Efficacy and safety of fingolimod in stroke: A systemic review and meta-analysis
Kai Zhao, Yu Guo, Ming-Fei Yang, Qiang Zhang
Kai Zhao, Yu Guo, Graduate School, Qinghai University, Xining 810016, Qinghai Province, China
Ming-Fei Yang, Qiang Zhang, Department of Neurosurgery, Qinghai Provincial People's Hospital, Xining 810007, Qinghai Province, China
Abstract BACKGROUND Brain tissue injury in stroke patients involves inflammation around the infarction lesion or hematoma, which is an important reason for disease deterioration and can result in a poor prognosis.The meta-analysis of animal experiments has concluded that fingolimod could treat stroke in animal models by effectively reducing lymphocyte infiltration.However, no evidence-based efficacy and safety evaluation of fingolimod in stroke patients is currently available.AIM To determine whether fingolimod could promote reduction of infarction lesion or hematoma and improve neurological prognosis in stroke patients.METHODS Data extracted for treatment effect included count of T-lymphocytes with cluster of differentiation 8 expression (CD8+ T cells, × 106/mL), lesion volume (infarction or hematoma, mL), and modified Barthel indexes.Data extracted for safety was risk ratio (RR).Overall standard mean difference (SMD) with its 95% confidence interval (95%CI) and pooled effect with its 95%CI were calculated with a fixedeffects model.I-square (I2) was used to test the heterogeneity.Funnel plot symmetry and Egger's regression were used to evaluate publication bias.RESULTS Four high-quality randomized controlled trials were included.There was a significant difference in CD8+ T cell count (I2 = 0, overall SMD = -3.59, 95%CI: -4.37-2.80, P = 0.737) and modified Barthel index (I2 = 0, overall SMD = 2.42, 95%CI: 1.63-3.21, P = 0.290) between the fingolimod and control groups.However, there was no significant difference in lesion volume (I2 = 10.6%, overall SMD = -0.17, 95%CI: -0.75-0.42, P = 0.917), fever (pooled RR = 0.93, 95%CI: 0.97-2.32, P = 0.864), suspected lung infection (pooled RR = 0.90, 95%CI: 0.33-2.43, P = 0.876), or any adverse events occurring at least once (pooled RR = 0.82, 95%CI: 0.36-1.87, P = 0.995) between the fingolimod and control groups.There was no publication bias.All of the results were stable as revealed by sensitivity analysis.CONCLUSION Fingolimod improves neurological function in stroke patients without promotion of lesion absorption.Taking fingolimod orally (0.5 mg/d, 3 consecutive days) is safe except for patients with rare severe adverse events.
Key Words: Acute ischemic stroke; Intracerebral hemorrhage; Fingolimod; Meta-analysis; Treatment
INTRODUCTION
Stroke, which mainly consists of acute ischemic stroke (AIS) with cerebral infarction and hemorrhagic stroke with intracerebral hematoma (ICH), is a common neurological disease with a high mortality and disability rate.Especially, during the coronavirus disease 2019 pandemic, related coagulopathy might be associated with ischemic stroke, cerebral hemorrhage, and cerebral venous thrombosis, which aggravated the financial burden in the world[1,2].For severe stroke patients whose condition has deteriorated rapidly, surgical intervention should be performed, which is considered the best way to save lives[3].Thus, to reduce the risk of disease deterioration and promote the recovery of nervous system function, it is important to perform timely intervention at the early stage of stroke.
Fingolimod is a newly approved drug for the treatment of multiple sclerosis.It can be metabolized to the active metabolite of fingolimod phosphateviasphingosine kinase.Fingolimod phosphate has a high affinity for sphingosine-1-phosphate receptors 1, 3, 4, and 5[4].Sphingosine-1-phosphate and its receptors can regulate lymphocyte migrationviaupstream cell signaling pathways.Thus, fingolimod phosphate can block the ability of lymphocytes to enter or exit the lymph nodes, which can reduce the number of lymphocytes in peripheral blood[5].Although fingolimod can be used for the treatment of multiple sclerosis with unknown mechanism, its role as an immunomodulator might involve the reduction of the migration of lymphocytes to central nervous system[6].
Brain tissue injury in stroke patients involves inflammation around the infarction lesion or hematoma, which is an important reason for disease deterioration and can result in a poor prognosis[7].The meta-analysis of animal experiments has concluded that fingolimod could treat stroke in animal models by effectively reducing lymphocyte infiltration[8].However, evidence-based efficacy and safety evaluation of fingolimod in stroke patients is currently not available.We hypothesized that fingolimod could effectively and safely promote reduction of infarction lesion or hematoma and improve neurological prognosis in AIS or ICH patients by reducing lymphocyte infiltration.In this study, we performed a systemic review and metaanalysis of recent randomized controlled trials (RCTs) to confirm this hypothesis.
