An ultra-robust fingerprinting method for quality assessment of traditional Chinese medicine using multiple reaction monitoring mass spectrometry
Zhenhao Li, Xiaohui Zhang, Jie Liao, Xiaohui Fan, Yiyu Cheng
Pharmaceutical Informatics Institute, College of Pharmaceutical Sciences, Zhejiang University, Hangzhou, 310058, China
Keywords:Multiple reaction monitoring Mass spectrometry-based fingerprinting Quality assessment Traditional Chinese medicine Robustness evaluation
ABSTRACT Chromatographic fingerprinting has been perceived as an essential tool for assessing quality and chemical equivalence of traditional Chinese medicine. However, this pattern-oriented approach still has some weak points in terms of chemical coverage and robustness. In this work, we proposed a multiple reaction monitoring(MRM)-based fingerprinting method in which approximately 100 constituents were simultaneously detected for quality assessment.The derivative MRM approach was employed to rapidly design MRM transitions independent of chemical standards, based on which the large-scale fingerprinting method was efficiently established.This approach was exemplified on QiShenYiQi Pill(QSYQ),a traditional Chinese medicine-derived drug product, and its robustness was systematically evaluated by four indices: clustering analysis by principal component analysis, similarity analysis by the congruence coefficient, the number of separated peaks, and the peak area proportion of separated peaks. Compared with conventional ultraviolet-based fingerprints, the MRM fingerprints provided not only better discriminatory capacity for the tested normal/abnormal QSYQ samples,but also higher robustness under different chromatographic conditions (i.e., flow rate, apparent pH, column temperature, and column).The result also showed for such large-scale fingerprints including a large number of peaks, the angle cosine measure after min-max normalization was more suitable for setting a decision criterion than the unnormalized algorithm. This proof-of-concept application gives evidence that combining MRM technique with proper similarity analysis metrices can provide a highly sensitive,robust and comprehensive analytical approach for quality assessment of traditional Chinese medicine.
1. Introduction
There has been a rising interest in traditional Chinese medicine(TCM)for not only primary health care,but also drug discovery and development.In contrast to synthetic or highly purified drugs,TCM generally contains a myriad of different phytochemicals, which may produce additive or synergistic effects on various biological targets [1,2]. Therefore, TCM holds significant promise for the treatment of chronic and multifactorial disorders such as diabetes and cardiovascular diseases [3,4]. Despite the great potential of TCM to the drug discovery community, scientific data for demonstrating their safety and efficacy are often lacking,as are evidencebased standards for quality and regulatory evaluation. Besides,these naturally-derived mixtures may exhibit considerable batchto-batch variability due to variations in geographical origins, agricultural practices, post-harvest processing and manufacturing processes [5]. Quality control therefore plays a critical role in assuring the safety, effectiveness and consistency of TCM.
Since its emergency, fingerprinting analysis has been increasingly perceived as a powerful tool for assessing quality and chemical equivalence of TCM. Numerous fingerprinting methods based on spectroscopy or chromatography have evolved [6,7], among which liquid chromatography is one of the most well-established methods for generating the fingerprints [8]. Compared with the determination of a few marker compounds which represent only a limited percentage of the whole herbal material, chromatographic fingerprints consider all detectable constituents to establish characteristic chemical profiles without necessarily requiring reference compounds, providing a more comprehensive summary of TCM quality. Till now, this pattern-oriented approach has been increasingly used for origin identification[9],authentication[10],process control [11] and quality assessment [12,13] of TCM and its derived drug products.
Although widely accepted as ideal approaches to assessing the quality of TCM, chromatographic fingerprints still have some limitations. For instance, a number of metrics (e.g., the congruence coefficient) have been applied to quantitatively compare the similarity between two fingerprints [7]. However, these parameters may fail to evaluate the actual chemical equivalence especially when there are large chromatographic peaks which predominantly determine the values of the parameters (e.g., the similarity index)or data vectors of fingerprints are similar in direction[10,14-16].To address such concerns, several methods have been proposed by modifying the method/algorithm of similarity analysis[10,17,18]or assigning appropriate weights to the peaks [15,19]. Another drawback of the chromatographic fingerprint involves the robustness,namely,the capacity of an analytical method to remain unaffected by small deliberate changes in method parameters[20].Generally,chromatographic performance is influenced by many factors including flow rate, apparent pH, column temperature and instrument. Therefore, a traditional medicine/herbal fingerprint can maintain sufficient robustness only under test conditions within small variations [21-23], which renders it difficult to reproduce test results and conduct interlaboratory comparisons. Algorithms for background/retention time drift correction [24,25], and peak alignment and extraction[26-29]may be helpful in improving the robustness.However,it is conceivable that these algorithms would possibly be incapacitated when the assay-related factors vary more considerably. Moreover, ultraviolet (UV) or diode array detector(DAD) based fingerprints, which represent the most commonly used detectors,suffer from relatively low sensitivity,resolution and coverage.
