Development of an analytical method for multi-residue quantification of 18 anthelmintics in various animal-based food products using liquid chromatography-tandem mass spectrometry
Kyun-Hee Yoo , D-Hee Prk , A.M. Ab El-Aty , Seon-Kwn Kim ,He-Ni Jun ,D-Hye Jeon ,Hee-Jun Cho ,e,Ahmet Hcimütüo˘lu ,Je-Hn Shim ,Ji Hoon Jeon , Ho-Chul Shin ,*
a Department of Veterinary Pharmacology and Toxicology, College of Veterinary Medicine, Konkuk University, Seoul,143-701, Republic of Korea
b State Key Laboratory of Biobased Material and Green Papermaking, College of Food Science and Engineering, Qilu University of Technology, Shandong Academy of Science, Jinan, 250353, China
c Department of Pharmacology, Faculty of Veterinary Medicine, Cairo University,12211, Giza, Egypt
d Department of Medical Pharmacology, Medical Faculty, Ataturk University, Erzurum, 25240, Turkey
e Department of Veterinary Physiology, College of Veterinary Medicine, Konkuk University, Seoul,143-701, Republic of Korea
f Natural Products Chemistry Laboratory, College of Agriculture and Life Sciences, Chonnam National University, Gwangju, 500-757, Republic of Korea g Department of Pharmacology, College of Medicine, Chung-Ang University, Seoul,156-756, Republic of Korea
Keywords:Anthelmintics Tandem mass spectrometry Animal-based food products Residue analysis Method validation
ABSTRACT In this study, we developed a simple screening procedure for the determination of 18 anthelmintics(including benzimidazoles, macrocyclic lactones, salicylanilides, substituted phenols, tetrahydropyrimidines, and imidazothiazoles) in five animal-derived food matrices (chicken muscle, pork, beef, milk,and egg) using liquid chromatography-tandem mass spectrometry. Analytes were extracted using acetonitrile/1% acetic acid (milk and egg) and acetonitrile/1% acetic acid with 0.5 mL of distilled water(chicken muscle, pork, and beef), and purified using saturated n-hexane/acetonitrile. A reversed-phase analytical column and a mobile phase consisting of (A) 10 mM ammonium formate in distilled water and (B) methanol were used to achieve optimal chromatographic separation. Matrix-matched standard calibration curves (R2≥0.9752) were obtained for concentration equivalent to ×1/2, ×1, ×2, ×3, ×4,and×5 fold the maximum residue limit(MRL)stipulated by the Korean Ministry of Food and Drug Safety.Recoveries of 61.2-118.4%, with relative standard deviations (RSDs) of ≤19.9% (intraday and interday),were obtained for each sample at three spiking concentrations(×1/2,×1,and×2 the MRL values).Limits of detection, limits of quantification, and matrix effects were 0.02-5.5 μg/kg, 0.06-10 μg/kg, and -98.8 to 13.9%(at 20 μg/kg),respectively.In five samples of each food matrix(chicken muscle,pork,beef,milk,and egg) purchased from large retailers in Seoul that were tested, none of the target analytes were detected. It has therefore been shown that this protocol is adaptable, accurate, and precise for the quantification of anthelmintic residues in foods of animal origin.