MATERIALS AND METHODS
Protocol used
This systemic review and meta-analysis were performed referring to the protocol published in the database of International Platform of Registered Systematic Review and Meta-analysis Protocols (INPLASY, https://inplasy.com/).
Literature search
A literature search was performed in the three public electronic databases: PubMed, Embase, and Cochrane.The strategy of literature search in PubMed was as follows: ("Fingolimod Hydrochloride"[Mesh]) OR (((((2-Amino-2-(2-(4-octylphenyl)ethyl)-1,3-propanediol hydrochloride[Title/Abstract]) OR (FTY?720[Title/Abstract])) OR (Gilenya[Title/Abstract])) OR (Gilenia[Title/Abstract])) OR (Fingolimod [Title/ Abstract])) AND (("Stroke"[Mesh]) OR (((((((((((((((((Stroke*[Title/Abstract]) OR (Cerebrovascular Accident*[Title/Abstract])) OR (CVA* [Title/Abstract])) OR (Cerebrovascular Apoplexy[Title/Abstract])) OR (Apoplexy, Cerebrovascular [Title/Abstract])) OR (Vascular Accident, Brain[Title/Abstract])) OR (Brain Vascular Accident*[Title/Abstract])) OR (Vascular Accidents, Brain[Title/Abstract])) OR (Cerebrovascular Stroke*[Title/Abstract])) OR (Stroke*, Cerebrovascular[Title/ Abstract])) OR (Apoplexy[Title/Abstract])) OR (Cerebral Stroke*[Title/Abstract])) OR (Stroke*, Cerebral[Title/Abstract])) OR (Stroke*, Acute[Title/Abstract])) OR (Acute Stroke*[Title/Abstract])) OR (Cerebrovascular Accident*, Acute[Title/Abstract])) OR (Acute Cerebrovascular Accident*[Title/Abstract]))).The strategy of literature search in Embase was as follows: (exp Stroke or Stroke$:ab,ti or 'Cerebrovascular Accident$':ab,ti or CVA$:ab,ti or 'Cerebrovascular Apoplexy':ab,ti or 'Apoplexy, Cerebrovascular':ab,ti or 'Vascular Accident, Brain':ab,ti or 'Brain Vascular Accident$':ab,ti or 'Vascular Accidents, Brain':ab,ti or 'Cerebrovascular Stroke$':ab,ti or 'Stroke$, Cerebrovascular':ab,ti or Apoplexy:ab,ti or 'Cerebral Stroke$':ab,ti or 'Stroke$, Cerebral':ab,ti or 'Stroke$, Acute':ab,ti or 'Acute Stroke$':ab,ti or 'Cerebrovascular Accident$, Acute':ab,ti or 'Acute Cerebrovascular Accident$':ab,ti) or (exp 'Cerebral Hemorrhage' or 'Hemorrhage$, Cerebrum':ab,ti or 'Cerebrum Hemorrhage$':ab,ti or 'Cerebral Parenchymal Hemorrhage$':ab,ti or 'Hemorrhage$, Cerebral Parenchymal':ab,ti or 'Parenchymal Hemorrhage$, Cerebral':ab,ti or 'Intracerebral Hemorrhage$':ab,ti or 'Hemorrhage$, Intracerebral':ab,ti or 'Hemorrhage$, Cerebral':ab,ti or 'Cerebral Hemorrhage$':ab,ti or 'Brain Hemorrhage$, Cerebral':ab,ti or 'Cerebral Brain Hemorrhage$':ab,ti or 'Hemorrhage$, Cerebral Brain':ab,ti).
Study selection
The articles involving the efficacy and safety of fingolimod in the treatment of stroke patients were screened.The inclusion criteria were: (1) Language and regions were not restricted; (2) Date of publication was up to December 31, 2020; (3) RCTs; (4) Patients suffered from AIS or ICH and the onset time was less than 6 h; (5) Fingolimod was used as the clinical intervention and the basic treatment methods were standard treatment according to the current American Heart Association guidelines; and (6) Outcomes involved assessment of immune cells, lesion volume, neurological function, and adverse events.The exclusion criteria were: (1) Duplicated publications; (2) Reviews, comments, letters, case reports, protocols of clinical trials, and conference papers; (3) Animal experiments; and (4) Articles with data irrelevant to this metaanalysis.