Featured with high sensitivity and selectivity, mass spectrometry (MS) has emerged as an essential tool in traditional medicine research[30,31].Among various MS scan modes,multiple reaction monitoring(MRM)is considered as a reliable method for detecting specific constituents of interest. The precursor-product filtering process of MRM provides high specificity and sensitivity,as well as the tolerance to variations of assay conditions (as unselected ions are all filtered).Moreover,the lack of chemical standards,which is a major obstacle in TCM research, particularly in MRM transition design, has been partially overcome by several modified MRM techniques such as pseudotargeted MRM[32],derivative MRM[33]and parallel reaction monitoring[34].This situation highlights the feasibility of MRM in developing selectivity, sensitive and robust fingerprints for TCM quality assessment. However, there are few studies reporting MRM-based fingerprints. Also, methodologies and similarity analysis algorithms for such large-scale fingerprints still lack.
Here, we proposed an MRM-based fingerprinting approach to quality assessment of TCM, in which, ideally, hundreds of constituents could be simultaneously monitored. The experimental workflow is outlined schematically in Fig.1.TCM samples were first subject to liquid chromatography-quadrupole time-of-flight mass spectrometry (LC-QTOF-MS) analysis for chemical identification.MRM transitions of the identified constituents were efficiently developed based on MS2of QTOF-MS without reference standards,which were then employed to generate the MRM fingerprints on liquid chromatography-triple quadrupole mass spectrometry (LCQqQ-MS). Robustness of the fingerprints was systematically investigated by four indices, i.e., clustering analysis by principal component analysis (PCA), similarity analysis by the congruence coefficient, the number of separated peaks (defined as peaks with resolution no less than 1.0 herein),and the peak area proportion of separated peaks. In addition, LC-UV fingerprints of the samples were generated and analyzed,and the results were compared with those of LC-MRM fingerprints.
QiShenYiQi Pill(QSYQ)is a TCM drug product widely prescribed for cardiac dysfunction, which is derived from four herbs: Astragalus membranaceus (Huangqi, HQ), Salvia miltiorrhiza (Danshen,DS), Panax notoginseng (Sanqi, SQ), and essential oil of Dalbergia odorifera(Jiangxiang,JX).As volatile components in JX essential oil can hardly be detected by conventional LC-UV [13,35], it is meaningful to develop an analytical approach capable of reflecting the quality of the four herbs in a single run.We therefore applied MRM fingerprinting to this complex preparation, and the result demonstrated the proposed method could detect different sorts of constituents in a sensitive and robust manner, thus making a more comprehensive assessment of the quality of the patent drug.
2. Experimental
2.1. Materials and reagents
HPLC-grade acetonitrile and formic acid were from Merk(Darmstadt, Germany) and Roe (Newark, USA), respectively. Ultrapure water was prepared by a Milli-Q Plus water purification system (Millipore, Billerica, USA). Other reagents used were all of analytical grade.Reference standards,including danshensu,caffeic acid, salvianolic acid B, salvianolic acid C, rosmarinic acid, ferulic acid, astragaloside IV, ononin, formononetin, calycosin, notoginsenoside R1, ginsenoside Rg1, Rb1, Rb3, Re and Rd were purchased from Winherb Medical Technology (Shanghai, China). Ten batches of QSYQ intermediates, consisting of 5 normal batches and 5 abnormal batches, were provided by Tasly (Tianjing, China), and sample information is shown in Table 1.The preparation process of QSYQ intermediate is detailed in Supplementary Material.
