Analytical methodologies for sensing catechol-O-methyltransferase activity and their applications
Fng-Yun Wng , Ping Wng , Dong-Fng Zho , Frnk J. Gonzlez , Yu-Fn Fn ,Yng-Liu Xi , Gung-Bo Ge ,*, Ling Yng ,*
a Institute of Interdisciplinary Integrative Medicine Research, Shanghai University of Traditional Chinese Medicine, Shanghai, 201203, China
b School of Life Science and Medicine, Dalian University of Technology, Panjin,124221, China
c Laboratory of Metabolism, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA
Keywords:Catechol-O-Methyltransferase (COMT)Enzymatic activity Probe substrate COMT inhibitor High-throughput screening
ABSTRACT Mammalian catechol-O-methyltransferases (COMT) are an important class of conjugative enzymes,which play a key role in the metabolism and inactivation of catechol neurotransmitters, catechol estrogens and a wide range of endobiotics and xenobiotics that bear the catechol group. Currently, COMT inhibitors are used in combination with levodopa for the treatment of Parkinson’s disease in clinical practice.The crucial role of COMT in human health has raised great interest in the development of more practical assays for highly selective and sensitive detection of COMT activity in real samples,as well as for rapid screening and characterization of COMT inhibitors as drug candidates. This review summarizes recent advances in analytical methodologies for sensing COMT activity and their applications. Several lists of biochemical assays for measuring COMT activity,including the probe substrates,along with their analytical conditions and kinetic parameters, are presented. Finally, the challenges and future perspectives in the field, such as visualization of COMT activity in vivo and in situ, are highlighted. Collectively,this review article overviews the practical assays for measuring COMT activities in complex biological samples, which will strongly facilitate the investigations on the relevance of COMT to human diseases and promote the discovery of COMT inhibitors via high-throughput screening.
1. Introduction
The mammalian catechol-O-methyltransferases (COMT, EC 2.1.1.6)are an important class of conjugative enzymes that catalyze O-methylation of catechol substrates,transferring the methyl of Sadenosyl-L-methionine(SAM)to one of hydroxyl groups of catechol substrates in the presence of Mg2+(Fig.1) [1-3]. COMT is widely distributed in both brain and peripheral tissues, as indicated by analysis of both protein and mRNA levels (Fig. 2). It is found that one single gene encodes two forms of COMT proteins, a soluble form (S-COMT) and a membrane-bound form (MB-COMT) [4,5].The COMT gene locates in the chromosome 22, band q11.2 and contains six exons [2]. As shown in Fig. 3, its expression can be controlled by two separate promoters P1 and P2,which encode the S-COMT and MB-COMT proteins, respectively. The MB-COMT is a type II transmembrane protein with the C terminus outside the membrane, which is different from the S-COMT in an extra 50 hydrophobic amino acids that serve as the transmembrane domain.The MB-COMT predominates in brain tissues, including hippocampus, cerebral cortical areas and hypothalamus [6,7], and MBCOMT is postulated to modulate the inactivation of dopaminergic and noradrenergic neurotransmitters such as dopamine (DA),epinephrine (E) and norepinephrine (NE)[8]. By contrast, S-COMT is distributed throughout the peripheral tissues and organs,including liver, kidney, heart, lung, intestinal tract, reproductive organs,gland,muscle,adipose tissue,skin and red blood cells.The highest S-COMT activities are found in the liver and kidney[3,9].SCOMT participates in the inactivation and detoxification of biologically-active or toxic catechol substances and their hydroxylated metabolites, such as catechol estrogens, including 2-hydroxyestradiol (2-OHE2), 2-hydroxtestrone (2-OHE1), 4-hydroxyestradiol (4-OHE2) and 4-hydroxyestrone (4-OHE1) [10].
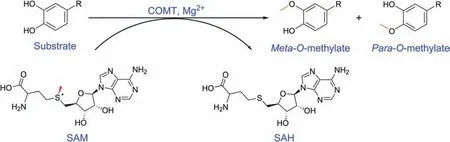
Fig.1. COMT-catalyzed methylation of catechol substrate to form Meta/Para-O-methylated products.

Fig. 2. The tissue distribution of COMT protein and mRNA in human body.

Fig.3. Structure of human COMT gene.The grey and orange boxes represent exons,and the blue lines between the boxes represent introns.The hatched boxes indicate the protein coding regions.The positions of the initiation codons for the transcription of S-COMT and MB-COMT mRNAs are indicated as S-ATG and MB-ATG.The two known promoters are P1(red bar) and P2 (green bar).
Recent investigations have revealed that the expression and function of COMT are influenced by various factors such as genetic polymorphism, gender, age and diet. Notably, human COMT is highly polymorphic and more than one hundred COMT alleles are thought to regulate its expression and catalytic activity [11-13].The most common COMT polymorphisms are Val158Met of MBCOMT and Val108Met of S-COMT, which can be used to predict the enzyme activities[14,15].The activity of COMT-Val homozygotes is~40% higher than that of COMT-Met homozygotes due to more hydrophobicity of Val residue than Met [16,17]. COMT activity is also related to gender.Commonly,COMT activity in males is much higher than that in females, which may be attributed to the negative regulation of estrogen on COMT activity [18]. In view of the significant individual differences in COMT activity,it is significant to accurately measure COMT activities in complex biological systems,which will strongly facilitate the diagnosis and treatment of COMTrelated diseases.