Data extraction
In consideration of the pharmacological action of fingolimod, absolute immunological cell counts (× 106/mL) in blood might be the most intuitive index.Especially, Tlymphocytes with cluster of differentiation 8 expression (CD8+T cells) play a key role in damaging the nervous system due to its cell killing effect, which could result in inflammatory edema[9].The edema around the infarction or hematoma could result in serious neurologic impairment in stroke patients[10,11].Thus, a rapid reduction of absolute infarction or hematoma volume might be the key of treatment in stroke patients.Lesion volume (mL) was defined as absolute infarction volume in AIS patients or absolute hematoma in ICH patients.The National Institute of Health stroke scale (NIHSS) is the specialized assessment to measure recovery of neurological function[12].The Modified Barthel index (mBI) is one of the earliest and the most commonly used methods for assessing ADL (activity of daily living) in rehabilitation medicine, which has high validity and reliability in assessment of stroke[13].Although risk ratio (RR) is often used as an effect size in cohort studies, it might be significant to assess the probability of adverse events like the number of patients to be harmed in clinical trials[14].In this study, the data extracted included CD8+T cell count, lesion volume (infarction or hematoma), NIHSS, mBI, and RR of safety.The data at time points near baseline, which might reflect condition of stroke at early treatment, were extracted.In addition, some confounders, which might result in errors, were adjusted, including characteristics of participation, period of treatment, and other factors.If the original data could not be acquired, other data were also extracted such as the difference between data at baseline and those at other time point.
Risk of bias
The quality assessment of included articles was performedviathe Cochrane Collaboration’s Tool of Assessing Risk of Bias using Review Manager 5.3 before data extraction.
Statistical analysis
We made the definition that the counts of studies selected or those with data extracted were positive events (+).The others were negative events (-).Kappa was calculated to test the agreement between Kai Zhao and Yu Guo.Kappa > 0.6 meant a high agreement.Zhang Q would make the final decision.Continuous variables with a normal distribution are expressed as the mean ± SD.Statistical difference of data before treatment in the intervention and control groups was tested by one-way analysis of variance (ANOVA) using SigmaStat version 4.0.If there was no statistical difference, data after treatment were directly used for meta-analysis.Relative numbers and their 95% confidence intervals (95%CI) are used to describe count data.Metaanalysis was performed using corresponding modules in Software for Statistics and Data Science (Stata, version 15.1; College Station, Texas 77845, United States).The overall standard mean difference (SMD) with its 95%CI and pooled effect with its 95%CI were calculated with a fixed-effects model.I-square (I2) was used to test the heterogeneity.Sensitivity analysis was performed to evaluate the stability of overall results by recalculating the overall SMD or pooled effect of the remaining studies after omitting the study with the highest quality or the fixed-effects model was switched to a random-effects model.Funnel plot symmetry and Egger's regression were used to evaluate publication bias.The methods to reduce heterogeneity and enhance sensitivity were the deletion of studies with publication bias or studies with the lowest quality and subgroup analysis.AllPvalues were two-sided with a significant level at 0.05.
RESULTS
Study selection and characteristics
Totally, 185 articles were retrieved from three databases according to the strategy.After screening according to the inclusion and exclusion criteria, four articles[15-18] reporting RCTs were enrolled ultimately (Figure 1).The Kappa of agreement of studies included by Kai Zhao and Yu Guo was 0.601 (P< 0.001; Table 1).One study emphasized the changes of inflammatory factors in patients with ICH after taking fingolimod orally[19].One study emphasized the role of rehabilitation nursing in AIS patients after taking fingolimod orally[20].The assessment of article qualityviaCochrane Collaboration’s Tool of Assessing Risk of Bias is shown in Figure 2.Three studies (Ying Fuet al[15], Ying Fuet al[16], and Zilong Zhuet al[17]) were evaluated as having insignificant risk bias in allocation concealment except for low risk in one study (De-Cai Tianet al[18]).There was no bias in performance, detection, attrition, or reporting.Other risk biases were insignificant in all studies.The articles finally included were published in 2014 to 2018 (Table 2).There were 67 stroke patients, including 56 AIS patients and 11 ICH patients, whose age ranged from 49 to 74 years.The period of oral fingolimod were 3 consecutive days after hospitalization.The oral dose was 0.5 mg/d.Conclusion with the same tendency was that fingolimod could effectively and safely treat AIS or ICH.

