Long-term effects of gravel-sand mulch thickness on soil microbes and enzyme activities in semi-arid Loess Plateau,Northwest China
ChengZheng Zhao,YaJun Wang,Yang Qiu,ZhongKui Xie,YuBao Zhang
1.Northwest Institute of Eco-Environment and Resources,Chinese Academy of Sciences,Lanzhou,Gansu 730000,China
2.University of Chinese Academy of Sciences,Beijing 100049,China
ABSTRACT In semi-arid areas of China,gravel and sand mulch is a farming technique with a long history.In this study,a sample survey was conducted on long term gravel sand mulch observational fields in the Northwest Loess Plateau to determine the effects of long term mulch on soil microbial and soil enzyme activities.We found that after long term gravel-sand mulch,compared with bare ground,soil organic matter,alkali nitrogen,conductivity decreased,while pH and soil moisture increased.Urease,saccharase and catalase decreased with increased mulch thickness,while alkaline phosphatase was reversed.The results of Illumina MiSeq sequencing shows that after gravel-sand mulch,the bacterial and fungal community structure was different from bare land,and the diversity was reduced.Compared with bare land,the bacteria Proteobacteria and Acidobacteria abundance increased with increased thickness,and Actinobacteria was opposite.Also,at the fungal genus level, Fusarium abundance was significantly reduced,and Remersonia was significantly increased,compared with bare land.Redundancy analysis (RDA) revealed that soil environmental factors were important drivers of bacterial community changes.Overall,this study revealed some of the reasons for soil degradation after long term gravel-sand mulch.Therefore,it is recommended that the addition of exogenous soil nutrients after long term gravel-sand can help improve soil quality.
Keywords:gravel and sand mulch;soil microbes;soil enzyme activities;soil degradation;soil quality
1 Introduction
In semi-arid areas,crop growth is greatly limited by moisture.Effective soil moisture can be increased through surface mulch and other soil management practices.Previous research studies have shown that:
(1) gravel-sand mixed mulch had the best effect in increasing soil temperature;(2) surface gravel-sand mulch inhibited evaporation,and gravel and sand mixed mulch layer reduces evaporation more effectively;(3)gravel and sand mulch reduce runoff and increase soil moisture compared to bare land;(4) gravel can effectively control wind erosion by blocking wind and sand (Liet al.,2000,2002;Li and Liu,2003;Xieet al.,2006,2010;Ma and Li,2011).Qiuet al.(2015)have shown that although gravel mulch maintains soil moisture,it may also lead to long term decline in unstable soil organic carbon content and total organic nitrogen content in the study area.
Microorganisms are an important part of the soil and play a vital role in the transformation of soil organic matter and energy,indicating soil fertility to some extent.Previous studies have shown that the diversity and abundance of bacteria and fungi decrease with increasing drought.Drought promotes changes in soil bacterial composition (Fernandoet al.,2015).The arid-soil microbiome stability is sensitive to aridity (Juliaet al.,2017).Abiotic factors such as extreme drought and intensive farming measures affect the abundance,diversity and function of soil microbial communities (Dominikaet al.,2020).The optimal temperature for fungal and bacterial growth rates is around 25-30°C,and our previous studies found lower values at higher temperatures (Jannaet al.,2005).The two treatments have significant effects on soil organic matter content,respiration,microbial biomass N,pH,cation exchange capacity,and essential plant nutrient concentrations (Soniaet al.,2002).Haoet al.(2017)showed that mulch has an effect on soil bacterial communities,and the richness and diversity of bacterial communities in the mulched samples were higher.Part of the microbial community is only present in the mulched soil samples.Gravel mulch can significantly alter the structure and composition of fungal communities (Qiuet al.,2018).Gravel and sand mulch have a certain influence on the structure and metabolic capacity of bacterial communities,which is one of the important reasons for crop yield(Haoet al.,2017).
Soil enzymes,as key indicators of soil microbial activity,are closely related to soil nutrient cycling processes,such as saccharase,urease and phosphatase participate in soil C,N,and P cycles,respectively(Yoo and Hojeong,2012;Wuet al.,2013).Soil enzyme activity reflects the vigour of various soil biochemical processes,biological activities and fertility characteristics of the soil(Minoriet al.,2011;Richardet al.,2013).Soil enzymes are sensitive to soil disturbance and are important indicators for assessing soil quality (Yuet al.,2006).Mulching has shown an increase in enzyme activity at least until 11 years,and then begins to decline significantly,indicating a decline in soil quality after long term mulch (Qiuet al.,2014a).