2.2. Sample preparation
Accurately weighted QSYQ intermediates(1 mg)were dissolved in 1 mL of 10%aqueous methanol(V/V).After vortexed for 30 s and centrifugation at 10,000 rpm for 10 min, the supernatants were subject to the fingerprinting analysis. Solutions of reference standards were individually prepared in methanol to corroborate chemical identification results.
2.3. Instrumentation
An Acquity UPLC system (Waters, Milford, MA, USA) coupled with a Triple TOF 5600plus MS (AB SCIEX, Framingham, MA, USA)was employed for chemical identification.Analysis was performed in negative electrospray ionization (ESI-) mode under the following parameters: scan range, m/z 100-1500; source voltage, -4.5 kV; source temperature, 550°C; curtain gas, 30 psi;gas 1(N2),50 psi;and gas 2(N2),50 psi.Declustering potential(DP),collision energy (CE) and collision energy spread (CES) of information dependent acquisition (IDA)-mediated MS2were 80 V,40 eV, and 20 eV, respectively.
An Agilent 1200 HPLC system (Agilent, Waldbronn, Germany)coupled with an API 4000 QqQ-MS (AB SCIEX, Framingham, MA,USA) was used for MRM fingerprinting. Parameters in the source were set as follows:source temperature,500°C;curtain gas,30 psi;gas 1, 40 psi; gas 2, 45 psi; and ionspray voltage, -4.0 kV. Fingerprinting was performed under MRM mode in which MS parameters for each analyte were derived from those of MS2of the QTOF-MS without routine optimizations via chemical standards. MRM transitions of 96 analytes are presented in Table S1.

Fig. 1. Schematic of multiple reaction monitoring (MRM)-based fingerprinting method for quality assessment of traditional Chinese medicine (TCM). LC-QTOF-MS: liquid chromatography-quadrupole time-of-flight mass spectrometry; UV: ultraviolet; LC-QqQ-MS: liquid chromatography-triple quadrupole mass spectrometry.
An Agilent 1100 HPLC system coupled with a variable wavelength detector(VWD)was used for LV-UV fingerprinting.The VWD wavelength was set at 203 nm.
The optimal chromatographic condition was set as follows:Zorbax SB C18column(1.8 μm,4.6 mm×100 mm,Agilent)at 30°C with mobile phase A (0.1% formic acid-water) and mobile phase B(acetonitrile); flow rate, 0.4 mL/min; linear gradient elution,0-5 min,10%-20%B;5-40 min,20%-55%B;40-50 min,55%-60%B; 50-60 min, 60%-90% B; 60-65 min, 90%-100% B; 65-70 min,100% B; injection volume,10 μL. In order to test the robustness of the LC-MRM and LC-UV fingerprinting methods, chromatographic conditions,including flow rate,apparent pH(i.e.,the concentration of formic acid in water), column temperature, and column, were deliberately changed with the one-variable-at-a-time (OVAT) procedure. The factor levels are shown below, and the resulting 15 conditions (C1 to C15) are detailed in Table S2: column temperature,20(C2),25(C3),30(C1,optimal),35(C4)and 40°C(C5);flow rate, 0.2 (C6), 0.3 (C7), 0.4 (C1, optimal), 0.5 (C8), and 0.6 mL/min(C9); concentration of formic acid in water, 0.03 (C10), 0.05 (C11),0.1 (C1, optimal), 0.2 (C12) and 0.3% (C13); column(Zorbax SB C18,Agilent), 1.8 μm, 4.6 mm × 100 mm (C1, optimal), 3.5 μm,2.1 mm × 150 mm (C14), and 5 μm, 4.6 mm × 250 mm (C15).
2.4. Robustness evaluation
In this work, robustness of the fingerprinting methods was evaluated in terms of clustering analysis, similarity analysis, the number of separated peaks, and the peak area proportion of separated peaks.PCA-based unsupervised exploratory analysis was used to visualize the fingerprint data and evaluate the cluster tendency of the normal and abnormal samples in different assay conditions. The congruence coefficient was employed to quantitatively characterize similarities of the fingerprints, in which both standard angle cosine measure[7] and angle cosine measure after min-max normalization were used to calculate the similarity index.The number of peaks represents the chemical information that can be captured by an analytical method,and the peak area proportion of separated peaks reflects how much of these information can be accurately obtained.Therefore,these two indices were also used to assess the fingerprint robustness under varied conditions.