Over two decades,increasing studies have indicated that COMT activity may be associated with a variety of human diseases, from cancers (such as breast cancer and pancreatic cancer) to mental disorders (such as Parkinson’s disease, pain, depression and schizophrenia) [19-21]. Human COMT has been highlighted as a target for the treatment of central nervous system disorders, such as pain and Parkinson’s disease[22].The symptoms of Parkinson’s disease are characterized by the degeneration of dopaminergic neurons and the accumulation of plaque-like amyloid in cerebral cortex[23-25].Currently,dopamine replacement therapy has been widely used to treat Parkinson’s disease by co-administration of levodopa, dopa-decarboxylase inhibitor and COMT inhibitor in clinical settings [26]. Numerous efforts are made to develop practical assays for sensing COMT activities in real samples, and for potential applications in disease diagnosis and drug discovery.Several COMT inhibitors (such as tolcapone, entacapone and opicapone) in the market are used for the treatment of Parkinson’s disease [27]. However, tolcapone, which acts both centrally and peripherally, can induce severe liver toxicity; entacapone, which acts mainly in the brain,can increase the risk of dyskinesia[28-30].Therefore, it is desirable to find efficacious and safe COMT inhibitors by using practical assays for rapid screening and characterization of COMT inhibitors.
Until now, a panel of methodologies including the antibodybased assay, the mass spectrometry-based proteomic technique and the substrate-based biochemical assay, have been developed for detecting the levels or activities of COMT in real samples[31-34]. Among all reported methodologies, substrate-based biochemical assays have been widely used in drug discovery and clinical-related studies, as these assays are capable of selectively and directly measuring the real activities of COMT in complex biological samples with good sensitivity and specificity. Many catechol substances with various skeletons including the physiological relevant substrates and synthetic optical substrates, are validated as probe substrates for sensing the COMT enzymatic activities in biological samples. This review summarizes the recent progress in the development of probe substrates for mammalian COMT and the corresponding analytical methodologies for sensing COMT activities, as well as their biomedical applications. Several lists of biochemical assays, including the probe substrates, along with their structural information,analytical conditions and kinetic parameters, are summarized comprehensively. Finally, the challenges and future perspectives in the field,such as in vivo or in situ visualization of COMT activities, are highlighted. The information and knowledge presented here can provide practical tools or methods for measuring COMT activities in complex biological samples, which is very helpful for the medicinal chemists to find more efficacious COMT inhibitors or activators via high-throughput screening techniques and for the pharmacologists to explore the physiological functions of COMT and the relevance of COMT to human diseases.
2. Structure and characteristics of mammalian COMT
2.1. Structural and catalytic features of mammalian COMT
The X-ray crystal structure of human COMT complexed with 3,5-dinitrocatechol(3,5-DNC)was first reported in 1994[35].Currently,more than 20 crystal structures of human and rat COMT complexed with a variety of ligands have also been presented, such as rat SCOMTcomplexed with a tight-binding nitrocatechol inhibitor and a bisubstrate inhibitor[36].The crystal structures of the mammalian COMT are well resolved at the atomic level, which provides deep insights into its catalytic mechanism. Investigations on the interactions between substrates and the targeted enzyme(s) are of importance for deeply understanding of the catalytic reaction mechanisms[37].As shown in Fig.4,human S-COMT has a typical α/β-folded structure containing seven β-sheets and eight α-helices.The two sets of α-helices (helices α1-α5 on one side and helices α6-α8 on the other side)are sandwiched together with the sevenstranded β-sheets [36]. Catechol-binding site is located in the shallow pocket of COMT, while the SAM-binding site consists of amino acid residues in the interior of COMT (Fig. 5A) [38]. The methionine portion of SAM forms several hydrogen bonds with residues Val42, Ser72 and Asp141, the ribose portion forms hydrogen bonds with Glu90,and the adenine ring forms hydrogen bonds with Ser119 and Gln120. Notably, Mg2+also acts in the process of substrate binding. It coordinates to the amino acid residues (Asp141,Asp169 and Asn170)of COMT, the two hydroxyls of catechol substrate and a water molecule, to form an octahedral coordination complex ultimately [39]. As shown in Fig. 5B, the meta-hydroxyl is surrounded by three positively charged groups:Mg2+,the amino group of Lys144 and the methyl group of SAM.The complex can release a proton to form the phenolate ion. The phenolate ion directly attacks the methyl group of SAM via an SN2nucleophilic substitution, which produces the meta-O-methylated product with breaking of C-S bond and forming of C-O bond[31].More recently, a mass spectrometry-based lysine reactivity profiling strategy reveals that the Lys144 of COMT is located at the catechol-ligand binding region and plays a pivotal role in the Omethylation reaction [32]. In addition, the initial rate and product inhibition studies suggest that the catalytic mechanism of mammalian COMT is an ordered Bi-Bi mechanism.SAM is the first and the catechol substrate is the second to bind on COMT,forming a ternary complex followed by the release of methylated product and SAH [33].