Table 1 Agreement of studies included by Zhao K and Guo Y
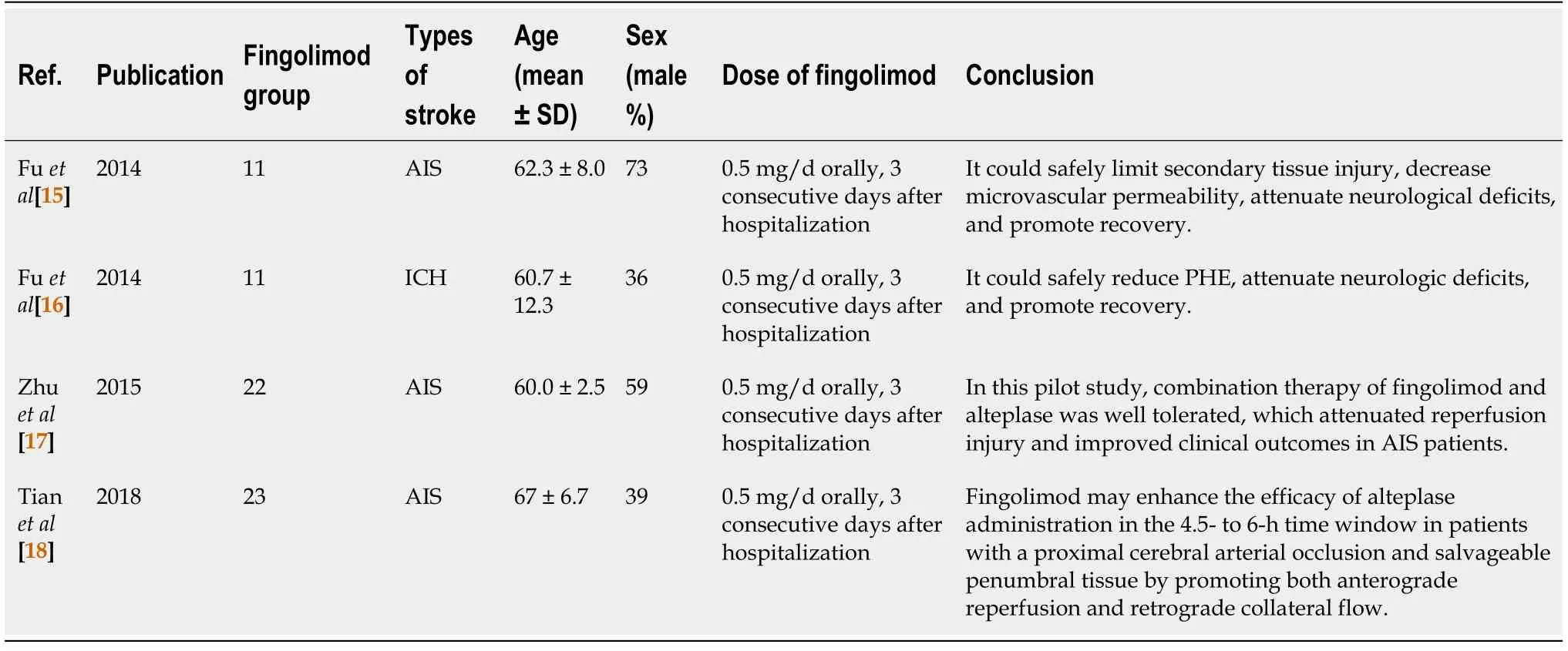
Table 2 Characteristics of studies used to perform the meta-analysis
Meta-analysis
Due to that some data were expressedviaonly one figure, some original data could not be acquired directly, which may result in missing of some data.The Kappa of agreement of data extraction between Zhao K and Guo Y was 0.634 (P< 0.001; Table 3).To acquire a more accurate result of meta-analysis, we tested the statistical difference of CD8+T cell counts at baseline and 1 day after taking fingolimod orally, lesion volume at baseline and 7 d after taking fingolimod orally, and mBI scores at baseline and 90 d after taking fingolimod orally between studiesviaANOVA.Unfortunately, there were significant statistical differences (P< 0.05) in all of continuous variables.Finally, we calculated difference between data at different time points manually to perform meta-analysis (Table 4).RRs and their 95%CIs of different adverse events, which consisted of fever, suspected lung infection, all adverse events occurring at least once, and other serious events involving hemorrhage of the digestive tract, cerebral hernia, bradycardia, atrial flutter, and thrombocytopenia, were calculated directly by referring to the count of adverse events occurring in patients of the fingolimod groups and control groups.Since no patients developed bradycardia, atrial flutter, or thrombocytopenia in the control groups, RRs of these adverse events were not calculated (Table 5).