2.5. Design of multiple reaction monitoring transition
The development of MRM transitions is generally labor-intensive,and the coverage is limited by the availability of chemical standards. Several efforts have been made to design MRM transitions for proteins/peptides [36,37], metabolites [32,38], and small molecules [39,40] negating the need for standards. Based on our previous studies [33,41], we employed herein the derivative MRM(DeMRM)method to rapidly transform parameters of MS2of QTOFMS to MRM parameters on QqQ-MS independent of standards. It consisted of four steps. First, nontargeted profiling and IDA-based MS2were performed for QSYQ on LC-QTOF-MS. Then, MS2information including the precursor-product ion pairs(usually the most intensive ions in MS1and MS2),and corresponding DP and CE were picked as initial parameters for MRM transitions. Next, moderate optimizations of DP and CE were performed on LC-QqQ-MS to achieve higher responses of several representative analytes,whereas parameters with minor impacts including entrance potential (EP) and collision cell exit potential (CXP) were fixed at-10 V and-12 V,respectively according to our previous studies[33,41].Finally,the MRM transition of each analyte was established based on the initial parameters and optimization results, and employed to generate the MRM fingerprints of QSYQ. For constituents employing adduct ions as the precursor ions(e.g.,compound 7 and 9), DP should be tailored to a relatively low level to circumvent declustering. Besides, scheduled MRM algorithm [42] was used during the whole procedure, in which analytes were monitored only around the expected retention time,thus enhancing the sensitivity. Among the 100 identified constituents, 96 of them exhibited good MS responses by applying the DeMRM method.Therefore, these constituents were selected for the MRM fingerprinting analysis, which reflected the majority of chemical information of QSYQ. MRM transitions of the analytes are detailed in Table S1.
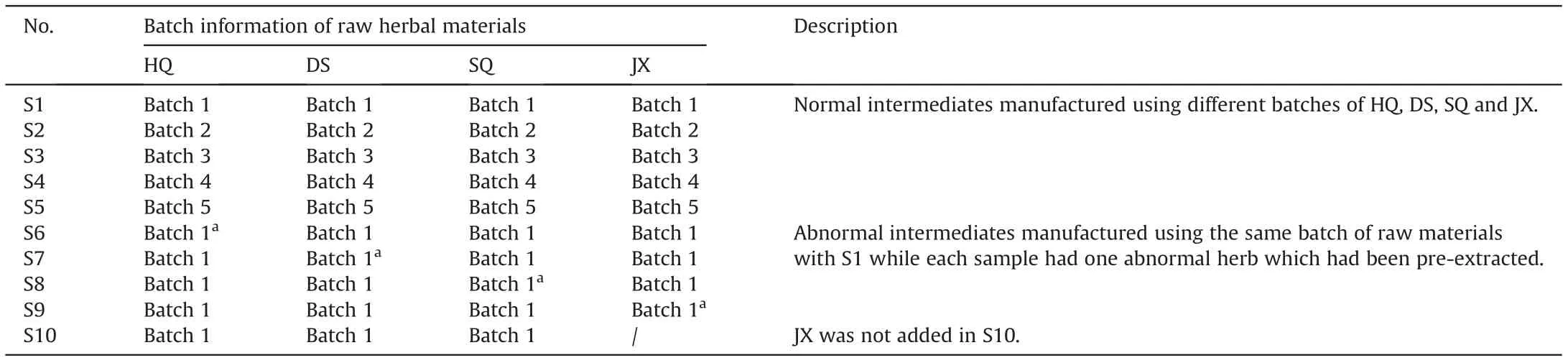
Table 1 Sample information of QiShenYiQi Pill.
2.6. Method validation
To ensure the proposed methodology was reliable for the intended use,both the LC-MRM and LC-UV fingerprinting methods were systematically validated under the optimal conditions in terms of intra- and inter-day precision, repeatability and stability.The selection of peaks for fingerprinting analysis was mainly based on two principles: (1) the peaks were common in all the test samples;(2)the resolution of the peaks should be no less than 1.0.Sample 1 was used to prepare the quality control(QC)samples for the method validation. Intra-day precision was estimated by analyzing one QC sample six times within one day,while inter-day precision was examined in duplicate per day over three consecutive days. Six replicates of the QC samples were prepared under the same condition and analyzed to measure repeatability.For stability assessment,the QC samples were stored at 4°C and then analyzed at 0, 2, 4, 8,12, and 24 h, respectively.