2.2. Substrate preference of mammalian COMT
The substrates of COMT are characterized by a catechol skeleton with other substituents on the aromatic nucleus. A series of endogenous substrates for mammalian COMT, including DA, E, NE and their hydroxylated metabolites, 3,4-dihydroxybenzoic acid(DHB), 3,4-dihydroxyphenylacetic acid (DHPA), 3,4-dihydroxyphenylethanol (DHPE), and 3,4-dihydroxyphenylglycol(DHPG), have been reported [40,41]. Similar to the catechol compounds, the catechol estrogens and the ascorbic acid with enediol group can also be O-methylated by COMT [34]. Exogenous substrates with the catechol group, including triphenols such as pyrogallol and gallic acid, and substituted catechols such as 3,4-dihydroxypropiophenone (DHPP), are also the substrates for mammalian COMT [40,42]. Several dietary, medicinal and natural products, such as dobutamine, α-methyldopa, benserazide, carbidopa, caffeic acid, green tea catechins, daphnetin, and flavonoids,and derivatives of hydroxyquinolines containing catechol groups,are also exogenous COMT substrates(Fig. 6) [43-46].
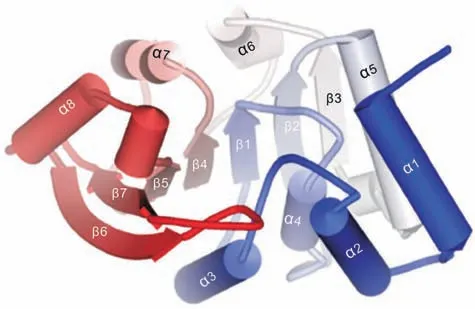
Fig. 4. The tertiary structure of the human S-COMT(PDB code: 3BWM), depicting the overall fold of a COMT structure.

Fig.5. (A)The crystal structure of rat COMT in complex with S-adenosyl-methionine(SAM),dinitrocatechol(DNC)and Mg2+,showing an octahedral complex.(B)Para-and metabound orientations of substituted catechol substrate in the active site of COMT and the interactions of Mg2+,H2O,amino acid residues and the catechol substrate.The black dashed lines indicate chelation.
Commonly, mammalian COMT catalyzes the catechol substrate to form two isomers,meta-O-and para-O-methylated products.The ratio of meta-O-to para-O-methylated metabolite is much different among substrates, dependent on the properties of the ring substituents [47]. In most cases, the catechol substrates show clear preference for meta-O-methylation, especially for the substrate with highly polar substituents [48,49]. However, for the substrate with a nonpolar substituent, such as 4-ethylcatechol, the amounts of meta-O- and para-O-methylates are found to be close to unity[41].The presence of a nonpolar region in the catechol-binding site of mammalian COMT can repel the binding of polar substrates in the orientation necessary for para-O-methylation, while nonpolar substrates can bind in a more random mode, resulting in the formation of nearly equal amounts of meta-O-and para-O-methylated products [1]. Both pyrogallol and gallic acid have three adjacent hydroxyl groups, while the intermediate hydroxyl group is more amenable to O-methylated by COMT[1].For the polycyclic catechol substrates,the preference of COMT is examined by using molecular docking simulations [50,51]. The molecular docking simulation suggested that human COMT predominantly catalyzes the formation of 8-O-methylated metabolite, for the reason that the 8-hydroxyl group is closer to the methyl of SAM than that of 7-hydroxyl(Fig. 7) [50].
3. Analytical methodologies for sensing COMT activity
Over the past decades, a number of analytical methodologies have been developed for measuring COMT activities in real samples, including colorimetry, liquid chromatography with UV or photodiode array (LC-PDA) detection, liquid chromatographyelectrochemical (LC-EC) detection, liquid chromatographyfluorescence (LC-FD) detection, liquid chromatographyradiochemical (LC-RC) detection, liquid chromatography-tandem mass spectrometry (LC-MS), gas chromatography-mass spectrometry (GC-MS), and the microplate reader based fluorescence detection. The details of these assays including the probe substrates, along with their analytical conditions and kinetic parameters,are provided in Table 1 [47, 52-71].
3.1. Colorimetry based assays
Colorimetry detection is based on the color change of reaction mixture due to the difference between the methylated product and the parent substrate in the absorption spectra. Without the extraction or other isolation technique, the colorimetry-based assay is able to measure the O-methylation products in the incubation mixture. Some catechol substrates, such as E, nitrocatechol(NC) and 3,4-dihydroxyacetophenone (DHAP), are usually employed in such assays. For example, the substrate epinephrine can react with hydroxylamine and ferric chloride, producing a colored-complex [52]. Quantitation can be performed by the color disappearance of the complex upon the addition of COMT enzyme.The O-methylation of NC can also be determined by the color disappearance [53]. The assay is based on the observation that NC can be methylated by COMTand the formed products do not exhibit the cherry-red color which is attributed to the ionization of two adjacent hydroxyls in strong alkali. Using DHAP as the substrate,the O-methylation products can be differentiated from the parent compound due to the red shift of their maximum absorptions from 265 nm to 344 nm [54]. Commonly, colorimetry-based assay is easily conducted and cost-effective, but drawbacks of poor selectivity and sensitivity limit its applications, such as the inability to discriminate meta/para-O-methylated products. Therefore, this method is not suitable for the determination of COMT activities in complex biological samples.
3.2. Liquid chromatography-UV based assays
Liquid chromatography (LC) is an essential tool for comprehensive analysis of complex samples due to its high separation capability. Compared to the colorimetry-based assay, LC coupled with UV or PDA detector allows the individual determination of meta-O- and para-O-methylated isomers. For example, an assay of COMT has been developed to determine the meta- and para-Omethylated products of 3,4-dihydroxybenzoic acid(DHBAc)with a detection limit of 1.8 pmol [55]. This method makes it possible to obtain the meta/para ratio of the O-methylation products.It has also been used to evaluate the inhibition potency of bifunctional polyhydroxy benzamides on COMT from porcine liver with 3,4,5-trihydroxybenzoate (THBAc) as a substrate [56].