1. Introduction
Helminths, or parasitic intestinal worms, come in three major types: cestodes (i.e., tapeworms); nematodes (i.e., roundworms);and trematodes(i.e.,liver flukes),all of which have been involved in livestock parasitic infestations [1]. These infestations have been controlled through use of anthelmintic agents,which are important for fattening and maintaining the reproductive performance of animals [1]. These anthelmintic agents are categorized into six main classes: benzimidazoles, macrocyclic lactones (avermectins and milbemycins), salicylanilides, substituted phenols, tetrahydropyrimidines,and imidazothiazoles [2]. In detail,benzimidazole anthelmintics,such as cambendazole and carbendazim,have been broadly used for the prevention and treatment of endoparasites in animals [3]. Macrocyclic lactone anthelmintics, such as avermectins,are used to treat parasitic infestations(in both larval and adult form) internally and externally [4]. Salicylanilides, such as closantel, rafoxanide and niclosamide, are used as primary antiparasitics,as they have been shown to be effective against many types of parasites, including ticks and mites, in several animal species [5].Tetrahydropyrimidines, such as morantel and pyrantel, are nicotinic receptor agonists that have been used for both pets and humans[6].Imidazothiazole anthelmintics,such as levamisole and tetramisole, are used to treat roundworms [7]. Additionally, praziquantel, cymiazole, nitroxynil, trichlorfon, fluazuron, and thiophanate have been used as anthelmintics [6]. Most anthelminthic agents are overused due to their being over-the-counter products and widely used for chemoprophylaxis [1]. Therefore, these drugs may remain in the edible tissues of chicken, pork, beef, milk, and eggs produced from anthelmintic-treated livestock,and may pose a threat to public health [8,9]. Consumption of these contaminated foods may lead to toxic effects,development of resistant strains of parasite,and/or allergic reactions in hypersensitive individuals[10].
In the Republic of Korea, residual veterinary drugs are managed through the criteria of maximum residue limits (MRLs) set by the Korean Ministry of Food and Drug Safety (MFDS)[11].MRLs are set according to theoretical maximum daily intake(TMDI)values,which are calculated based on food intake and levels of acceptable daily intake(ADI)values[11].Some of the anthelmintics have established MRLs in foods of animal origin, but others (such as niclosamide,rafoxanide,emamectin,cymiazole,praziquantel,morantel,pyrantel,and cambendazole) have no MRL (Table 1) [12]. In other countries,MRLs for anthelmintics have been partially established for animal muscle, milk, and eggs, including the Food Chemicals Codex(0.05-0.2 mg/kg), United States Department of Agriculture(0.1-1 mg/kg), European Union (0.01-0.05 mg/kg), and Australian Agricultural and Veterinary Chemicals Code (0.001-2 mg/kg)[12-16].
Several studies have developed detection methods for anthelmintics in animal-derived foods involving single and simultaneous compound analyses using liquid chromatography-tandem massspectrometry (LC-MS/MS) [1,7,8,17-27]. Extraction methods for anthelmintics have involved both liquid-liquid extractions (LLEs)[8,21] and quick, easy, cheap, effective, rugged, and safe (QuECh-ERS) method [23,24] for animal tissues and egg. Many studies,especially for milk, have involved several anthelmintic extraction methods, including LLEs [7], QuEChERS [1,25], and solid-phase extractions (SPEs) [22,26-28]. Among these, the SPE extraction method has the advantages of reduced noise due to matrix effects(MEs) and increased drug sensitivity; however, it has the disadvantages of long experimental time and poor repeatability [29].QuEChERS extractions are simpler than SPEs; however, the more analytes that need to be simultaneously detected, the most likely the SPE sorbent may irreversibly bind analytes [30]. Currently,single anthelmintic agent analyses methods are not very effective because anthelmintics have been less important than other xenobiotics, such as pesticides, in terms of public health. Therefore,there is a need for the development of a simple, accurate, quick,multi-analyte, low-cost method for monitoring anthelmintic agents. In this study, a method was developed to analyze 18 anthelmintic agents from five animal matrices (pork, milk, egg,chicken muscle,and beef)using simple,fast,and conventional LLE.

Table 1 Maximum residue limit (MRL) criteria defined by the Korean Ministry of Food and Drug Safety [12] for five animal-derived food product anthelmintic residues.