Extensive research has proven that thickness of the gravel-sand mulch is one of the important factors affecting the soil environment.Gravel-sand mulch could effectively reduce evaporation.The thicker the covering,the more obvious inhibition effects evaporation (Zhou and Xie,2015).The relationships between mulch resistance and mulch stratum thickness or grain size of gravel are represented by logistic curves (Qiuet al.,2014b).There is a close negative relationship between mulch thickness and soil evaporation,with exponential function (Wanget al.,2014).Gravel-sand mulch not only inhibits evaporation,but also increases infiltration.Compared with CK,cumulative infiltration increased by 4.3%,11.8%,12.8% and 17.1% under gravel-sand thickness of 6,9,12 and 15 cm,respectively,and average infiltration rate increased by 25.8%,38.7%,50.0% and 62.9%,respectively (Baiet al.,2017).In addition,the thickness of gravel cover is also an important factor affecting soil respiration.Also,the thickness of gravel mulch is also an important factor affecting soil respiration,microorganisms and crops.The peak time of soil respiration after gravel mulched lags behind that of bare land,and the peak value decreases with increases of mulch thickness(Zhaoet al.,2020).It is optimum for microbial developing when the thickness of gravel-sand is in the range of 7-9 cm (Panget al.,2012).Moreover,7-8 cm of mulch thickness appears to be the most appropriate option for gravel-sand mulch to sustain high watermelon yield and water use efficiency (Wanget al.,2014).
In summary,gravel mulch thickness changes the soil microenvironment and has a certain impact on soil microbes and soil enzyme activities.Gravel-sand mulch farmland is a traditional farming method for large-scale application in semi-arid areas of Northwest China.Thus,research on ecosystem service functions cannot be ignored.Studying the soil biological and enzyme activities in the mulch tillage system can help to understand the impact of ground mulch conditions on soil health and provide a basis for sustainable use and scientific management of farmland ecosystem functions.
2 Materials and methods
2.1 Site description
This experiment was conducted at Gaolan Ecological Agriculture Research Station,Northwest Institute of Eco-environment and Resources,Chinese Academy of Sciences.
The research station is located in the Loess Plateau in northwest China (Gaolan,Lanzhou,Gansu Province,36°13'N,103°47'E),with an elevation of about 1,800 m.The average annual precipitation is 263 mm,of which rainfall from May to September accounts for nearly 70%,and annual average evaporation is 1,786 mm.The annual average temperature in the region is 8.4 °C,the temperature in January is -9.1 °C,which is the lowest monthly average temperature,and the temperature in July is 20.7 °C,which is the highest monthly average temperature.The soil belongs to Haplic Orthic Aridisol with silt loam texture of loess origin (sand 123 g/kg;silt 669 g/kg;clay 208 g/kg).The bulk density of the soil surface averaged 1.33 g/cm3.
2.2 Experimental design
Samples of non-planted crops were collected in the long term gravel-sand mulched observation field(set in 2006) to determine the effects of gravel-sand mulch on soil microorganisms.The experiment was carried out in 2017,and the gravel mulch period was 11 years.There were four mulch thicknesses:bare land (CK);gravel-sand mulch thicknesses 5 cm(GM5);gravel-sand mulch thicknesses 7 cm (GM7)and gravel-sand mulch thicknesses 9 cm (GM9).Mixed layer contained 30% coarse pebbles,40% 2-4 cm medium gravel and 30% 0.3-2 cm fine sand.The control(CK)treatment was bare land.The experimental treatments were arranged in a randomized complete block design (RCBD),and each treatment was sampled three times,with a plot area of 4.5 m2(1.5m×3m).In this experiment with no crops planted,soil microbes,enzyme activity and physicochemical properties were determined in the gravel-sand mulched areas and bare ground.
2.3 Soil sampling and measurement
Three representative points were selected in each plot.The surface litter of bare ground and gravel-sand mulch was removed,and then sampled with a soil drill.The visible plant debris in the soil was picked out.The soil samples were then divided into three parts:(1) a fresh soil sample was passed through a 2 mm sieve and quickly placed in a liquid nitrogen tank for the determination of soil microbial diversity;(2) a fresh soil was placed in an aluminum box and placed in an oven for soil moisture content;(3) the other soil was naturally air-dried for the determination of soil nutrients.The physical and chemical properties were determined by the Soil Physical and Chemical Laboratory of the Cold and Arid Regions Environmental and Engineering Research Institute of the Chinese Academy of Sciences;and (4) the last soil was naturally air-dried,and soil enzyme activity (saccharase,urease,alkaline phosphatase,and catalase) was determined using a colorimetric detection method.