2.7. Data processing and statistical analysis
Raw data of QTOF-MS, MRM fingerprints and UV fingerprints were processed by PeakView 1.2.0.3 (AB SCIEX), Analyst 1.51 (AB SCIEX) and ChemStation B.04.02 (Agilent), respectively. Multivariate data were analyzed by SIMCA-P 14.1(Umetrics,Umeå,Sweden)for PCA-based clustering analysis using peak areas of the constituents.
3. Results and discussion
3.1. Characterization of chemical constituents in QSYQ
Representative chromatograms obtained by UPLC-QTOF-MS and HPLC-VWD are shown in Fig.S1.In order to gain more information from the chromatograms, multiple MS data processing methods were employed complementarily for chemical identification.Briefly,molecular formulae were generated according to the highresolution MS data, and putative identification of the peaks was assigned based on literature and database matching, then further confirmed via MS2fragmentations. Strategies such as preclassification [43], diagnostic ion filtering [44] and the targeted following nontargeted(TFNT)approach[41]were implemented to accelerate the dereplication process. Besides, on-line MS2spectra databases including the Global Natural Products Social Molecular Network (GNPS), MassBank, the human metabolome database(HMDB), and MassGraph were also used for direct compound identification. By applying these approaches, a total of 100 constituents were identified or tentatively characterized from QSYQ,including 45 saponins, 19 phenolic acids, 14 flavonoids, one anthraquinone, one phenanthraquinone, and 20 miscellaneous compounds,16 of which were unambiguously identified by comparison with reference standards in terms of retention time and mass spectra. Taking calycosin (15) from HQ as an example, the predominant quasi-molecular ion[M-H]-at m/z 283.0608 gave the formula C16H12O5.The fragment ion at m/z 268.0361 was attributed to a CH3loss from the methoxy group of the compound.Fragment ions at m/z 239.0331 and 211.0384 corresponded to successive neutral losses of one and two CO moieties after a CH4loss. Moreover,fragment ions at m/z 135.0085 and 91.0198 were characteristic ions formed by the retro-Diels-Alder (RDA) reaction of the C-ring([M-H-C9H8O2]-) and the subsequent loss of CO2, respectively. In addition, this peak showed identical retention time and similar mass spectra to those of the reference standard calycosin. Thus compound 15 was unambiguously assigned as calycosin. The proposed fragmentation pathways of calycosin are shown in Fig. S2.Characterization of 100 peaks and detailed MS information are listed in Table S3.
3.2. Validation of the proposed method
Validation results of the LC-MRM and LC-UV fingerprinting methods are summarized in Tables S4 and S5 respectively, which are expressed as relative standard deviation (RSD) of peak areas.For MRM fingerprinting,the variation ranges of intra-and inter-day precision, repeatability and stability were 0.70%-13%, 0.81%-15%,0.72%-14%,and 0.42%-13%,respectively.For UV fingerprinting,the variation ranges of intra-and inter-day precision,repeatability and stability were 0.091%-4.2%, 0.22%-5.6%, 0.21%-5.0%, and 0.21%-5.8%,respectively.Due to the nature of mass spectrometry,RSDs of MRM fingerprinting were bigger than those of UV fingerprinting,but still at an acceptable level.These results supported that the two fingerprinting methods were of reasonable reliability and applicable to quality assessment of QSYQ.
3.3. Similarity analysis and robustness evaluation
The validated fingerprinting methods were subsequently applied to assess the consistency of 10 batches of QSYQ intermediates. Four indices, i.e., clustering analysis, similarity analysis, the number of separated peaks (NS), and the peak area proportion of separated peaks (PS) were employed to characterize differences and similarities between the normal and abnormal batches, and meanwhile evaluate the robustness under different test conditions.
3.3.1. Clustering analysis by PCA
PCA is a variable reduction approach which allows visualization of multidimensional data.This unsupervised exploratory technique was used here to see whether the normal/abnormal batches of QSYQ could be precisely distinguished by the two fingerprinting methods,and whether the clustering result would be influenced by the varied chromatographic conditions. The peak area was used herein for the clustering analysis.