2. Experimental
2.1. Chemicals and reagents
Levamisole HCl (98% purity), closantel (99% purity), trichlorfon(99% purity), praziquantel (99.6% purity), nitroxynil (99% purity),tetramisole HCl (99% purity), guaifenesin (99% purity), carbendazim (99% purity), rafoxanide (98% purity), morantel tartrate (99%purity), emamectin benzoate (99% purity), cambendazole (99%purity), pyrantel tartrate (99% purity), fluazuron (99% purity),eprinomectin (96% purity), acetic acid (99.5% purity), and ammonium formate(97%purity)were purchased from Sigma-Aldrich(St.Louis, MO, USA). Cymiazole (99% purity) was supplied by the Korean Ministry of Food and Drug Safety (MFDS, Cheong-Ju, Republic of Korea).Thiophanate(99.5%purity)and niclosamide(99%purity) were procured from BOC Sciences (Shirley, NY, USA) and Aladdin Biochemical Technology Co., Ltd. (Shanghai, China),respectively.Ultra-high purity water obtained from a Milli-Q water purification system (Millipore, Bedford, MA, USA) was used for mobile phase preparation. HPLC-grade acetonitrile (ACN), methanol, and n-hexane were acquired from Pharmaco-Aaper (Brookfield, CT, USA).All other chemicals and reagents were of analytical grade, unless otherwise specified.
2.2. Standard preparation
Stock solutions(1000 mg/L)were prepared for all drugs,except carbendazim (100 mg/L), by dissolving 10 mg of the standard reagents in methanol.Working solutions in methanol were prepared from the stock solutions at six different concentrations. The working solutions were used to produce both standard and matrixmatched calibration curves. The standard solutions were stored at -20°C in the dark.
2.3. Sample preparation
The samples used in the experiments were chicken muscle,pork, beef, milk, and egg. These were purchased from markets in Seoul, the Republic of Korea. After chopping, 2 g of the samples(2 mL for milk and egg) were weighed and transferred into 50 mL conical tubes. The samples were spiked with 0.2 mL of working solution and equilibrated for 10 min [31]. To the pork, beef, and chicken samples,0.5 mL of water was added,and the samples were vortex-mixed for 3 min.To all of the samples,10 mL of acetonitrile/1% acetic acid solution was added, and the samples were vortexmixed for 10 min followed by centrifugation (Union 32 R Plus;Hanil Science Industrial Co., Ltd., Incheon, Republic of Korea) at 4,000 rpm for 10 min at 4°C.The supernatants were transferred to new 50 mL conical tubes containing 10 mL of saturated n-hexane/acetonitrile. After vortexing for 10 min, the mixtures were centrifuged at 4000 rpm for 10 min.The subnatants were then collected in 15 mL conical tubes and dried under nitrogen gas at 45°C (<0.3 mL). The residues were dissolved in 1 mL of mobile phase B(methanol), vortexed, and centrifuged (MEGA 17R; Hanil Science Industrial Co., Ltd., Incheon, Republic of Korea) at 10,000 rpm at 4°C for 10 min. The supernatants were transferred to new 15 mL tubes and dried under nitrogen gas at 45°C(<0.3 mL).Finally,the residues were reconstituted with 0.5 mL of methanol, mixed for 10 min, and centrifuged at 10,000 rpm at 4°C for 10 min. The samples were then filtered using 0.2 μm syringe filters (MILLEX,Merck Millipore Ltd, Co. Cork, Ireland) and stored in vials for analysis.
2.4. LC-MS/MS analysis
Anthelmintic residue concentrations were quantified via high performance liquid chromatography (HPLC) using an Agilent 1100 series instrument (Palo Alto, CA, USA) equipped with a quart pump (G1311A), degasser (G1322A), autosampler (G1313A), column oven (G1316A), and API 3200TM MS/MS detector (Applied Biosystems, New York, NY, USA). Electrospray ionization (ESI) in both positive and negative ion-switching and multiple reaction monitoring (MRM) modes, with a dwell time of 50 ms, was used.In both the positive and negative ion modes, ion source gas 1(GS1) and ion source gas 2 (GS2) were optimized, the ion spray voltage was set to ±4.5 kV, and the capillary temperature was 350°C. Single standard solutions (0.1 mg/mL) were injected, and MS precursor ions were detected as fragments [M+H]+or[M-H]-. The MRM transition results are summarized in Table 2.