Soil organic matter was determined by potassium dichromate (K2Cr2O7) oxidation at 170-180 °C,followed by titration with 0.1 mol/L ferrous sulphate(Walkley and Black,1934).Alkaline hydrolysis of nitrogen was determined by the NaOH alkali diffusion method (Bremner and Shaw,1955).Available phosphorus was extracted using 0.5 M sodium bicarbonate(NaHCO3) (pH 8.5) and ammonium acetate(C2H7NO2),respectively,and examined using the colorimetric method (Olsen,1954).The activities of soil urease,saccharase,catalase,and alkaline phosphatase were measured according to the colorimetric detection,according to the methods presented by Alef and Nannipieri (1995).The soil moisture is measured by the drying method.The soil pH was determined according to ISO 10390:soil pH was measured using a pH meter according to a 1:2.5 soil/water suspension(ISO,1994a).Soil conductivity was determined according to ISO 11265:soil conductivity is measured by a conductivity meter according to a 1:5 soil/water suspension(ISO,1994b).
2.4 Data analysis
2.4.1 Soil microbial sequencing
Soil microbial diversity was analyzed using bare soil and soil gravel-sand mulch of different thicknesses,and each sampling was repeated three times.Microbial sequencing of soil samples was completed by Beijing Novo Biotech Co.,Ltd.,China (http://www.novogene.com).The main experimental procedures are as follows:Sample DNA was extracted by CTAB and SDS methods.We used agarose gel electrophoresis to check DNA concentration and purity.We diluted an appropriate amount of sample with sterile water to 1 ng/μL.Using the diluted genomic DNA as a template,the specific primers of Barcode,Phusion®High-Fidelity PCR Master Mix with GC Buffer of New England Biolabs,and high-efficiency high-fidelity enzyme were used to amplify the V3-V4 region of the 16SrRNA gene and the ITS1 region of the fungal 18SrRNA gene.The mixture was homogenized according to the concentration of the PCR product.After thorough mixing,the PCR product was detected by electrophoresis on a 2% agarose gel.The product was recovered through the gel recovery kit provided by Qiagen.The TruSeq® DNA PCR-Free Sample Preparation Kit constructed the DNA library.When the library was qualified,the constructed library is quantified by Qubit and Q-PCR,and sequenced using HiSeq2500 PE250.Sequence analysis was performed using Qiime software (Version 1.7.0),which also analyzed alpha and beta diversity.All the effective Tags of all samples were clustered by Uparse software(Uparse v7.0.1001)(Robert,2013).By default,the sequences were clustered into OTUs (Operational Taxonomic Units)with 97%identity,and representative sequences were selected.Spectral annotation analysis was performed using the RDP Classifier (Version 2.2)method and the Green Gene database (set thresholds 0.8-1).The community composition of each soil sample was counted at seven classification levels:kingdom,phylum,class,order,family,genus,and species(DeSantiset al.,2006;Wanget al.,2007).
2.4.2 Sequencing data analysis
The raw data (Raw Data) obtained by sequencing is spliced and filtered to obtain valid data(Clean Data).Based on valid data,OTU clustering and species classification analysis are conducted.According to OTUs clustering results,the species sequence of each OTU representative sequence is annotated.Thus,we obtain corresponding species information and abundance distribution based on species level.Simultaneously,the abundance calculation analysis of OTUs is carried out to obtain information on species richness and uniformity in samples,and OTU information that is common or different between different samples or groups.
In addition,there is a sparse curve (Lundberget al.,2013),from which several individuals are randomly selected from the sample,and the number of species represented by these individuals is counted.A curve for verifying the amount of sequencing data is established.Sparse curves are sufficient to reflect species diversity and indirectly reflect species richness in the sample.
2.5 Statistic data analysis
Statistical analysis and graphing were performed using SPASS 25.0,Origin 2018,SIMCA 14.1,and Canoco5.0.