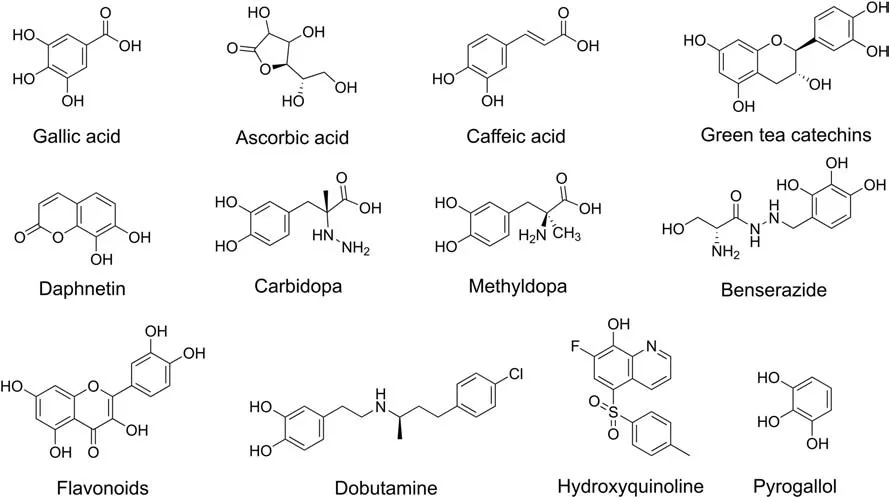
Fig. 6. Chemical structures of exogenous substrates for COMT.
3.3. Liquid chromatography-electrochemical detection (LC-EC)based assays
LC-EC is an extremely selective and sensitive detection technique with an enormous linear dynamic range.Catechol substrates themselves and their corresponding mono-methylated metabolites, which contain the phenolic hydroxyls, can be oxidized to respond the electronical detector. This method has proved the advantages such as high sample throughput, high chromatogram resolution and low blank values. A wide range of catechol substrates, such as DA [57], 3,4-dihydroxybenzylamine (DHBAm) [58]and dDHBAc[59],have been used for sensing COMT activities with electrochemical method.With DA as the substrate,the selectivity of electrochemical method allows the individual determination of the two product isomers and improves the precision to less than 4%RSD with the addition of an internal standard, 3-methoxy-4-hydroxybenzylamine [57]. In addition, COMT activities can be measured in various tissues with a detection limit of 1 pmol by using DHBAm as the substrate[58].The method is also suitable for screening COMT inhibitors and for quantifying the meta- to paramethylated products.An enhanced electrochemical electrode,such as three electrodes in series, has been developed to quantitate COMT activity in striatal homogenates with a detection limit of~0.5 pmol[59].In addition,this electronical method can be applied to assess the inhibitory effects of nitrocatechol compounds on COMT[9].
3.4. Liquid chromatography-fluorescence detection (LC-FD) based assays
The O-methylated products by COMT can be analyzed with the fluorescence detector if they exhibit fluorescence emission or they can be modified with the fluorogenic reagents.Generally,the LC-FD based assay is quite sensitive with the lower detection limit of the target fluorescent metabolite around 0.1 pmol[60].With NE or DA as a natural substrate,S-COMT and MB-COMT activities in different brain areas and erythrocytes can be readily evaluated by the native fluorescence of methylation products [61,62]. With 3,4-dihydroxybenzaldehyde (DHBAd) as the substrate, the O-methylated products can be detected after derivatization with 2,2′-dithiobis (1-aminonaphthalene) (DTAN) [63]. For the determination of low COMT activity in erythrocytes or brain tissues, it is urgent to develop fluorogenic substrates with high sensitivity. The first synthesized fluorescent substrate for COMT, 2-(3,4-dihydroxyphenyl)naptho-[1,2-d]-thiazole (DNT) has been successfully applied to assess the inhibition effect of tolcapone on COMT in erythrocytes [64]. The second synthesized fluorescent substrate,5,6-dihydroxyindole-2-carboxylic acid (5,6-DHI2C), can also be used to monitor COMT activities in melanocytes [65].
3.5. Liquid chromatography-radiochemical (LC-RC) detection methods
It is well-known that the sensitivity of LC-RC based method is always higher than that of other analytical assays,including LC-UV,LC-EC and LC-MS/MS. For COMT activity assay, the radioactiveisotopes can be labeled in either the catechol substrate or SAM.For example, with14C-labeled dopamine as the substrate, COMT activity can be measured with a low detection limit of 0.04 pmol[66]. The 2-hydroxy [3H]estrone is also employed as the substrate to detect COMT activity in human erythrocyte[67].An advantage in using labeled SAM is that it is easier to separate from the Omethylated product than the parent compound, due to higher hydrophobicity difference between the two molecules.For instance,a direct extraction radiochemical assay is developed for COMT activity by using [3H] methyl labeled SAM and 3,4-dihydroxyphenylacetic acid (DHPAc) as the donor and acceptor of methyl group, respectively [68]. DHPAc is converted to radioactively-labeled homovanillic acid in the presence of [3H]methyl labeled SAM. This product is extracted into an organic solvent-scintillant mixture, followed by the scintillation counting.In addition, the steric course of the SAM methyl transfer is also investigated by using SAM labeled with [1H], [2H] and [3H] [31].