The residue samples were separated using a C18 column(Gemini-NX C18 110A, 100 mm × 2.0 mm, 3 μm particle size;Phenomenex, Torrance, CA, USA) at 30°C. The mobile phase consisted of (A) 10 mM ammonium formate in distilled water and (B)methanol. The flow rate was set at 0.15 mL/min and the injection volume was 10 μL.The mobile phase gradient was started at 5%(A)for 2 min(0-2 min),increased to 90%(A)at 4 min(2-4 min),then after 4 min(4-8 min),decreased back to 5%(A)at 9 min(8-9 min),and maintained for 6 min (9-15 min).
2.5. Method validation
In accordance with the criteria of the MFDS, the developed anthelmintic method was validated in terms of linearity,precision,accuracy,limit of detection(LOD),and limit of quantification(LOQ).To measure the linearity of the method,all metrics were fortified at six concentrations based on the MRLs listed in Table 1 (×1/2,×1,×2,×3,×4,and×5)or set to 0.01 mg/mL for analytes without a specified MRL.LODs and LOQs were estimated based on signal-tonoise (S/N) ratios of ≥3 and ≥10, respectively. Accuracies of intraday (single day, n = 3) and interday (three consecutive days,n=3)recovery values were calculated at three spiking levels(×1/2,×1,and×2 the MRL values).Repeatability,in terms of the relative standard deviation (RSD) of precision, was estimated at the same concentrations. MEs were also measured for all 18 anthelmintic drugs in all matrices.
3. Results and discussion
3.1. Sample preparation
For animal-based food samples, common initial extraction solvents are acidic or basic ACN or MeOH [9]. Thus, a comparison of the use of 10 mL of ACN vs. MeOH as an extraction solvent was conducted. Extractions using MeOH produced final analyte solutions which were cloudy.As a result,ACN was selected as the initial extraction solvent. Initial recoveries with ACN were between 46%and 77% for all 18 drugs. Three acids ((a) 0.5% trifluoroacetic acid;(b) 0.5% formic acid; and (c) 0.5% acetic acid) were added to improve the recovery efficiency of ACN.Overall,the recoveries were 65%-80% higher with added acid, compared to ACN alone. A comparison of the acids showed that (a) and (b) generated peaks for levamisole, tetramisole, and cymiazole that were split and broadened, while the recoveries of the negative ionization drug compounds were 81%-90% using solvent (c). Four concentrations(0.1%,1%, 2%, and 5%) of acetic acid were then tested to compare their effects on recovery. Based on the obtained recoveries, a 1%acetic acid that was added to the extract of chicken,pork,and beef samples,gave a good recovery yield of 80%-90%for all drugs,with the exceptions of levamisole, pyrantel, morantel, cymiazole, andtetramisole.To further increase drug recovery from animal muscle(chicken, pork, beef), samples were tested under various conditions: storage of 30 min [32], extraction using 15 mL of solvent,increased homogenization time, and dichloromethane [19]extraction.Comparatively,recoveries of the five sulfur heterocyclic drugs, i.e., tetramisole, levamisole, cymiazole, pyrantel, and morantel(Fig.1),were high(81%-82%, 79%-80%, 73%-81%,77%-80%,and 86%-94%, respectively) in milk and egg (liquids), while recoveries in the three animal muscle samples (chicken, pork, and beef)were low(45%-49%,47%-51%,51%-57%,62%-64%,and 64%-66%,respectively).Based on these results,1 mL of water was added to the three matrices,followed by a strong homogenization process for 3 min.Although the mean recoveries of the five drugs increased by 10%-20%,the main disadvantage was a longer preparation time due to nitrogen evaporation.The longer evaporation time lowered the drug sensitivities and the amount of water that could be used(reduced to 0.5 mL);thus a more efficient sample preparation was achieved(Fig. 2).