3 Results
3.1 Effect of mulch thickness on bacterial and fungal communities
The sparse curves (Figure 1) were obtained after sequencing with 16S rDNA,and ITS1,and trends of the curves reflect the rationality of the amount of sequencing data and indirectly reflect species abundance in the sample.The sequence is clustered into OTUs with 97%identity.
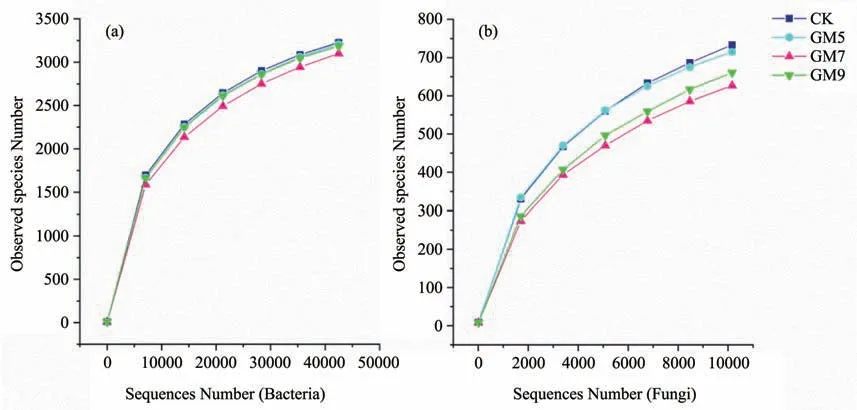
Figure 1 Sparse curves of bacteria and fungi
After clustering of OTUs,the relative abundance of bacterial and fungal components in different mulch thicknesses (levels) is presented in Figure 2 according to species annotation at the phylum level.
Bacteria:Among the abundance of the top ten species(top 10),most of the three treatments and control belonged to Proteobacteria,Actinobacteria,Acidobacteria,Gemmatimonadetes,Firmicutes,Chloroflexi,Planctomycetes,Nitrospirae,Crenarchaeota and Verrucomicrobia.The relative abundance patterns of the 10 dominant phylum varied.In all treatments and controls,Proteobacteria represented the most important category,accounting for about 20%.The abundance of Proteobacteria,Acidobacteria increases with increasing gravel mulch thickness.After the gravel was mulched,the abundance of Actinobacteria decreased,with the lowest value at GM7.The relative abundance of Verrucomicrobia increased after gravel-sand mulch.Gemmatimonadetes,Nitrospirae and Crenarchaeota have the highest abundance when mulching GM7 and Chloroflexi has the lowest abundance at GM7.
Fungi:Among the abundance of the top ten species (top 10),most of the three treatments belonged to Ascomycota,Basidiomycota,Chytridiomycota,Zygomycota,Glomeromycota and Neocallimastigomycota(Figure 2).Among all mulch treatments and control,Ascomycota was the most important category,with an average of more than 50%.The relative abundance of Ascomycota is lowest at the mulch thickness of GM7,and the relative abundance of Basidiomycota is opposite.The relative abundance of Zygomycota and Glomeromycota decreased after gravel mulched,while the relative abundance of Chytridiomycota was reversed.The relative abundance of Neocallimastigomycota did not change in the control and mulch treatments.
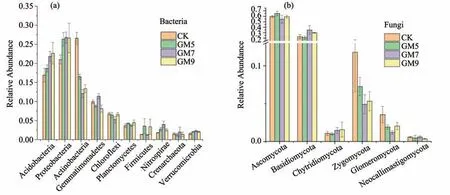
Figure 2 Abundance of bacterial and fungal phylum levels
From the fungal point of view,according to the findings of species annotation,the abundance ofFusariumandRemersoniain the non-mulched and mulched samples was significantly different among the top 10 species,with the highest abundance at the fungal genus level.As presented in Figure 3,with an increase of gravel mulch thickness,the abundance ofFusariumwas significantly reduced.In the mulch thickness of GM5 the abundance was reduced by 78.84% compared with bare ground,and in the mulch thickness of GM9,it was reduced by 91.00% compared with the control,whileRemersoniawas opposite.Remersoniahad the highest abundance at a thickness of GM7,which was 5.86 times higher than the control.