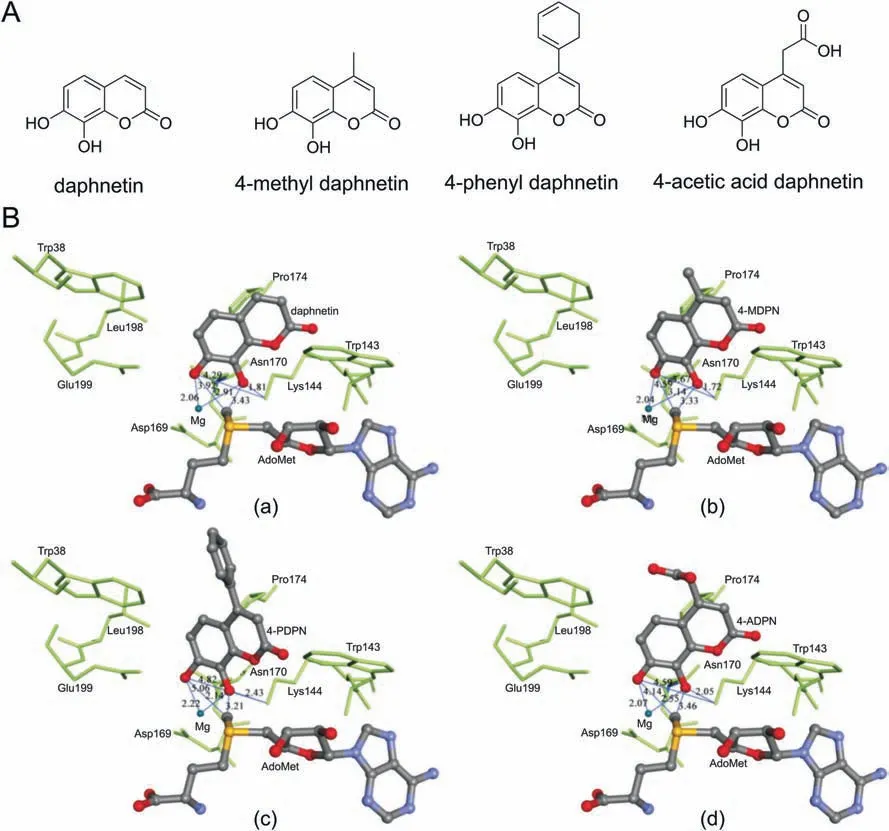
Fig. 7. (A) Chemical structures of daphnetin, 4-methyl daphnetin (4-MDPN), 4-phenyl daphnetin (4-PDPN), and 4-acetic acid daphnetin (4-ADPN). (B) Docking simulations of (a)daphnetin, (b) 4-MDPN, (c) 4-PDPN, and (d) 4-ADPN into human S-COMT.
3.6. Liquid chromatography-mass spectrometry (LC-MS) based assays
LC-MS is routinely used for detection of the activity of target enzyme(s),via monitoring the formation rates of the metabolite(s).Over the past two decades, many LC-MS/MS based methodologies for sensing COMTactivities in real samples have been reported with various physiological substances or drugs bearing catechol group as substrates. Among all reported LC-MS/MS based assays, electrospray ionization (ESI) source is frequently selected for the ionization of both catechol substrates and the O-methylated metabolites.A sensitive LC-MS/MS based assay has been developed for the detection of O-methyltransferase activity by monitoring the conversion of SAM to SAH, which is suitable for investigating the methyltransferases of interest [69]. Meanwhile, the LC-MS/MS based assay is also used for measuring COMT activities in real samples by using catechol estrogen as the substrate with the limit of detection around 1.5 pmol [70].
3.7. Gas chromatography-mass spectrometry(GC-MS)based assays
GC-MS can also be used to monitor the formation of O-methylated product(s) of a given COMT substrate, for the polarity of Omethylated product(s) is lower than that of the substrate. The endogenous catechol estrogens, including 2-OHE2, 2-OHE1, 4-OHE2 and 4-OHE1, have been used as the substrates to assess COMT activities by GC-MS [71]. In most cases, the derivatization procedure is essential for GC-based assays, which not only improves the volatility and stability of analytes,but also provides high sensitivity.Dependent on the specific applications,the selection of the appropriate chemical reagents is important for successful derivatization[72].A GC-MS-based method has been developed to measure COMT activity in liver cytosolic preparations using dopamine as the substrate. The product 3-methoxy-dopamine is quantified after the acetylation with acetic anhydride (AA) [73]. Others have proved that the levels of many catechol estrogens as neuroactive steroids in rat brains are regulated by mammalian COMT[74,75].The derivatization of catechol estrogens is a necessary stepprior to GC separation,for the reason that they themselves are not easy to be volatilized even at a high temperature. When derivatization with O-methyl hydroxylamine (MHA) or N,Obis(trimethylsiyl)trifluoroacetami-ide (BSTFA), the catechol estrogens can be identified by comparison with the MS spectra of the authentic sample.A stable-isotope dilution GC-MS/MS method has been described for quantitative determination of the catecholamine metabolite DHPG in human urine using a two-step derivatization process with pentafluorobenzyl bromide (PFB-Br) and BSTFA[76].In summary,compared to other reported methods,the mass spectrometry for sensing COMT activity can provide high sensitivity, precision, accuracy and so on.