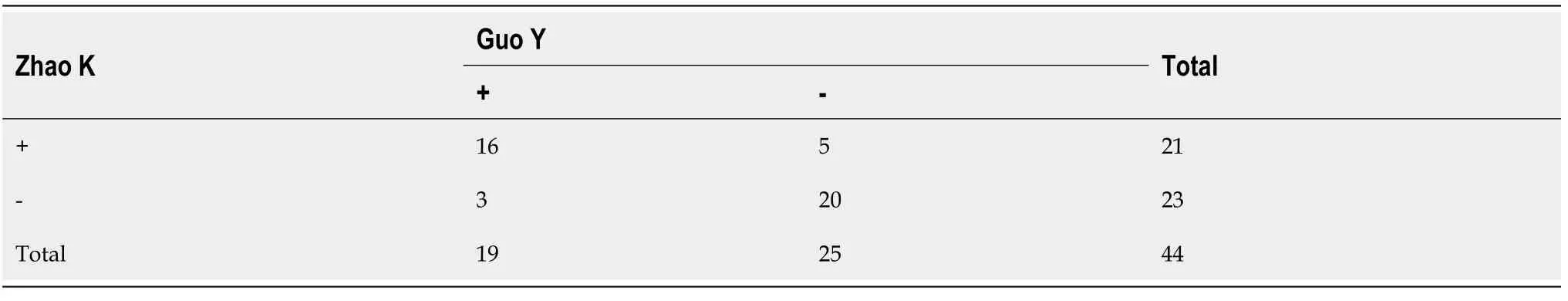
Table 3 Agreement of data extraction between Zhao K and Guo Y
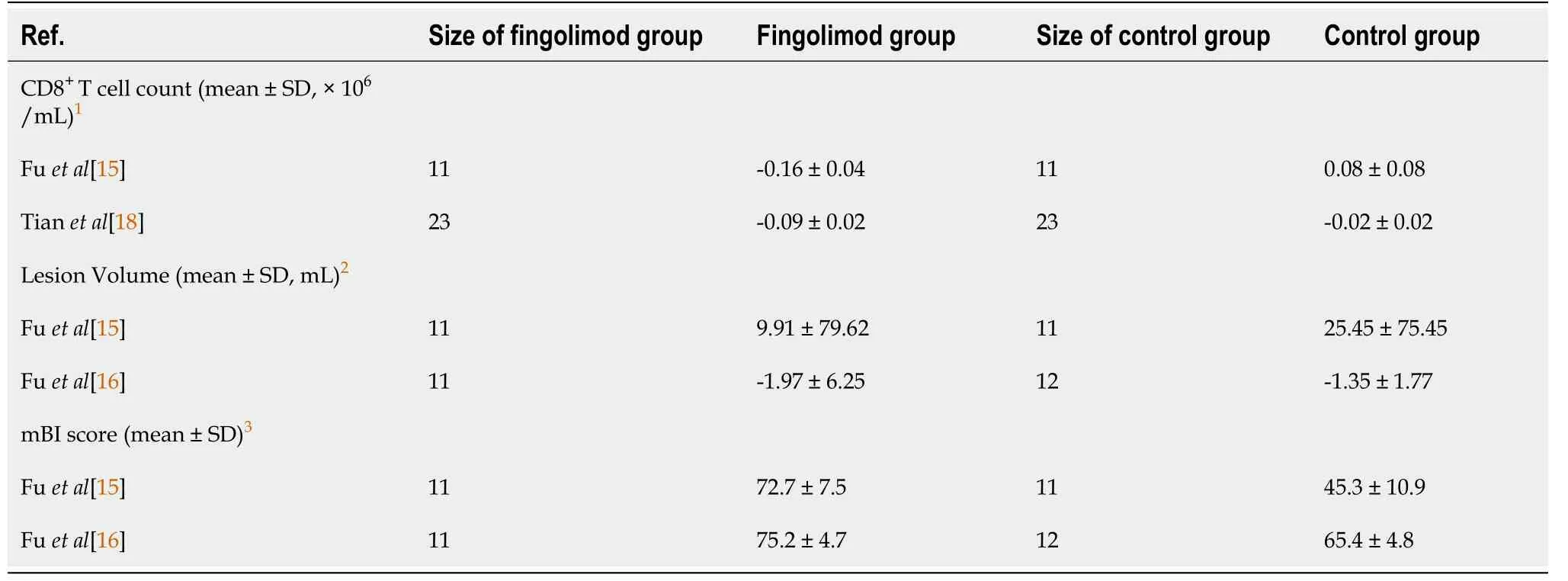
Table 4 Changes of T-lymphocytes with cluster of differentiation 8 expression/infarction or hematoma volume/modified Barthel index scores in the fingolimod and control groups
Analysis with a fixed-effects model showed that there was no heterogeneity (I2= 0, Figure 3) in the two studies used for comparing overall SMD difference in CD8+T cell counts and lesion volume except for two articles used for comparing overall SMD difference in mBI scores (I2= 10.6%, Figure 3).There was a significant difference in CD8+T cell counts (overall SMD = -3.59, 95%CI: -4.37--2.80,P= 0.737, Figure 3) and mBI (overall SMD = 2.42, 95%CI: 1.63-3.21,P= 0.290, Figure 3) between the fingolimod and control groups.However, there was no significant difference in lesion volume between the fingolimod and control groups (overall SMD = -0.17, 95%CI: -0.75-0.42,P= 0.917, Figure 3).Pooled RRs and their 95%CIs were calculated with a fixed-effects model.The RRs of fever (I2= 0, pooled RR = 0.93, 95%CI: 0.97-2.32,P= 0.864, Figure 4), suspected lung infection (I2= 0, pooled RR = 0.90, 95%CI: 0.33-2.43,P= 0.876, Figure 4), and all adverse events occurring at least once (I2= 0, pooled RR = 0.82, 95%CI: 0.36-1.87,P= 0.995, Figure 4) showed no significant difference between the fingolimod and control groups.Meta-analysis was not performed in RRs of other serious events because there was only one study reporting hemorrhage of the digestive tract or cerebral hernia and RRs of bradycardia, atrial flutter, and thrombocytopenia could not be calculated.