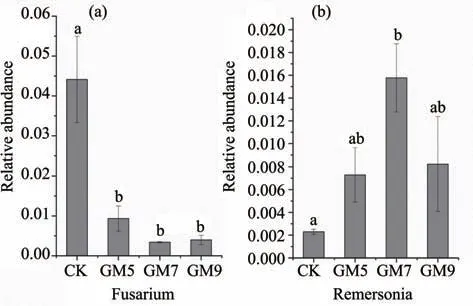
Figure 3 Fungal genus levels:changes in abundance of Fusarium and Remersonia
Principal component analysis (PCA) was based on OTUs levels,and the results are presented in Figure 4.
Bacteria:Principal component 1 explained variance of 21.5%,principal component 2 explained variance of 10.6%,and all samples were divided into three groups.It can be seen that except for CK (control),all other groups of samples can be aggregated,indicating that gravel-sand mulched constituent communities were similar overall.This displayed clear differences in the bacteria community structure between CK and gravel-sand mulch treatments.
Fungi:In PCA,principal component 1 explained variance of 15.5%,principal component 2 explained variance of 12.8%,and all samples were divided into three groups.It can be seen that except for CK (control) and GM5,the compositional communities of GM7 and GM9 were similar.This displayed clear differences in the fungal community structure between CK and different thickness gravel-sand mulch treatments.
3.2 Effect of mulch thickness on enzyme activity
In Figure 5,the level of enzyme activity for the four treatments shows that urease,catalase,saccharase,and alkaline phosphatase exhibited different responses to gravel-sand mulch.
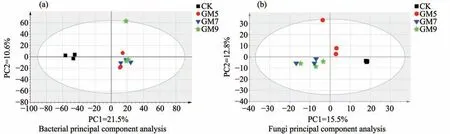
Figure 4 PCA analysis of bacteria and fungi based on OTUs levels
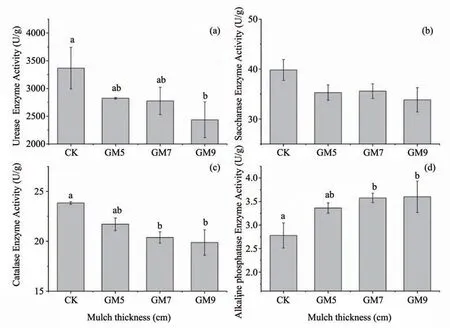
Figure 5 Activity of four enzymes under different gravel-sand mulch thickness(levels)(a,urease;b,saccharase;c,catalase;d,alkaline phosphatase)
Urease activity decreased with increased gravelsand mulch thickness and significantly lower than the control (by 27.66%),with a mulch thickness at GM9.Saccharase activity decreased as the thickness of the gravel mulch increased.Catalase activity also decreased significantly with an increase of gravel-sand mulch thickness and when the mulch thickness reached GM7 and GM9,the catalase activity was significantly lower than CK (control) (by 14.49%,16.61%,respectively).Alkaline phosphatase activity increased with an increase of mulch thickness.When the thickness reached GM9,alkaline phosphatase activity increased significantly compared with the control(by 28.62%).
3.3 Soil properties with different levels of mulch thickness
The soil properties of different mulch thickness(levels) treatments are presented in Figure 6.After gravel sand mulching,soil moisture significantly increased by nearly 2x as compared to the control,while soil organic matter decreased significantly after gravel mulching,but soil moisture and organic matter did not significantly differ between treatments.The content of alkali nitrogen is the lowest at GM5 and GM7.The available phosphorus content significantly increased with an increase of mulch thickness.When the mulch thickness reached GM9,it was also significantly higher than the control.The pH significantly increased after gravel mulching,average increased by about 1.The conductivity was significantly reduced by nearly 10 times relative to the control after gravel mulched.
3.4 Correlation of soil enzyme activity,microorganisms and environmental factors
In the results of Zhouet al.(2019) RDA was used to analyze the relationship between soil physical and chemical properties,soil enzyme activities and soil microorganisms.The results of RDA analysis show that the first RDA axes explained 61.04% and second RDA axes explained 27.92% of total variation between soil bacterial communities at the phylum level(Figure 7).Through CANOCO's forward selection,some environmental factors that have a greater contribution to the bacterial community were screened out.The ranking of these factors are as follows:pH >Conductivity >Soil Moisture >Soil Organic Matter >Catalase >Urease >Alkali-Hydrolyzable Nitrogen >Alkaline phosphatase >Saccharase >Available phosphorus (Figure 7).Furthermore,pH(P=0.004);Conductivity (P=0.001);Soil Moisture(P=0.004);Soil Organic Matter (P=0.002);Catalase(P=0.01);Urease (P=0.024);Alkali-Hydrolyzable Nitrogen (P=0.02);Alkaline phosphatase (P=0.032)were the most important environmental reasons related to bacterial communities' changes.The phyla Nitrospirae and Verrucomicrobia were correlated to pH and Soil Moisture,which was positive,whereas Actinobacteria was the opposite.Actinobacteria was positively correlated to conductivity and soil organic matter while Chloroflexi was positively correlated to saccharase.
