Is dose modification or discontinuation of nilotinib necessary in nilotinib-induced hyperbilirubinemia?
You-Wen Tan
You-Wen Tan, Department of Hepatology, The Third Hospital of Zhenjiang Affiliated Jiangsu University, Zhenjiang 212003, Jiangsu Province, China
Abstract Nilotinib is a specific breakpoint cluster region-Abelson leukemia virus-tyrosine kinase inhibitor that is used as an effective first- or second-line treatment in imatinib-resistant chronic myelogenous leukemia (CML) patients.Hepatotoxicity due to nilotinib is a commonly reported side effect; however, abnormal liver function test (LFT) results have been reported in asymptomatic cases.When alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels are more than five-fold the upper limit of the normal (ULN) or when the serum total bilirubin level is more than three-fold the ULN, dose modification or discontinuation of nilotinib is recommended, resulting in decreased levels of hematological indicators in certain patients with CML.Nilotinib-induced hyperbilirubinemia typically manifests as indirect bilirubinemia without elevated ALT or AST levels.Such abnormal liver functioning is thus not attributed to the presence of a true histologic lesion of the liver.The underlying mechanism may be related to the inhibition of uridine diphosphate glucuronosyltransferase activity.Therefore, nilotinib dose adjustment is not recommended for this type of hyperbilirubinemia, and in the absence of elevated liver enzyme levels or presence of abnormal LFT findings, physicians should consider maintaining nilotinib dose intensity without modifications.
Key Words: Tyrosine kinase inhibitors; Nilotinib; Chronic myelogenous leukemia; Hyperbilirubinemia; Drug induced liver injure; Liver injury
INTRODUCTION
Chronic myelogenous leukemia (CML) originates in hematopoietic stem cells of malignant hyperplastic diseases and accounts for 15%-20% of all leukemia cases, with an incidence of 1-2/100000[1].CML is one of the most common types of leukemia and is marked by the presence of a breakpoint cluster region (BCR)-Abelson leukemia virus (ABL) on the (9, 22)(C34, C11) chromosome translocation fusion gene,i.e., the gene encoding P210-type fusion protein which exerts a type of tyrosine kinase activity.The subsequent abnormal clonal proliferation of myeloid hematopoietic cells is the primary cause of CML.
BCR-ABL tyrosine kinase plays an important role in cell differentiation, division, adhesion, and stress response, and its constitutive activation can lead to the development of leukemia.BCR-ABL is expressed in tumor cells of 95% of patients with CML, whereas it is not expressed in normal cells.Therefore, specific BCR-ABL tyrosine kinase inhibitors (TKIs) can be used to effectively treat CML[2].
TKIs are known to induce hepatotoxicity[3].A systematic study conducted on the basis of 12 previous studies demonstrated that TKIs are associated with a higher risk of hepatotoxicity, compared to placebos[4].Regarding hepatotoxicity of grade 3 or higher, the instructions recommend dosage reduction and discontinuation to allow recovery of liver functioning.The objective of this study was to confirm that the distinction between true hepatotoxicity and hyperbilirubinemia alone does not require dosage changes or discontinuation of treatment for hyperbilirubinemia caused by nilotinib alone.
PRESENT STATUS OF NILOTINIB TREATMENT
Nilotinib is a molecularly targeted kinase inhibitor that competitively binds to the platelet-derived growth factor C-kit and ABL kinases, thus it is effective for treating CML.A five-year follow-up survey showed that the 5-year cumulative rate of complete cytogenetic remission was 87%, the estimated 5-year survival rate was 89%, and the rate of progression-free survival was 93%[5].Up to 98% of the patients achieved hematological remission, and cytogenetic response was achieved in 86% of the patients at the chronic stage after treatment.After 1, 2, 3, and 4 years of continuous treatment, only 3.4%, 7.5%, 4.8%, and 1.5% of the patients, respectively, were expected to experience deterioration.Nilotinib is also considered an effective second-line treatment for imatinib-resistant cases[6,7].
HEPATOTOXICITY OF NILOTINIB
Common adverse reactions to nilotinib include dermatological, digestive, and cardiovascular side effects.Abnormal liver function is a common side effect in nilotinib-treated patients, and a considerable proportion of the patients show elevated levels of liver enzymes and bilirubin, based on analyses of phase II and III trials (Table 1).Up to 70% of the patients exhibit elevated levels of liver enzymes[8], and most patients are asymptomatic, presenting with grade 1-2 abnormal liver function.This typically occurs within 10-14 d of initial treatment, and only 4%-9% of the patients demonstrate a five-fold upper limit of normal (ULN) alanine aminotransferase (ALT) or aspartate aminotransferase (AST) activity, which can be improved by dosage adjustment and treatment discontinuation.