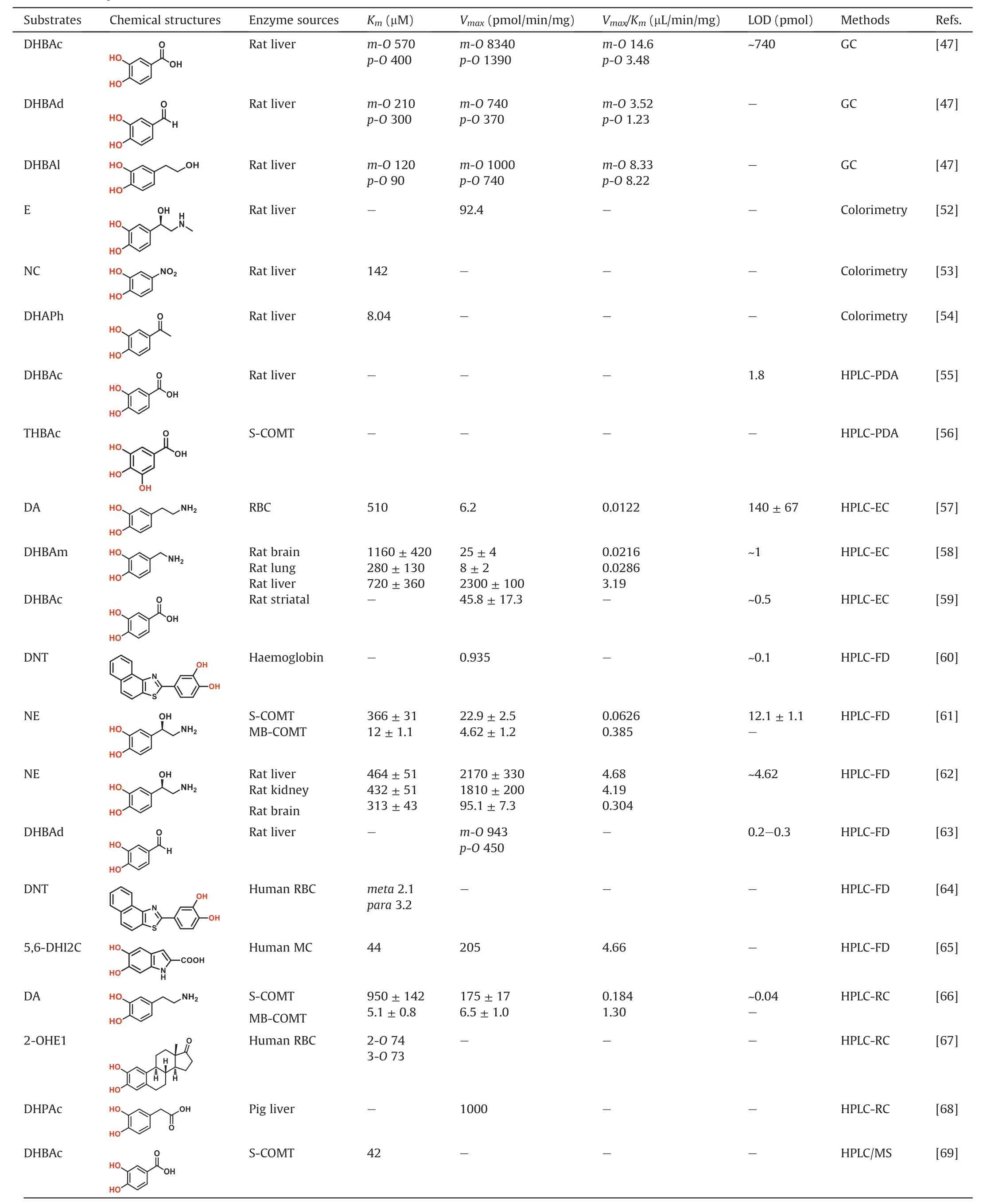
Table 1 Non-fluorescent probe substrates and detection methods for COMT.
(continued on next page)

Table 1 (continued)
3.8. Microplate reader-based fluorescence assays
Although the above-mentioned assays are frequently used to measure the COMT activities in real samples,these methodologies are laborious and expensive with the need for derivatization or sample preparation. By contrast, microplate reader-based fluorescence detection with specific fluorescent probes is applicable for high-throughput detection of COMT activities without separation and derivatization, and is low-cost, time-efficient and easily performed [77,78]. The highly specific fluorescent probes with excellent optical properties can also achieve in situ and in vivo visualization of the localization of target enzyme(s) and the realtime monitoring of target enzyme(s) with good temporal and spatial resolution [79]. Therefore, it is highly desirable to design and develop more practical fluorescent probe substrates for sensing COMT activities in living systems via a feasible way.