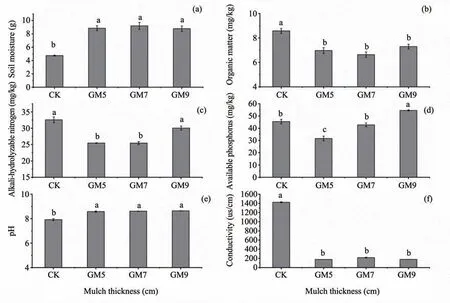
Figure 6 Soil physical and chemical properties of different mulch thickness(levels)
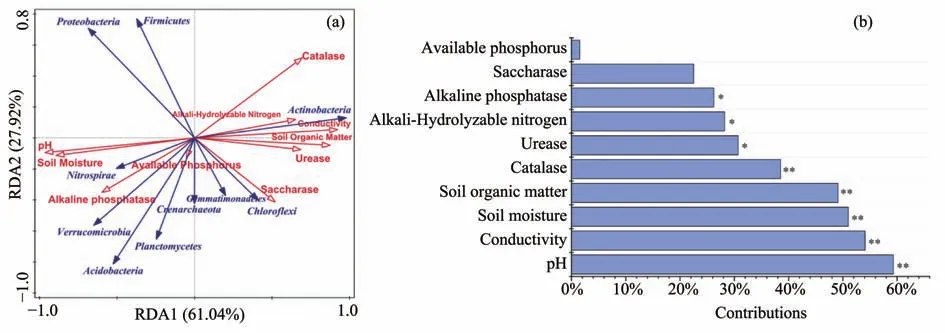
Figure 7 Redundancy analysis(RDA)between bacterial phylum and selected environmental factors and the contribution and significance of each environmental variable to the bacterial community.*indicates that the significance level of the difference is 0.05.**indicates that the significance level of the difference is 0.01
The results of RDA analysis shows that the first RDA axes explained 69.68% and second RDA axes explained 22.55% of the total variation between soil fungal communities at the phylum level (Figure 8).Through CANOCO's forward selection,some environmental factors that have a greater contribution to the fungal community were screened out.The ranking of these factors are as follows:Catalase >Urease >pH >soil organic matter>Alkaline phosphatase >Soil moisture>Available phosphorus >Conductivity >Alkali-Hydrolyzable nitrogen >Saccharase (Figure 8).The correlation between these environmental factors and changes in fungal communities was not significant.Glomeromycota was positively correlated to soil organic matter while Basidiomycota was positively correlated to pH and soil moisture.
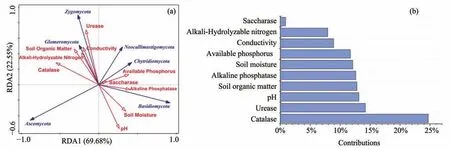
Figure 8 Redundancy analysis(RDA)between fungal phylum and selected environmental factors and the contribution and significance of each environmental variable to the bacterial community.*indicates that the significance level of the difference is 0.05.**indicates that the significance level of the difference is 0.01
4 Discussions and conclusions
4.1 Soil properties
Our results show that gravel mulched can effectively increase soil moisture.These findings are consistent with other results (Liet al.,2002;Li and Liu,2003).Compared to the control,soil characteristics of gravel mulch are different from control while Soil Organic Matter and Alkali-Nitrogen significantly decreased after long term gravel mulched soils.There are three explanations for this decrease in Soil Organic Matter and Alkali-Nitrogen in the mulched treatment:First,the gravel-sand mulch layer obstructed plant litter in the natural environment from entering the soil,which is a supplement to the lack of exogenous nutrients in the soil.Second,the fact that long term gravel mulch caused reduce soil organic matter and alkali-hydrolyzable nitrogen was supported in this study (Figure 6).Previous studies have shown that after 11 years of mulching,the main nutrient elements and enzyme activities of the soil are reduced compared to bare land.Therefore,adding organic matter after 11 years of mulching is an option to reverse soil degradation (Qiuet al.,2014a,2015).The third cause of this phenomenon:sand of the gravel layer will enter into the surface soil,also,the soil content per unit volume will be lower than the original soil,in the sample,accordingly reducing soil organic matter and Alkali-Nitrogen content.Studies have shown that in the case of gravel-sand mulching,soil under the mulch layer will enter the mulch layer,and the soil content will reach 7.5% in the mulch layer after 5 years (Wanget al.,2010).We also found that conductivity dropped significantly by nearly 10 times.This suggests that long term gravel-sand mulch inhibited the upward movement of salt and removes the upper salt,which is more conducive to crop growth.