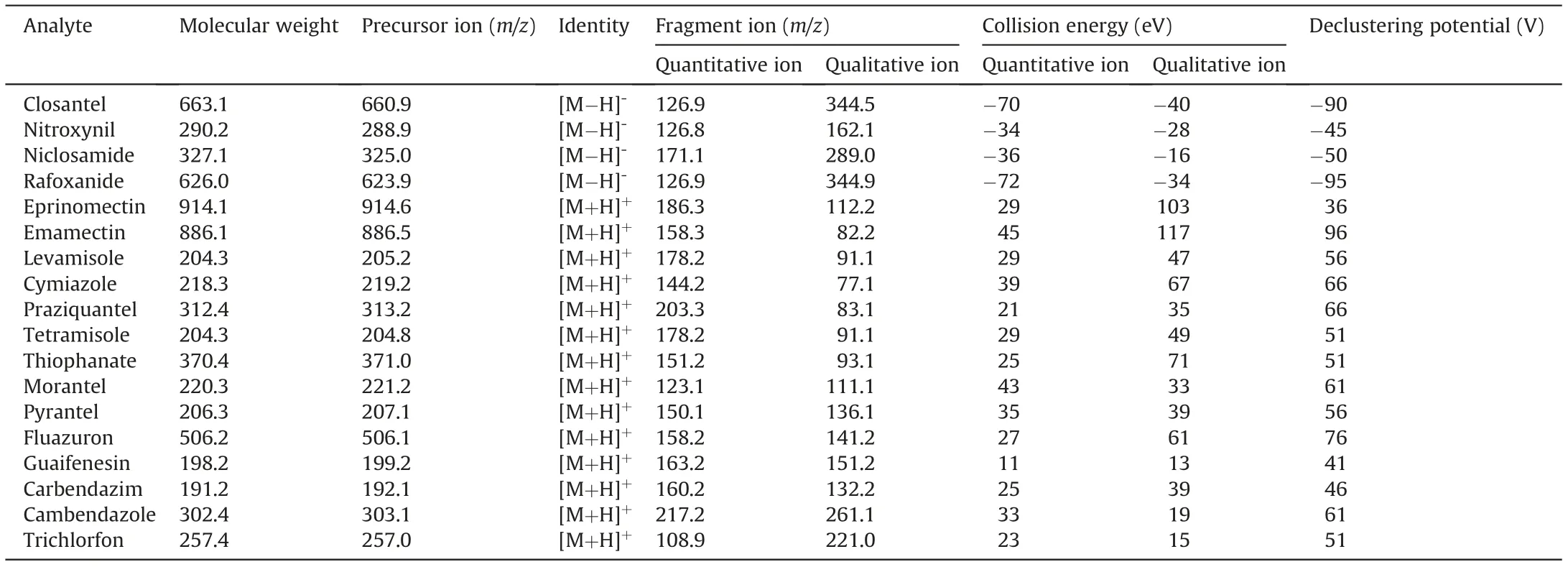
Table 2 Multiple reaction monitoring (MRM) transitions of the 18 anthelmintics.

Fig.1. Chemical structures of the tested anthelmintics.

Fig. 2. Comparison of extraction methods used for chicken, pork, and beef samples (mean ± SD).

Fig. 3. Recovery rates using QuEChERS (EN) methodology.
The QuEChERS method has been applied to residue analyses of veterinary drugs [33], and its use in sample pretreatment is adaptable to complex matrices rich in fats and proteins.Compared to SPE, the QuEChERS method can reduce extraction time, labor,and cost [33]. Therefore, based on previous studies [1,25] using QuEChERS(EN or AOAC)methodology,this method was applied to the five animal-derived products. However, the overall drug recoveries were 22%-62%(Fig.3),which were lower than those using LLE. Therefore, we decided to use LLE method throughout the experimental work. n-hexane is mainly used for removing fatty acids, and when added to ACN, it has been shown to increase extraction efficiency [34]. For this reason, saturated n-hexane in ACN was used and the LLE method was ultimately employed for the determination of the analytes. After concentrating the extracted solvents,1 mL of MeOH was added, and the samples were evaporated down to pellets before LC-MS/MS analysis.