Publication bias and influence analysis
There was a symmetrical distribution in funnel plots of continuous variables (Figure 4A) and RRs (Figure 4B).Because only two studies were used to perform metaanalysis of treatment effect, we recalculated overall SMD of CD8+T cell count (I2= 0, overall SMD = -3.59, 95%CI: -4.37--2.80,P= 0.737, Table 6), lesion volume (I2= 0, overall SMD = -0.17, 95%CI: -0.75-0.42,P= 0.917, Table 6), and mBI scores (I2= 10.6%, overall SMD = 2.43, 95%CI: 1.59-3.26,P= 0.290, Table 6) after the fixed-effects model was switched to a random-effects model.Pooled RRs and their 95%CIs of fever (I2= 0, pooled RR = 0.93, 95%CI: 0.37-2.32,P= 0.723, Table 6), suspected lung infection (I2= 0, pooled RR = 0.90, 95%CI: 0.33-2.43,P= 0.723, Table 6), and all adverse events occurring at least once (I2= 0, pooled RR = 0.82, 95%CI: 0.36-1.87,P= 0.969, Table 6) were analyzed using the same method as above.In addition, for sensitivity analysis, the pooled effect of the remaining studies was recalculated after omitting the study with the highest quality because more than two studies were used to perform metaanalysis of treatment safety.We considered that the study might be assessed to have higher quality for its larger number of included patients in studies with the same assessment in Cochrane Collaboration’s Tool of Assessing Risk of Bias.Finally, after omitting the study with the highest quality, we recalculated pool RRs and their 95%CIs of fever (Zhuet al[17] omitted,I2= 0, pooled RR = 0.78, 95%CI: 0.23-2.68,P= 0.723, Table 6), suspected lung infection (Zhuet al[17] omitted,I2= 0, pooled RR = 0.78, 95%CI: 0.23-2.68,P= 0.723, Table 6), and all adverse events occurring at least once (I2= 0, pooled RR = 0.84, 95%CI: 0.33-2.18,P= 0.969, Table 6).

Table 5 Complications and adverse events in the fingolimod and control groups

Table 6 Overall standard mean difference or pooled risk ratio via random-effects model and pooled risk ratio of remaining studies after the study with the highest quality is omitted
DISCUSSION
There were many clinical application of fingolimod due to its original medicine indication of reducing the migration of lymphocytes to the central nervous system, such as Alzheimer's disease and Susac syndrome[21,22].Although the mechanisms of action of fingolimod in the treatment of AIS or ICH might be different, absorption of infarction lesion in AIS or hematoma in ICH both involved cytophagyviamicroglia in brain tissue or immunocyte in peripheral blood across injured brain-blood barrier[23,24].Edema around lesion has probable results of microcirculation obstruction, injured brain-blood barrier, and inflammatory response caused by lymphocyte infiltration in stroke patients, which may strongly affect recovery of neurological function and prognosis due to its space occupying and toxic effect.Thus, fingolimod might reduce secondary damage after strokeviaits pharmacological action.In our meta-analysis of RCTs, although we did not find that fingolimod could accelerate reduction of lesion volume (I2= 0, overall SMD = -0.17, 95%CI: -0.75-0.42,P= 0.917, Figure 3)viareducing lymphocyte counts in peripheral blood (I2= 0, overall SMD = -3.59, 95%CI: -4.37--2.80,P= 0.737, Figure 3), there was an effective improvement in ADL in stroke patients (I2= 10.6%, overall SMD = 2.42, 95%CI: 1.63-3.21,P= 0.290, Figure 3), which might be the evidence that fingolimod might cause reduction of secondary damageviareducing lymphocyte infiltration in the brain effectively.