4.2 Enzyme activity
In addition,urease,saccharase,and catalase enzyme activity decreased as the thickness of the mulch increased compared with CK.Qiuet al.(2015)reported that soil enzyme activity decreased after more than 11 years of gravel-sand mulch,indicating a decline in soil quality(Yuet al.,2006).Alkaline phosphatase activity increased as the thickness of the mulch increased,and alkaline phosphatase is sensitive to pH.After mulching,the soil pH changed from neutral to mildly alkaline,therefore,subsequent changes in alkaline phosphatase activity may affect the P cycle (Juma and Tabatabai,1978).Studies have shown that there is a positive correlation between phosphatase activity in soil and soil organic phosphorus content.In our results,both available phosphorus and phosphatase activities increased with increasing thickness (Spiers and McGill,1979).
4.3 Bacterial and fungal communities
After long term mulch,Proteobacteria and Acidobacteria abundance increased with increased thickness compared with bare land,while Actinobacteria abundance decreased.This is consistent with the results of Qiuet al.(2017) and Haoet al.(2018).The richness of Acidobacteria increased and Actinobacteria abundance was reduced compared to the control.The average abundance of Proteobacteria in the mulched samples was higher than that of the bare land samples.Compared with CK,Proteobacteria and Acidobacteria abundance was high,while the content of organic matter was low.In soils with high organic carbon content,Proteus is more abundant than acid bacteria,which may be due to the difference in organic carbon content in organic matter (Axelroodet al.,2002).In addition,based on the results of the 16S rRNA gene sequence,between various parts of the bacteria,the ratio of Proteobacteria and Acidobacteria reflects the level of soil nutrients (Ericet al.,2001).After gravel-sand mulch,Gemmatimonadetes,Nitrospirae and Verrucomicrobia abundance increased,which is consistent with the results of Qiuet al.(2018).Gemmatimonadetes,Nitrospirae and Crenarchaeota have the highest abundance when the treatment was GM7.Studies have shown that the mulching thickness of 7-9 cm is more suitable for the growth of bacteria(Panget al.,2012).The positive effect of gravel-sand mulch on microbial biomass may be due to the ability of mulch to increase soil temperature and moisture (Wanget al.,2009).Therefore,the thickness of 7 cm may be more suitable for the growth of these types of bacteria.The abundance of Chloroflexi is the lowest in the gravel mulching 7 cm thickness.Studies have shown that members of Chloroflexi are responsible for degrading carbohydrates and cellular materials in municipal sewage (Yukiet al.,2007).The organic matter content is the lowest when the treatment was GM7,so the decrease in Chloroflexi abundance may be related to the reduction of carbohydrates and cell material in organic matter.
At the level of fungal phylum,Ascomycota and Basidiomycota are the main categories,which is consistent with previous studies.The previous sequencing platforms Ascomycota and Basidiomycota are the most abundant phylum,accounting for more than 60% (McGuirelet al.,2013;Schmidtet al.,2013).The abundance of Glomeromycota decreases after gravel mulch,which is consistent with the results of Qiuet al.(2018).The decrease in Zygomycota abundance after gravel mulch is contrary to the results of Qiuet al.(2018).Studies have shown that Chytridiomycota is often distributed in aquatic and moist soils(Karling,1977;Fuller and Jaworski,1987).The soil water content increases after gravel mulch,which may lead to an increase in Chytridiomycota abundance.Neocallimastigomycota is not a dominant fungus,and its abundance is extremely low in the control and all treatments.Seatonet al.(2020) showed that compared with bacteria,fungi are less susceptible to the physical and chemical properties of the habitat.Therefore,the abundance of Neocallimastigomycota may not change much in all controls and treatments.