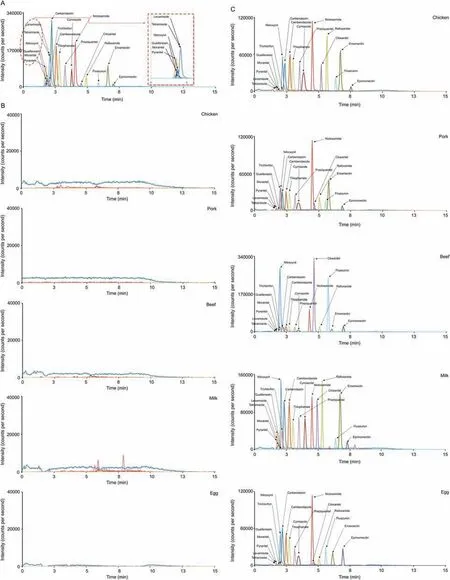
Fig. 4. Chromatograms of the 18 anthelmintics: (A) standards, (B) blank samples, and (C) 200 μg/kg spiked blank samples (chicken, pork, beef, milk, and egg).
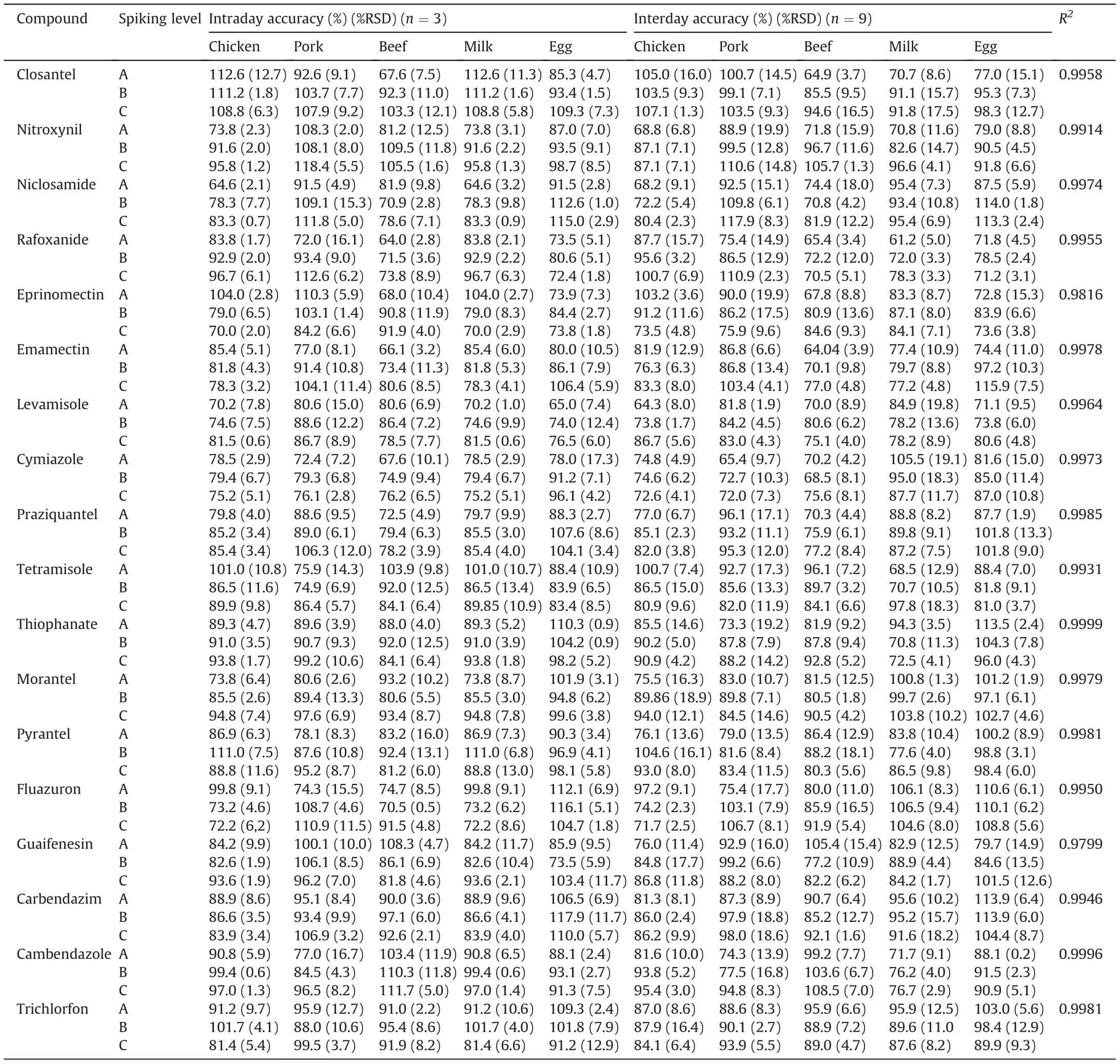
Table 3 Intraday and interday accuracy and precision of the 18 anthelmintics in chicken,pork, beef, milk, and egg.