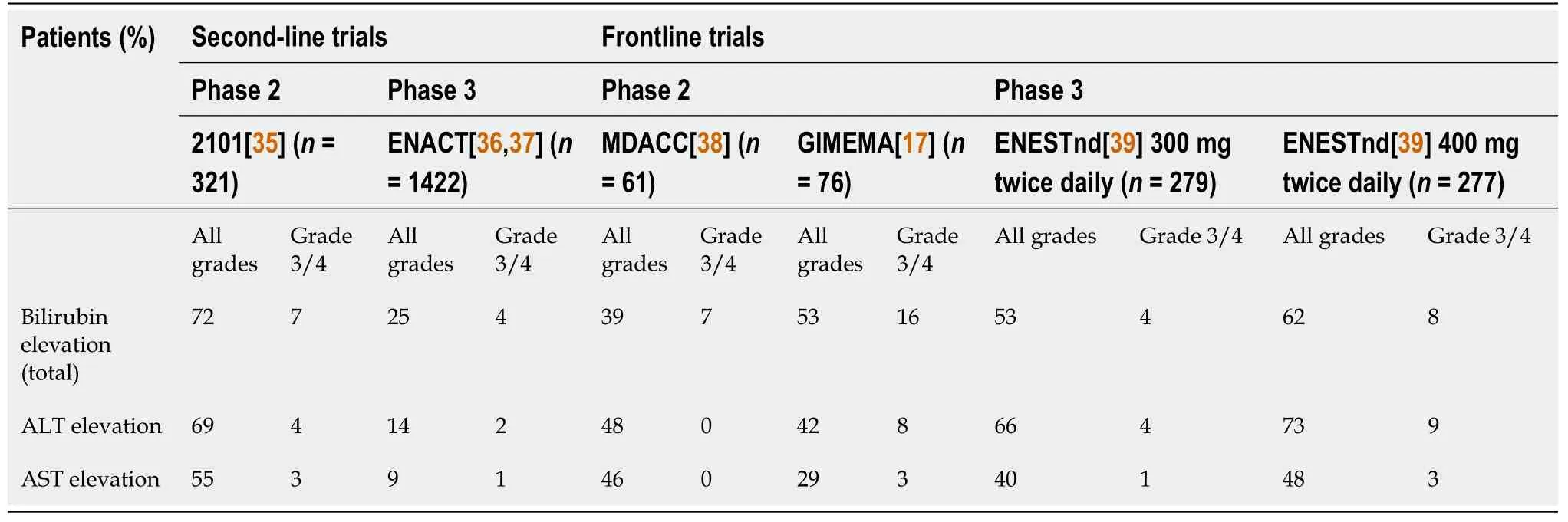
Table 1 Incidence of biochemical abnormalities across the second-line and frontline nilotinib trials
In a real-world study of nilotinib, a marked elevation of ALT and AST levels was not common, and the incidence of an increase to more than five-fold the ULN was 1%-4%.Concurrently, the manifestation of jaundice was mainly attributed to indirect bilirubin levels and self-remission, and it was attributed to Gilbert syndrome (GS)[9].However, in a nilotinib phase I and II study spanning 36 mo and conducted in Japan, 29% of the patients showed elevated bilirubin levels, 24% showed elevated ALT levels, but only 3% showed over five-fold the ULN[10].Severe liver failure caused by nilotinib treatment is rare.For example, a 34-year-old woman with CML developed progressive jaundice (bilirubin 14.5 mg/dL, ALT 1856 U/L, and ALP 254 U/L) after 8 mo of nilotinib treatment.She was subsequently diagnosed with liver failure and underwent liver transplantation.
COMPARISON OF HEPATOTOXICITY INDUCED BY NILOTINIB AND IMATINIB
Nilotinib is clinically used because of resistance to or side effects of imatinib treatments.In the ENESTnd trial, a phase 3 randomized trial including 846 patients[11], two different doses of nilotinib showed ALT activity rates of over five-fold the ULN at 4% and 9%, respectively, compared with 3% with imatinib.Only two patients showed relatively severe liver dysfunction (data not reported), indicating that the frequency of liver dysfunction due to nilotinib treatment was higher than that of imatinib.During the 5-year follow-up of the same group of patients, the rate of abnormal liver function was 50%-60%, but liver failure was implicated in none of the reported 50 cases of death[12].A 47-year-old woman with CML presented with liver function deterioration (bilirubin level 20 mg/dL, alanine aminotransferase level 828 U/L prothrombin time 24 s) during imatinib therapy administrated before liver transplantation, and the postoperative treatment with nilotinib did not result in liver damage[13].In a group of 88 imatinib-resistant CML patients who received nilotinib for 3 years, 14% showed elevated ALT and ALP levels.Interestingly, five patients with imatinib-induced hepatotoxicity did not exhibit any recurrence of nilotinib-induced hepatotoxicity[14].
