score matching[J].Clin Neurol Neurosurg,2020,191:105712.
[18] Xie H,Kong Y X,Zhang Q,et al.Value of Serum Tumor Marker Isocitrate Dehydrogenase 1 in the Diagnosis of Lung Cancer[J].Acta Academiae Medicinae Sinicae,2019,41(6):813-817.
[19] Tang D,Liu M Y,Zhang Q,et al.Isolation and characterization of chloroplastic glutamine synthetase gene (CsGS2) in tea plant Camellia sinensis[J].Plant Physiology and Biochemistry,2020,155:321-329.
[20] Wang C,Piao C,Liu J,et al.Mammalian SIRT4 is a tumor suppressor of clear cell renal cell carcinoma by inhibiting cancer proliferation, migration and invasion[J].Cancer Biomark,2020,29(4):1-10.
[21] An A,Yka B,Mh A,et al.ALDH4A1 expression levels are elevated in postmortem brains of patients with schizophrenia and are associated with genetic variants in enzymes related to proline metabolism-Science Direct[J].Journal of Psychiatric Research,2020,123:119-127.
[22] Zhao X,Huang Y,Yuan G,et al.A novel tumor and mitochondria dual-targeted photosensitizer showing ultra-efficient photodynamic anticancer activities[J].Chem Commun (Camb),2019,55(6):866-869.
[23] Zhang N,Tan Y,Yan L,et al.Modulation of Tumor Hypoxia by pH-Responsive Liposomes to Inhibit Mitochondrial Respiration for Enhancing Sonodynamic Therapy[J].Int J Nanomedicine,2020,15:5687-5700.
[24] Marciano R,David H B,Akabayov B,et al.The Amuvatinib Derivative, N-(2H-1,3-Benzodioxol-5-yl)-4-{thieno[3,2-d]pyrimidin-4-yl}piperazine-1-carboxamide, Inhibits Mitochondria and Kills Tumor Cells under Glucose Starvation[J].Int J Mol Sci,2020,21(3):1041.
(收稿日期:2020-09-16) (本文編辑:张爽)