3.2. LC-MS/MS conditions
Optimized signals were obtained for all target analytes in both ESI turbo-positive and -negative ion modes. The 18 drugs, i.e.,emamectin, eprinomectin, morantel, pyrantel, levamisole, carbendazim, cambendazole, praziquantel, tetramisole, thiophanate,cymiazole, trichlorfon, fluazuron, and guaifenesin in positive ion mode; and rafoxanide, niclosamide, nitroxynil, and closantel in negative ion mode, were well signaled (Table 2). A polarity switching method (combining both positive and negative ion modes) was used in this study, as it has the advantage of not requiring multiple injections [25]. For simultaneous multi-drug analyses, the dwell time parameter determines cycle time (CT).CT increases proportionally with the number of drugs analyzed as the dwell time becomes longer. In addition to reducing analyte sensitivity, this also reduces the smoothness of peaks [35]. Therefore,the dwell time was set to 50 ms,rather than 150 ms,resulting in better peaks due to reduced CT.
Solvent A, which was prepared as an aqueous solution, was compared at five different compositions,and solvent B was tested as either methanol or acetonitrile,as the mobile phase of the method.Based on previous studies, ammonium formate, formic acid, and ammonium acetate were used as solvent A [1,7,8,25,36,37]. As a result,5 mM ammonium formate,10 mM ammonium formate,0.1%formic acid,10 mM ammonium acetate,and 0.1 mM acetic acid were tested. When formic acid was used, tailing was observed for the peaks representing niclosamide, rafoxanide, and nitroxynil. The intensities of levamisole and tetramisole were weak when ammonium acetate and acetic acid were used.When using ammonium formate,which showed good intensities and peak shapes, the overall drug signals were the strongest when a 10 mM concentration was used.Solvent B was tested as either acetonitrile or methanol.Although the peaks of pyrantel and morantel were slightly broadened using methanol, it was selected because of the improved intensities of trichlorfon and fluazuron observed. Additionally, peak tailing and peak splitting of niclosamide occurred when acetonitrile was used.
Isocratic gradients holding mobile phase solvents A and B at constant ratios of 5:95, 10:90, 20:80, 30:70, and 40:60 were also conducted.The 5:95 ratio was found to afford the strongest signals;thus, optimal gradient conditions were selected using this ratio.Under these conditions, peaks were evenly distributed between 1 and 8 min during a 15 min run time. To obtain the best peak separation using this mobile phase, six types of columns (a Phenomenex Gemini C18, Phenomenex Gemini-NX C18, Phenomenex Kinetex EVO, Phenomenex Luna C18, Waters XBridge C18, and an Agilent Eclipse XDB-C18) were compared and analyzed. The Phenomenex Kinetex EVO and Waters XBridge C18columns showed two peaks for pyrantel and morantel, and a poor signal for trichlorfon. All of the columns, with the exception of the Phenomenex Gemini-NX C18column,showed low signals.The Gemini-NX C18column (100 mm × 2.0 mm, 3 μm particle size) was therefore determined to provide the best chromatographic separation quality with the optimized mobile phase at a flow rate of 0.15 mL/min.