Fusariumwas significantly reduced in the mulching treatment,at the level of the fungus genus,compared with CK.Fusariumis the main pathogen of blight (Mardiet al.,2002).Studies have shown that adding a thick layer of mulch to the surface of the soil helps to control weeds and optimize soil moisture to keep the soil cool,which helps to create adverse conditions for soil-borne pathogens and thus control disease (Bawa,2016).Studies have shown that soil sun exposure and two-layer mulch reduced the rate of fungal infection in plants (Garibaldi and Gullino,1991).In addition,we also found thatRemersoniaabundance increased significantly compared to CK.
Wanget al.(2014) has shown that gravel-sand mulch can reduce the temperature difference between day and night.In the semi-arid loess area of northwest China,after the gravel-sand mulched,the soil temperature at a depth of 10 cm is 0.5°-4.5° higher than that of the bare ground (Li,2003).Remersoniais considered to be a thermophilic fungi,so the abundance ofRemersoniaincreased significantly after gravel-sand mulch,which may be due to the warming effect of gravel-sand mulch(Morgensternet al.,2012).
4.4 Correlation of soil enzyme activity,microorganisms and environmental factors
In this study,through PCA results,soil bacterial and fungal communities under the mulch treatment were different from the bare land.This finding is consistent with previous studies where gravel-sand mulch altered the structure of soil bacterial and fungi communities(Lvet al.,2019).In addition,the diversity of bacteria and fungi (Chao,Shannon and ACE index)indicated that coverage reduces the α diversity of bacteria and fungi(Qiuet al.,2018).
Notably,RDA in this study shows a negative correlation between Proteobacteria and Acidobacteria and organic matter content.The RDA also shows that Acidobacteria was positively correlated with pH,which is consistent with previous studies.The results of Ryanet al.(2009)can explain the abundance of Acidobacteria,indicating that pH is closely related to the abundance of Acidobacteria bacteria.Previous studies have shown that non-metric multidimensional scale(NMS) ranking analysis and Pearson's product-moment correlation reveal a strong correlation between the investigated Actinobacteria community and various limnological parameters such as conductivity,total phosphorus,alkalinity or primary production(Martinet al.,2007).As in our RDA model,Actinobacteria was positively correlated with soil organic matter,alkaline nitrogen,and urease.In the study by Yinet al.(2019),sacchaarase activity was closely related to Basidiomycota and Chloroflexi.Chloroflexi has proven to be a denitrifying agent involved in the soil nitrogen cycle because they use NO2-or NO3-as electron acceptors(Yukiet al.,2007;Tomonoriet al.,2012).It is worth noting that Chloroflexi is positively correlated with soil organic matter,alkaline nitrogen and sacchaarase in the RDA model.
In addition,RDA also shows that the contribution rate of environmental factors to fungal community composition is relatively low.Mohammadet al.(2018) studied the global topsoil microbial community structure and function and found that environmental factors have a greater impact on bacterial groups than fungi.The study of Seatonet al.(2020) also shows that compared with bacteria,fungi are less susceptible to the physical and chemical properties of the habitat.The results of this research give us a better explanation of the relationship among microbial communities and soil properties under gravel-sand mulch.
In conclusion,long-term gravel-sand mulch reduced the diversity of bacteria and fungi and altered the structure of microbial communities.Long-term gravel-sand mulch led to the loss of soil nutrients and the reduction of soil enzyme activity is an important cause of soil degradation.RDA analysis also shows that changes in soil environmental factors significantly affected bacterial communities.After a long period of gravel mulch,organic fertilizer should be added to improve the soil microenvironment which is conducive to the sustainable use of farmland.
Acknowledgments:
This study was funded by the National Key R&D Program(Grant No.2016YFC0501403-3).
 Sciences in Cold and Arid Regions2021年6期
Sciences in Cold and Arid Regions2021年6期
- Sciences in Cold and Arid Regions的其它文章
- Cryogenic wedges on the NE Qinghai-Tibet and Ordos Plateaus:Their characteristics,origin and OSL dating
- High-precision measurements of the inter-annual evolution for Urumqi Glacier No.1 in eastern Tien Shan,China
- Editor-in-Chief Yuanming Lai
- Satellite-measured water vapor isotopologues across the Tianshan Mountains,central Asia
- Climate response and radial growth of Pinus tabulaeformis at different altitudes in Qilian Mountains
- Seasonal variation of airborne fungi of the Tiantishan Grottoes and Western Xia Museum,Wuwei,China