Figure 1 Process of study screening.

Figure 2 Quality assessment for studies included.
With regard to safety of oral fingolimod in stroke patients, infectious diseases might be the main adverse events.Especially, patients with hemiplegic paralysis may be aggravated to severe hypostatic pneumonia.In our meta-analysis, fever (I2= 0, pooled RR = 0.93, 95%CI: 0.97-2.32,P= 0.864, Figure 4) and suspected lung infection (I2= 0, pooled RR = 0.90, 95%CI: 0.33-2.43,P= 0.876, Figure 4) were both considered as infectious diseases.We did not find that fingolimod might increase the risk of infection.Regarding other adverse events, there were some rare severe adverse events in the fingolimod group.Risk of cerebral hernia was not increased after the medication (RR = 0.35, 95%CI: 0.06-1.90, Table 3).The reason might be the large lesion volume at baseline.In the clinical trial by Tianet al[18], fingolimod was applied based on delayed alteplase administration.Hemorrhage of the digestive tract is definitely the potential risk during thrombolytic therapy.However, we found that the risk of digestive tract hemorrhage was not increased in the fingolimod group (RR = 0.42, 95%CI: 0.04-4.30, Table 3).Sphingosine-1-phosphate can regulate calcium ion movement in cells, which can affect myocardial contractility[25].Therefore, fingolimod-phosphate can bind to sphingosine-1-phosphate receptors, which might result in arhythmia, such as bradycardia and atrial flutter.Likewise, sphingosine-1-phosphate can be produced from platelets and participate in platelet activation, which might result in thrombocytopenia in the fingolimod group[26].Therefore, the application of fingolimod should be cautious in patients with a history of cardiac disease and platelet disorder.In general, according to pooled RR of adverse events occurring at least once (I2= 0, pooled RR = 0.82, 95%CI: 0.36-1.87,P= 0.995, Figure 4), fingolimod might be safe for stroke patients.
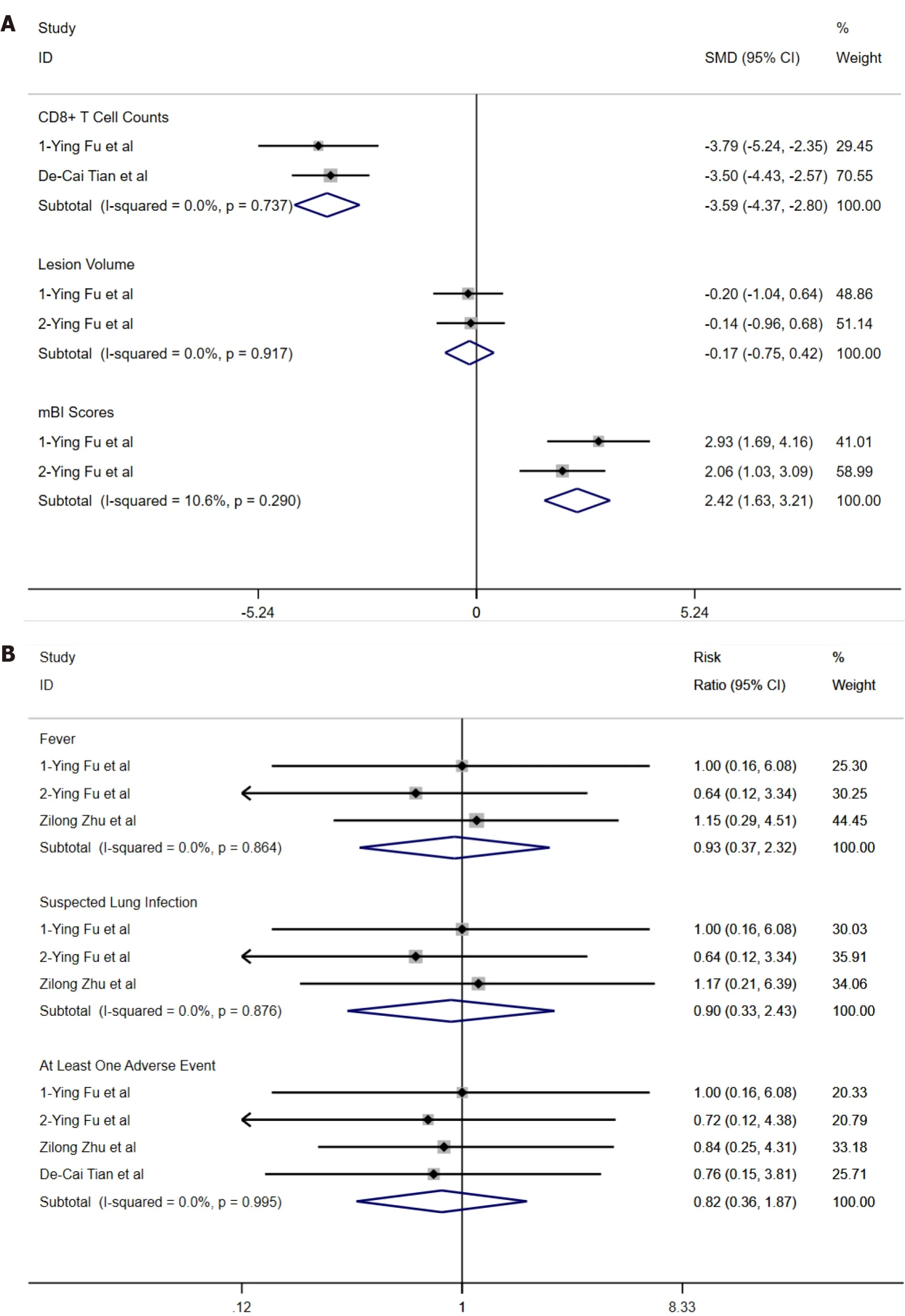
Figure 3 Forest plots of overall standard mean difference and pooled risk ratio.