3.3. Method performance
3.3.1. Specificity and linearity
Specificity was validated through analyses of working standards(spiking level = 10 μg/L) and five blank samples (n = 5). No potential interferants were observed around the retention time of the 18 drugs (Fig. 4). Matrix-matched calibration curves from the responses of the 18 drugs were calculated using six working standards with concentrations of ×1/2, ×1, and ×2 to ×5 the MRL values (n = 3). Linearity was satifactory with correlation coefficients (R2)≥0.9752 (Table 3).
3.3.2. LODs, LOQs, and MEs
In this study,LODs of 0.02-5.5 μg/kg and LOQs of 0.06-10 μg/kg were achieved. As shown in Table 4, the LODs and LOQs have lower levels than those of the MRLs set for each drug. MEs were determined by comparing the signals of the individual analytes in pure solvent with those of spiked analyte blank matrix samples processed by the same sample preparation methods [38].MEs were calculated as follows: ME (%) = (peak area of the standard in matrix - peak area of the standard in pure solvent)/peak area of the standard in pure solvent × 100, where the spiking concentration = 20 μg/kg. MEs of -98.1% to 13.3%, -98.7% to 1.1%, -98.8% to 4.0%, -95.0% to 13.91%,and-96.8%to-11.0%were acquired for chicken,pork,beef,milk,and egg, respectively,and ion suppression was observed for all of the drugs. The suppression effects of fats and phospholipids may decrease or increase signal strengths of measured analytes depending on their proportions in sample matrices. However,MEs cannot be ruled out [39].
3.3.3. Accuracy and precision
Herein,the accuracy and precision of the developed method are defined as recovery and RSD,respectively.The intraday and interday accuracy and precision values of the 18 compounds at three different concentrations are listed in Table 3. The method was validated as accurate,reliable, and reproducible,with recovery values of 61.2%-118.4% and RSDs of ≤19.9% (intraday and interday), which suitably met Codex criteria (for spiking concentrations = 1-10 and 10-100 μg/kg,recoveries were 60%-120%and 70%-120%,and RSDs were ≤30%and 20%,respectively)[40].
3.4. Method application
The developed method was used to measure anthelmintic residues in five different samples (beef, pork, chicken, milk, and egg)purchased from large markets in Seoul, the Republic of Korea.Notably, the results demonstrated that none of the samples contained the target anthelmintics at detectable concentrations.
4. Conclusions
Methanol-based 10 mM ammonium formate solution was adopted as the mobile phase for the developed LC-MS/MS method.This method involved liquid-liquid extractions using acetonitrile/1%acetic acid/water(chicken,pork,and beef)and acetonitrile/1%acetic acid (milk and egg). The samples were purified with saturated nhexane/acetonitrile for detection and quantification of the 18 anthelmintics. This method was found to be highly efficient,inexpensive,and less time-consuming than other established methods.LODs and LOQs demonstrated that the developed protocol is feasible under established MRL criteria for the detection and quantification of anthelmintic residues in animal-derived food products.

Table 4 Limits of detection (LODs) and limits of quantification (LOQs) of the 18 anthelmintics in chicken,pork, beef, milk, and egg using the proposed method.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This study was supported by a grant(18162MFDS523)from the Ministry of Food and Drug Safety Administration in 2019.
 Journal of Pharmaceutical Analysis2021年1期
Journal of Pharmaceutical Analysis2021年1期
- Journal of Pharmaceutical Analysis的其它文章
- Comprehensive metabolic profiling of Alismatis Rhizoma triterpenes in rats based on characteristic ions and a triterpene database
- Evaluation of apoptotic effects of mPEG-b-PLGA coated iron oxide nanoparticles as a eupatorin carrier on DU-145 and LNCaP human prostate cancer cell lines
- Drug target discovery by magnetic nanoparticles coupled mass spectrometry
- Analytical methodologies for sensing catechol-O-methyltransferase activity and their applications
- Applications and challenges of low temperature plasma in pharmaceutical field
- Solid phase microextraction chemical biopsy tool for monitoring of doxorubicin residue during in vivo lung chemo-perfusion