It is well-known that there are many factors affecting the sensitivity and practicability of the fluorescence substrates for sensing COMT in complex biological systems.An ideal fluorescence substrate is expected to have excellent selectivity and good response to the target enzyme, as well as strong anti-interference capability [80,81]. In the future, more practical fluorescence substrates for COMT with improved properties such as higher brightness, lower photo-toxicity, minimized autofluorescence, deeper tissue penetration ability and higher spatial and temporal resolution should be designed and developed for sensing COMT activities in living systems, including the brain [82,83]. A major advance in this methodology is that 7,8-dihydoxylcourmarins (DHC) can be catalyzed by COMT with high region-selectivity at C-8 phenolic group to form only 8-O-methylation product which can give off a strong fluorescence signal [84]. For instance, the developed coumarin-based fluorescence probe, 7,8-dihydroxy-4-methylcoumarin (DHMC), is catalyzed by COMT to a single methylated product,7-hydroxy-8-methoxy-4-methylcoumarin(HMMC)with a turn-on fluorescence signal at 520 nm. After that, a two-photon fluorescent probe, 3-(benzo[d]thiazol-2-yl)-7,8-dihydroxy-2Hchromen-2-one(3-BTD), which based on coumarin fluorophore,is also optimized [85]. The probe 3-BTD can be methylated at C-8 phenolic group by COMT, and the 8-O-methylation metabolite exhibits the strong two-photon absorption and emission properties.The fluorescent probe 3-BTD is very suitable for sensing and imaging of COMT activities in living cells and rat brain tissues with good cell permeability, tissue penetration capability and high optical resolution (Fig. 8). The two fluorescent probes serve as a promising tool to provide visualization information of COMT in vivo, to explore the COMT-related biological and pathological functions and to discover novel COMT inhibitors. These fluorescence probe substrates are summarized in Table 2.

Fig. 8. (A) Design and optimization of 3-BTD that can be selectively methylated at the 8-OH site to form 3-BTMD with strong fluorescence response. (B and C) Imaging of endogenous COMT activity in U87 cells and rat brain tissues, respectively.
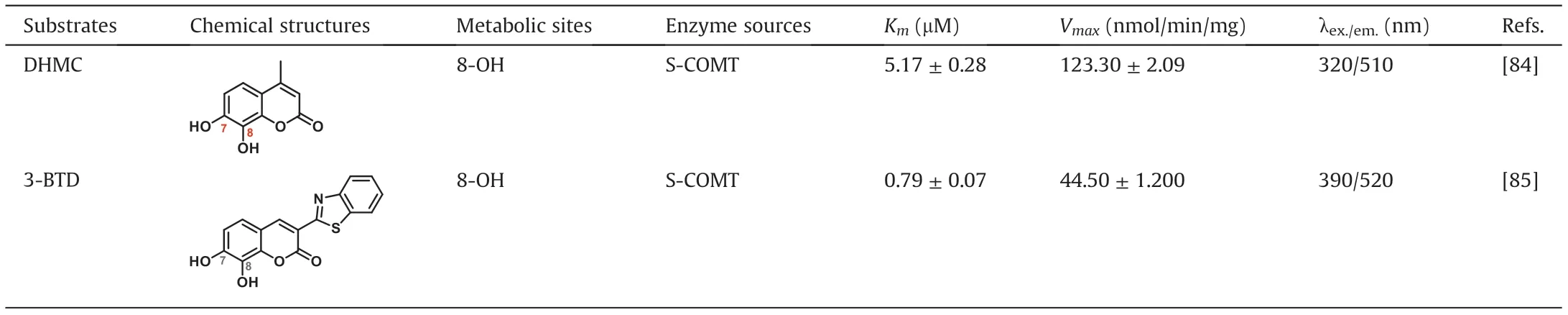
Table 2 Fluorescent probe substrates for COMT.
4. Biomedical applications of COMT activity assays
4.1. Sensing COMT activities in biological systems
Mammalian COMT is a crucial enzyme that modulates the dopamine exposure in the brain and participates in the regulation of mood,cognition,pain perception and response to stress[86,87].COMT has become a promising therapeutic target for ameliorating the cognitive deficits related with mental diseases [88-90]. Inhibition of COMT activity via serotonin binding contributes to pain hypersensitivity, which can provide additional strategies for the treatment of clinical pain conditions[91].Meanwhile,COMT is also responsible for the metabolism of endogenous catechol estrogens and exogenous catechol drugs in peripheral tissues.Reduced COMT activity has been suggested to be a risk factor for various estrogenassociated cancers of breast and ovarian cancers [13,92]. High COMT activity is significantly correlated with the longer overall survival of pancreatic cancer patients, suggesting that COMT has a protective nature and becomes a new target for pancreatic cancer therapy [93]. COMT levels are significantly decreased in human renal cell cancers (RCCs) tissues, implying its suppressive role in tumor development [94]. In addition, patients with low COMT activities may be especially susceptible to tolcapone-induced hepatotoxicity [6]. About 8-fold interindividual variations in COMT activities have been demonstrated in human erythrocytes, indicative of the differences of the disposal and metabolism of catecholdrugs [85]. Recently, the interspecies variations of COMT activities have been evaluated using liver S9 as enzyme sources from human,rat, monkey, dog, mouse, minipig, guinea pig and New Zealand rabbit.The results show that the highest COMT activity is found in the rat liver S9[95].The accurate measurement of COMT activity in biological system will facilitate the studies on COMT-associated human diseases and personalized medicine in clinical practice.

Table 3 Three marketed COMT inhibitors.
4.2. Screening and characterization of COMT inhibitors
The inhibition of COMT activity has served as an effective treatment strategy for the Parkinson’s disease in clinical settings[96-99]. As shown in Table 3, three marketed COMT inhibitors(opicapone, tolcapone and entacapone) have been validated to improve the bioavailability of levodopa by blocking the peripheric COMT activity [94]. Considering that the current inhibitors may cause some side-effects such as hepatotoxicity and severe diarrhea,it is necessary to find more efficacious COMT inhibitors with improved safety profiles [28-30].