As shown in Fig. 2A, the MRM fingerprinting showed excellent discriminatory capacity under the optimal condition (C1). Normal samples S1 to S5,which were produced by different batches of raw herbal materials,could be clustered in one group in the PCA scores plot. Abnormal samples S6 to S10, each with an abnormal herbal material(while the other materials were identical to S1),were well differentiated from S1 or other normal samples. This high discriminatory capacity was attributed to wide coverage of the MRM fingerprint, in which a large number of constituents from each compositional herb could be simultaneously detected,including those in JX essential oil. In contrast, the discriminatory capacity of UV fingerprinting was much inferior. As depicted in Fig. 2B, only S6 (with abnormal HQ) and S7 (with abnormal DS)could be differentiated from S1, while S8 (with abnormal SQ), S9(with abnormal JX) and S10 (without JX) were grouped with S1.This is mainly because saponins in SQ only have weak end absorption at 203 nm in UV,while volatile components in JX essential oil can hardly be detected by the LC-UV method due to their low polarity and poor ultraviolet absorption [13].
The clustering result was further investigated under varied assay conditions (C2 to C15). As shown in Figs. S3-S6, changes in column temperature, flow rate, concentration of formic acid, and column, had relatively minor impacts on the clustering result of both MRM and UV fingerprinting, which indicated that the clustering result was not sensitive to the assay conditions. In some certain conditions,S9 and S10 could not be well differentiated from normal samples by MRM fingerprinting,as only 9 constituents in JX essential oil were detected and their contributions to the clustering were relatively minor. The PCA scores plot only reflected the (dis)similarity of samples on the principal components, while not providing information regarding the quality of the chromatographic method.Therefore,more indices were necessary to make a more comprehensive evaluation of the robustness and adaptability of the fingerprinting methods.
3.3.2. Similarity analysis by the congruence coefficient
The congruence coefficient is widely adopted to quantitatively characterize the similarity between two fingerprints by calculating the cosine of the angle.Generally,an average or median fingerprint of a set of samples[16],or the fingerprint of a qualified sample[19],serves as the reference fingerprint to compare with.In this work,the fingerprint of S1 was employed as the reference since all the abnormal samples were derived from this sample.We first used the standard angle cosine measure (Supplementary Material) to calculate the similarity between the reference and the samples. As depicted in the upper two panels of Fig. 3, for both MRM and UV fingerprinting, all the tested samples including the abnormal ones,showed high similarities to the reference (with similarity index above 0.95) under all the tested conditions, indicating that this algorithm was not suitable for differentiating the normal and abnormal samples in the test condition. One reason was that, as mentioned above, peaks accounting for a large proportion of the fingerprint predominantly determined the value of the similarity index.In each abnormal sample,only a small part(ranging from 10%to 31%)of the peaks were influenced in a moderate degree;thus the impacts on similarity were not obvious.Another reason was that,as 96 and 25 peaks were detected in MRM and UV fingerprints respectively, the contribution of each peak to the similarity was therefore reduced. To address this problem, we employed the minmax normalization to normalize the areas of individual peaks between 0 and 1, so that each peak would contribute approximately proportionately to the similarity.As depicted in the lower two panels of Fig.3,after normalization,abnormal samples including S6,S7,S8 and S10 could be obviously differentiated from the normal samples by MRM fingerprinting. Notably, the similarity indices between the reference and S6, S7 or S8 were all below 0.85, indicating that the modified congruence coefficient could be used for similarity analysis of the large-scale fingerprints. It was noteworthy that, though decreased, the abnormal sample S9 still showed relatively high similarity to the reference after normalization, mainly due to the limited number of constituents detected in JX essential oil.This may be further improved by including more constituents from JX, or employing statistical indices of multivariate modeling, such as Hotelling T2and DModX [19,45]. Moreover, the similarity indices under different conditions showed good consistency, which suggested good robustness of the MRM fingerprinting method. In contrast, the UV fingerprinting after normalization could only identify S6 and S7 as abnormal samples,while failing to differentiate S8, S9 and S10 from S1. In addition, the similarity index of UV fingerprinting varied more considerably under different assay conditions, especially that of S6 and S7, indicating the inferior robustness of UV fingerprints compared with that of MRM fingerprints.