THE MAIN SYMPTOM OF NILOTINIB-INDUCED HEPATOTOXICITY IS UNCONJUGATED HYPERBILIRUBINEMIA
Elevated levels of bilirubin are a common adverse drug reactions to nilotinib treatment, which occurs in 3%-16% of the patients, but constitutes no evident pathological significance[15].In a previous study, 119 patients with CML with imatinib resistance were treated with nilotinib; nine patients subsequently showed elevated levels of indirect bilirubin, and three showed elevated ALT levels.None of the increased parameters influenced the hematological remission effect[16].
In studies on nilotinib-induced hepatotoxicity, hyperbilirubinemia is the most common etiology of hepatotoxicity and is the main reason reducing or discontinuing nilotinib (Table 1).Hyperbilirubinemia is mainly characterized by the presence of unconjugated bilirubin.The initial median time of increase was 18 d, and the duration was approximately 8 d.A spontaneous decrease in bilirubin levels may occur without medication or phototherapy.
In a case reported by Corteset al[17], a 39-year-old man with CML was administered with low-dose (300 mg/d) nilotinib for continuous treatment in the frontline GIMEMA trial, and his bilirubin levels increased gradually, resulting in the development of grade-4 hyperbilirubinemia.Ten days after drug discontinuation, the hyperbilirubinemia was resolved.Moreover, the original dose of nilotinib was continued, and hyperbilirubinemia development was observed along with four recurrences.Bilirubinemia was maintained at grade 2-3, and notably, the patients demonstrated a complete molecular response 1 year after treatment.Therefore, hyperbilirubinemia is considered a benign condition[18].
NILOTINIB-INDUCED HYPERBILIRUBINEMIA IS ASSOCIATED WITH URIDINE DIPHOSPHATE GLUCURONOSYLTRANSFERASE (UGT1A1) ACTIVITY
UGT1A1 is a membrane protein that binds to the endoplasmic reticulum.It is an enzyme that plays a crucial role in phase II biotransformation of several endogenous and exogenous substances[19].TheUGT1A1gene is predominantly expressed in the human liver, but also in bile tissue, the large intestine, and the stomach, and it is responsible for bilirubin binding[20].UGT1A1 is a key enzyme involved in the metabolism of bilirubin.UGT1A1 expression is correlated with UGT1A1 activity, which leads to abnormal synthesis of unconjugated bilirubin in the blood and excretion of conjugated bilirubinin vitro[21], resulting in hyperbilirubinemia[22].Hyperbilirubinemia has been observed in patients treated with TKIs.A correlation between the inhibition of UGT1A1 activity and an increase in bilirubin level was observed in patients treated with TKI.The gene polymorphism ofUGT1A1is the main reason underlying the function of theUGT1A1gene[23].
The promoter region of theUGT1A1gene exhibits a certain polymorphism, and its atypical TATA box region contains 5-8 TA repeats.The most common genotypes included six TA repeats[24].UGT1A1expression decreases with increasing TA repeats.A variant ofUGT1A1containing an untypical TATA box region of theUGT1A1* 28 promoter contains seven TA repeats; it is associated with decreased UGT1A1 expression and frequently causes hyperbilirubinemia of unknown etiology[25].Furthermore, new variants have been reported, includingUGT1A1*6/*6, *6/*28, and *28/*28[26].
Nilotinib inhibited UGT1A1 activity in a dose-and time-dependent manner.Abumiyaet al[27] investigated 34 CML patients treated with low and high dosages of nilotinib, and hyperbilirubinemia was more common in patients treated with high dosages and over long periods.The proportion of patients harboringUGT1A1*6/*6 and *6/*28 genotypes (poor metabolizers) was also higher[27].This suggests that variations in theUGT1A1gene should be considered when administering nilotinib.