Recently, numerous enzyme activity-based assays have been developed to screen drug-like inhibitors using different enzyme sources. For example, a fluorescence polarization (FP) assay has been reported to identify specific MB-COMT inhibitors from a series of 4-pyridinone compounds using recombinant MB-and S-COMTas the enzyme sources [4]. A 96-well microplate-based fluorescence assay has been also developed to screen the inhibitors of human SCOMT with 6,7-dihydroxycoumarin as substrate [79]. To explore the feasibility of PC12 cell line as an in vitro drug screening platform, the cell line (from male rat adrenal pheochromocytoma) is first used as the enzyme source to screen COMT inhibitors [100].Based on structural design,some potential bisubstrate inhibitors of COMT have been developed and synthesized, which offers the guidelines for the design of novel COMT inhibitors [101].
An MS-based method can evaluate the COMT inhibitors and gain insights into the structure-activity relationships in binding to COMT [102]. In facts, the effects of herbal medicine on human metabolism-enzymes have not been well-investigated even though several COMT inhibitors are obtained from natural products[103,104]. With human liver cytosol as the enzyme source, Liang et al. [105] found that natural daphnetin and its O-methylated metabolite can inhibit COMT-mediated dopamine O-methylation in a dose-dependent manner by using HPLC/MS.With a radiochemical assay,it has been shown that COMT from human liver and placenta can be inhibited by tea catechins, flavonoids, polyphenols and caffeic acid [106,107]. The inhibitory effects of plant-derived alkaloids and phenolics on human COMT were evaluated in the fluorescence-based biochemical assay [108]. All these compounds could be regarded as the potential lead compounds to design novel potent COMT inhibitors.
Over the past two decades, computer-aided drug design has been applied to the design and development of COMT inhibitors.For example, Lerner [38] reported a fragment-based screening approach to discover noncatechol-type COMT inhibitors which bind in the SAM binding pocket.Jatana et al.[42,109]has revealed the bisubstrate-type of COMT inhibitors as the new leading compounds by using pharmacophore modeling and virtual screening.In near future,the highly selective and efficacious COMT inhibitors may be designed and developed by using molecular modeling approaches in combination with HTS biochemical assays presented in this review article.
5. Conclusion and perspectives
Mammalian COMT is a class of phase II conjugative enzymes that participate in the metabolism of catecholamine neurotransmitters and the inactivation and detoxification of catechol estrogens and exogenous catechol drugs. The crucial roles of COMTs in human health have promoted the development of practical assays for sensing COMT activities in real samples and the highthroughput screening platform of COMT inhibitors for future clinical translation.In this review,recent advances in the structural and catalytic features of mammalian COMT have been outlined, with emphasis on the substrate preferences and the methodologies for measuring COMT activity. A variety of biochemical assays for sensing COMT activity, including the probe substrates, along with their analytical conditions and kinetic parameters, as well as their biomedical applications have also been reviewed. Although LCbased methodologies (such as LC-PDA, LC-EC and LC-MS/MS) are routinely used for sensing COMT activities in real samples, microplate reader-based fluorescence assays hold great promise for COMT detection owing to its inherent advantages such as without sample preparations, derivation and separation, applicable for high-throughput screening and in situ and in vivo visualization of enzymatic activity. However, the reported fluorescent probes for COMT show the shorter emission wavelength, which limits their applications in living systems. Recently, near-infrared (NIR) fluorescence probes have become a powerful tool for both fundamental research and clinical practice. The use of NIR probes can deepen photon penetration in the tissues, reduce photo-damage to biological samples and produce low background autofluorescence from biomolecules in living systems[110-112].NIR probes open an avenue to the biomedical imaging such as the imaging of enzyme activities and some biological molecules like the biomarkers.In vivo NIR imaging in animal models has strongly facilitated the translational link between fundamental research and clinical applications. In the near future, it will be necessary to develop practical NIR probe substrates for in vivo and in situ imaging of COMT activities, to facilitate the measurement of COMT activities in deeper organs such as the brain and liver,as well as to explore the physiological functions of COMT and the relevance of COMT to human diseases.
Declaration of competing interest
The authors declare that there are no conflicts of interest.Acknowledgments
This work was supported by the National Key Research and Development Program of China (2017YFC1700200,2017YFC1702000), the National Natural Science Foundation of China (81922070, 81703604, 81973286, 81773687 and 81603187),Natural Science Foundation of Shanghai (18ZR1436500), Program of Shanghai Academic/Technology Research Leader(18XD1403600)and Shuguang Program(18SG40)supported by Shanghai Education Development Foundation and Shanghai Municipal Education Commission, the project sponsored by the development fund for Shanghai talents (2019), and the Key Science and Technology Program of Shenyang supported by Shenyang Science and Technology Bureau(17-230-9-05).
 Journal of Pharmaceutical Analysis2021年1期
Journal of Pharmaceutical Analysis2021年1期
- Journal of Pharmaceutical Analysis的其它文章
- Comprehensive metabolic profiling of Alismatis Rhizoma triterpenes in rats based on characteristic ions and a triterpene database
- Evaluation of apoptotic effects of mPEG-b-PLGA coated iron oxide nanoparticles as a eupatorin carrier on DU-145 and LNCaP human prostate cancer cell lines
- Development of an analytical method for multi-residue quantification of 18 anthelmintics in various animal-based food products using liquid chromatography-tandem mass spectrometry
- Drug target discovery by magnetic nanoparticles coupled mass spectrometry
- Applications and challenges of low temperature plasma in pharmaceutical field
- Solid phase microextraction chemical biopsy tool for monitoring of doxorubicin residue during in vivo lung chemo-perfusion