Figure 4 Funnel plots of publication bias.
Only four RCTs were included in the meta-analysis finally.Furthermore, two of them were completed by the team led by the same professor.The studies included was insufficient to perform a meta-analysis with high quality due to an insufficient number of patients.Although publication bias was not found, the outcome might be influenced by tendentiousness of articles with the same first authors.As some results were only shown in figures in some articles, these results were not extracted, which may lead to missing data at other time points and some variables, such as counts of other types of lymphocytes, edema around lesion, and NIHSS scores.In addition, there was potential influence of non-original data because data of continuous variables used in metaanalysis were processedviamathematical transformation owing to statistical difference before meta-analysis.Although a few stroke patients used fingolimod as a therapeutic medication, we believe that our research would bring about a new direction of stroke medication.
CONCLUSION
Fingolimod might improve neurological function in stroke patients by reducing lymphocyte infiltration in the brain effectively; however, we did not find the evidence that it could promote infarction lesion or hematoma absorption.In general, oral fingolimod (0.5 mg/d, 3 consecutive days) is safe in stroke patients except for some rare severe adverse events.
ARTICLE HIGHLIGHTS
Research background
Brain tissue injury in stroke patients involves inflammation around the infarction lesion or hematoma, which is an important reason for disease deterioration and can result in a poor prognosis.The meta-analysis of animal experiments has concluded that fingolimod could treat stroke in animal models by effectively reducing lymphocyte infiltration.
Research motivation
The evidence-based of efficacy and safety evaluation of fingolimod in stroke patients is currently unavailable.
Research objectives
We hypothesized that fingolimod could effectively and safely promote reduction of infarction lesion or hematoma and improve neurological prognosis in AIS or ICH patients by reducing lymphocyte infiltration.
Research methods
In this study, we performed a systemic review and meta-analysis of recent randomized controlled trials to confirm the above hypothesis.
Research results
There was a significant difference in CD8+T cell count and modified Barthel index between the fingolimod and control groups.However, there was no significant difference in lesion volume, fever, suspected lung infection (pooled RR = 0.90, 95%CI: 0.33-2.43,P= 0.876), and all adverse events occurring at least once between the fingolimod and control groups.
Research conclusions
Fingolimod might improve neurological function in stroke patients by reducing lymphocyte infiltration in the brain effectively.Fingolimod might not promote infarction lesion or hematoma absorption.Oral fingolimod (0.5 mg/d, 3 consecutive days) is safe in stroke patients except for some rare severe adverse events.
Research perspectives
More high-quality randomized controlled studies involving more patients are needed to provide more clinical research evidence.
 World Journal of Meta-Analysis2021年6期
World Journal of Meta-Analysis2021年6期
- World Journal of Meta-Analysis的其它文章
- MicroRNAs as prognostic biomarkers for survival outcome in osteosarcoma: A meta-analysis
- Hydroxychloroquine alone or in combination with azithromycin and corrected QT prolongation in COVID-19 patients: A systematic review
- Prediabetes and cardiovascular complications study: Highlights on gestational diabetes, self-management and primary health care
- Newer developments in viral hepatitis: Looking beyond hepatotropic viruses
- Gastrointestinal tumors and infectious agents: A wide field to explore
- Preclinical safety, effectiveness evaluation, and screening of functional bacteria for fecal microbiota transplantation based on germ-free animals