MECHANISM UNDERLYING NILOTINIB-INDUCED HYPERBILIRUBINEMIA
Nilotinib inhibits the activities of cytochrome P450 enzymes CYP3A4, CYP2C8, CYP2C9, CYP2D6, and UGT1A1[28].Fujitaet al[29] have proven nilotinib to be an effective non-competitive drug for human UGT1A1in vitro.In this study, SN-38 was used as a substrate, and human liver microsome and recombinant human UGT1A1 were used as enzyme sources to study the inhibitory effect of nilotinib on UGT1A1-catalyzed SN-38 glucuronidation.The results showed that nilotinib exerted a noncompetitive inhibitory effect on SN-38 glucuronization of human liver microsome UGT1A1 and recombinant human UGT1A1 with K (i) values of 0.286 ± 0.0094 and 0.079 ± 0.0029 μm, respectively.
UGT1A1 GENOTYPE DOES NOT AFFECT NILOTINIB EFFICACY AND SAFETY
TKIs are currently used for the treatment of CML.Nilotinib can increase the level of bilirubin by inhibiting UGT1A1 activity[30,31] In imatinib- and dasatinib-treated GS patients, elevated levels of bilirubin may occur; thus, it is not known whether such a condition of hyperbilirubinemia affects the efficacy and safety of nilotinib.We previously reported the case of a 24-year-old CML patient with unconjugated hyperbilirubinemia treated with nilotinib[32].Reduction and discontinuation of treatment can improve bilirubin levels immediately, but complete cytogenetic response (CCyR) worsens.CCyR can be achieved using a normal dose, but hyperbilirubinemia without ALT and other abnormal enzyme activities can be induced again.After conducting repeated trials four times, a normal dose of nilotinib was administered, and no pathological damage was observed in liver pathology.Although high bilirubin (grade 3-4) levels persisted, the patient achieved continuous CCyR.
In a 16-year retrospective study[33], long-term hematological toxicity and hepatocellular toxicity of imatinib, nilotinib, and dasatinib were observed.The effect of the GS genotype on progression-free survival was evaluated.One hundred and five patients with CML-CP who were consecutively treated with either first- or secondgeneration TKIs were evaluated.Gilbert's syndrome genotypes were distributed as follows: 17 (16.2%) patients with 7/7, 44 (41.9%) patients with 6/7, and the remaining cases with wild-type genotypes.The results showed that there was no difference in either the major molecular response or complete cytogenetic response observed among the different GS genotypes.Hyperbilirubinemia was observed in 26 patients, and grade 3/4 was observed in 9 patients.However, no true endothelial toxicity was observed in patients who continued to use TKIs.
Therefore, the European LeukemiaNet recommendations state that hyperbilirubinemia caused by TKIs is more common during nilotinib treatment and that this characteristic is the most frequently reported laboratory adverse event, rather than representing a true liver injury.This phenomenon is more common in the UGT1A1-mutated population and should be monitored closely, while dose adjustment has not been suggested[34].
This type of hyperbilirubinemia does not necessitate dose adjustment to achieve improvements, but bilirubin levels may spontaneously decline, a phenomenon which has been recognized by several scholars[18].
CONCLUSION
In conclusion, nilotinib is a commonly used drug in the treatment of CML, during which hyperbilirubinemia is commonly observed, and hyperbilirubinemia with indirect bilirubin elevation is not accompanied by abnormal hepatocyte enzymes.This abnormality is not real drug-induced liver damage, but the variation in UGT1A1 leads to inhibition of UGT1A1 activity, resulting in disorders of the bilirubin metabolism[34].Therefore, neither dose modification nor discontinuation is recommended for patients with hyperbilirubinemia at more than three-fold the ULN, and the benefits of maintaining the original dose to CML outweigh the disadvantages caused by dose reduction or treatment discontinuation.A different abnormality, mainly accompanied by elevated levels of liver enzymes, should follow the standard of reduction or drug discontinuation at more than three-fold the ULN.
 World Journal of Meta-Analysis2021年6期
World Journal of Meta-Analysis2021年6期
- World Journal of Meta-Analysis的其它文章
- Efficacy and safety of fingolimod in stroke: A systemic review and meta-analysis
- MicroRNAs as prognostic biomarkers for survival outcome in osteosarcoma: A meta-analysis
- Hydroxychloroquine alone or in combination with azithromycin and corrected QT prolongation in COVID-19 patients: A systematic review
- Prediabetes and cardiovascular complications study: Highlights on gestational diabetes, self-management and primary health care
- Newer developments in viral hepatitis: Looking beyond hepatotropic viruses
- Gastrointestinal tumors and infectious agents: A wide field to explore