3.3.3. Robustness evaluation by the number and peak area proportion of separated peaks
NS reflects the resolution of a chromatographic method and is a direct metric for robustness evaluation.Similarly,PS represents the information that can be reliably acquired by a fingerprint. These two indices were thus used to assess the robustness of the MRM and UV fingerprinting methods.Peaks with resolution no less than 1.0 were counted in Analyst or ChemStation,and their proportions were added to obtain the peak area proportion of separated peaks.Under the optimal method(C1),96 and 25 peaks were detected in MRM and UV fingerprinting respectively, which accounted for 80.7% and 87.5% of their respective fingerprints. This manifested that the basic method had been properly optimized and was suitable for fingerprinting analysis of QSYQ. As shown in Fig. 4, the variations of assay conditions had minor impacts on NS and PS of MRM fingerprinting, which ranged from 87 (C15) to 96 (C1), and 75.1% (C15) to 80.7% (C1), respectively. In comparison, PS of UV fingerprinting was significantly influenced by the changed conditions with a wide range from 46.9% (C14) to 87.5% (C1). The NS of UV fingerprinting was, on the surface, not significantly changed.However,many minor peaks were overlapped or merged by larger peaks in the UV fingerprint when the conditions varied, as indicated by the peak purity. In MRM mode, peak overlap and merger can be largely avoided as analytes are monitored in separate channels by the paired mass filters. These results highlighted the superiority of MRM-based fingerprinting method in terms of robustness, sensitivity and coverage.

Fig.3. Similarity indices between the reference(S1)and other QSYQ intermediates(S2-S10)calculated by the standard angle cosine measure(upper two panels)and the modified angle cosine measure (lower two panels). Fingerprint data were acquired by MRM fingerprinting (left two panels) or UV fingerprinting (right two panels).

Fig. 4. The number of separated peaks (solid lines) and the peak area proportion of separated peaks (dotted lines) of MRM fingerprinting (blue lines) and UV fingerprinting (red lines) under different chromatographic conditions (C1-C15).
4. Conclusion
This work demonstrated the feasibility of MRM-based fingerprints for quality and consistency assessment of traditional Chinese medicine.Through the derivative MRM approach,MRM transitions of herbal constituents could be rapidly constructed independent of chemical standards.The proposed method was successfully used to assess the quality and consistency of different batches of QSYQ intermediates, and capable of differentiating normal/abnormal samples in a reliable and accurate manner.Moreover,robustness of the MRM fingerprints was systematically investigated in terms of clustering analysis, similarity analysis, NS, and PS. The results supported that the MRM-based fingerprint possessed much better coverage and robustness than the conventional UV fingerprint,and was applicable to quality assessment of complex traditional medicine. However, it is important to mention that two different MS platforms are necessary in the workflow.Therefore,there is a need for integrated MS techniques such as parallel reaction monitoring(PRM) that allow simultaneous qualitative and quantitative analyses on one MS platform, which can reduce instrument cost and variations between instruments. Moreover, although angle cosine measure after min-max normalization was proposed herein for similarity analysis of MRM fingerprints, more appropriate algorithms or indices need to be developed for such large-scale fingerprints in which hundreds of constituents are detected.
Declaration of competing interest
The authors declare that there are no conflicts of interest.Acknowledgments
This work is financially supported by the National Natural Science Foundation of China (Grant No. 81803714) and the Fundamental Research Funds for the Central Universities (Grant No.2019QNA7041). We would like to thank Dr. Yi Wang and Dr.Xiaoping Zhao for their helpful comments and discussions.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpha.2020.01.003.
 Journal of Pharmaceutical Analysis2021年1期
Journal of Pharmaceutical Analysis2021年1期
- Journal of Pharmaceutical Analysis的其它文章
- Comprehensive metabolic profiling of Alismatis Rhizoma triterpenes in rats based on characteristic ions and a triterpene database
- Evaluation of apoptotic effects of mPEG-b-PLGA coated iron oxide nanoparticles as a eupatorin carrier on DU-145 and LNCaP human prostate cancer cell lines
- Development of an analytical method for multi-residue quantification of 18 anthelmintics in various animal-based food products using liquid chromatography-tandem mass spectrometry
- Drug target discovery by magnetic nanoparticles coupled mass spectrometry
- Analytical methodologies for sensing catechol-O-methyltransferase activity and their applications
- Applications and challenges of low temperature plasma in pharmaceutical field
