Corchorus olitorius aqueous extract attenuates quorum sensing-regulated virulence factor production and biofilm formation
Hanan M.Al-Yousef, Perwez Alam, Zakia Khanam, Musarat Amina, Wafaa H.B.Hassan
1Department of Pharmacognosy, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia
2University Malaysia Kelantan, Campus Jeli, 17600, Kelantan, Malaysia
3Department of Pharmacognosy, Faculty of Pharmacy, Zagazig University, 44519 Zagazig, Egypt
ABSTRACT
KEYWORDS: Corchorus olitorius L; Anti-quorum sensing activity;Pseudomonas aeruginosa; Chromobacterium violaceum; Virulence factors
1.Introduction
Corchorus
olitorius
(C
.olitorius
) L.(Family: Malvaceae), which is commonly known as “Saluyot,” is an edible leafy vegetable plant that is distributed in the tropical and subtropical regions of Asia and Africa.C
.olitorius
is considered as a valuable food and a major source of fiber[1].In English,C
.olitorius
is named as Jew’s mallow and Egyptian spinach asC
.olitorius
is a staple food among the Jewish and Egyptian communities since ancient times[2].The leaves ofC
.olitorius
are a rich source of iron, vitamins A, C, and E, calcium, protein, riboflavin, folate,niacin, thiamin, and dietary fibers[3].Various studies have reported the pharmacological properties ofC
.olitorius
, including anti-microbial,anti-fungal[4], anti-oxidant[5], anti-tumor[6], anti-inflammatory,analgesic[7], and anti-pathogenic properties.Additionally,C
.olitorius
can be used to treat gestational diabetes in pregnant women[8].Pseudomonas
aeruginosa
(P
.aeruginosa
) is associated with many infectious diseases, such as pneumonia, bacteremia, endocarditis,meningitis, brain abscess, otitis media, keratitis, osteomyelitis, and diarrhea[9].There are ongoing research efforts to develop strategies to control bacterial infection through anti-pathogenic agents, which exert anti-bacterial effects by inhibiting quorum sensing (QS), a bacterial communication process.The quorum communication system regulates thePseudomonas
virulence factors, such as protease,elastase, pyocyanin, alginate, biofilm formation, bacterial motility, and toxins[10].QS inP
.aeruginosa
is regulated byN
-acylated homoserine lactones (AHLs), which are signaling molecules.The concentration of these auto-inducers increases with the increase in bacterial population.These signaling molecules regulate bacterial pathogenicity[11].Therefore, the inhibition of QS can be an alternative strategy to manage bacterial virulence and anti-microbial resistance[12].This study aimed to investigate the quorum sensing inhibitory (QSI) effect ofC
.olitorius
L.stem aqueous fraction (COAF) using the reporterChromobacterium violaceum
(C
.violaceum
).Additionally, the anti-pathogenic potential of COAF, which exhibited QSI activity, against the virulence factors ofP
.aeruginosa
PAO1, including biofilm formation, pyocyanin, elastase production, and swarming motility was examined.2.Materials and methods
2.1.Plant material
The fresh stem ofC
.olitorius
was purchased from a supermarket in Riyadh, Saudi Arabia in June 2016.The plant was authenticated by Prof Mahmoud Abdul Aziz Mahmoud at the College of Food and Agricultural Sciences, King Saud University.A voucher specimen(HM37-2) was deposited at the department’s herbarium.The plant material was air-dried and powdered to obtain a coarse powder (165 g).2.2.Media, chemicals, and solvents
Luria-Bertani (LB) broth, LB agar, and tryptone were obtained from Lab M Limited (Lancashire, United Kingdom).Mueller-Hinton broth, Mueller-Hinton agar, Tryptone soya broth, and Tryptone soya agar were purchased from Oxoid (Hampshire, UK).Dimethyl sulfoxide (DMSO) was purchased from Sigma (St.Louis, USA).The analytical grade solvents, such as ethanol (95%), petroleum ether (boiling point 40–60 ℃), chloroform, methanol,n
-butanol, and ethyl acetate were purchased from BDH (UK), which were distilled before use.2.3.Extraction and fractionation
The air-dried and powdered stem ofC
.olitorius
was subjected to exhaustive extraction with 95% ethanol (2 L×3 L, each) in a Soxhlet apparatus for 12 h at room temperature (18 ℃).The pooled ethanol extracts were evaporated under vacuum to obtain 63.3 g of dark green residue.The residue was suspended in 1 L water/methanol (9:1) and subjected to successive extraction with petroleum ether (boiling point 40-60 ℃) (3 L×0.5 L), chloroform (3 L×0.5 L), ethyl acetate (3 L×0.5 L),n
-butanol (4 L×0.5 L).Each fraction was individually concentrated to obtain the petroleum ether(5.4 g), chloroform (6.2 g), ethyl acetate (9.5 mg),n
-butanol (8.5 g), and aqueous (19.0 g) fractions.The COAF rich in quantity and chemical components were re-dissolved in 2.5% DMSO and used for evaluating the QSI activity.DMSO at a concentration of 1.5% does not inhibit the growth of microorganisms[13].2.4.Qualitative phytochemical investigation
All prepared fractions were subjected to phytochemical screening in order to scrutinize their bioactive constituents.For this, 3-5 mg of each fraction was separately suspended in 1 mL of ethanol or distilled water in clean glass test tubes.
2
.4
.1
.Detection
of
flavonoids
A total of 1 mL of stock solution was taken in a test tube and a few drops of 5% of ethanolic KOH was added.The appearance of intense yellow in the test tube which completely turned colorless on the addition of a few drops of 5% HCl indicated the presence of flavonoids[14].
2
.4
.2
.Test
for
triterpenes
/steroids
The stock solution of plant fractions was treated with acetic anhydride (1 mL) and five drops of concentrated HSO.The formation of a bluish green ring and conversion of violet color into blue indicated the presence of terpenoids and steroids[15,16],respectively in the plant fraction.
2
.4
.3
.Detection
of
tannins
A total of 3 mL of sample solution of plant fraction was diluted with chloroform and 1 mL of acetic anhydride was added to this solution.Existence of tannins in the test samples was confirmed by the appearance of green color on addition of 1 mL of concentrated HSO[17].
2
.4
.4
.Test
for
coumarins
A volume of 3 mL of 2 mol/L NaOH solution was mixed with 2 mL of the test sample and appearance of yellow color indicated the existence of coumarin.Confirmation test was carried out by adding 5 mol/L HCl (1 mL) and formation of a colorless upper layer in the test solution is considered positive[18].
2
.4
.5
.Test
for
alkaloids
The stock solution of crude ethanolic extract of the plant was dissolved in a minimum solvent and applied to pre-coated TLC plates using toluene: ethyl acetate: diethylamine (70:20:10) as a solvent system.The detection of alkaloids was confirmed by the appearance of red or orange spots on TLC after spraying with Dragendroff’s reagent[19].
2
.4
.6
.Test
for
sesquiterpene
lactones
Picric acid (1%) dissolved in 10% of NaOH solution (Baljet reaction) was used for the detection of sesquiterpene lactones in the extracts.Equal volumes of reagents (1:1 ratio) were mixed and added to the 1 mL of stock solution.The color change of sodium picrate solution from yellow to orange-red indicated a positive reaction[20].
2
.4
.7
.Test
for
quinones
Equal volumes of stock solution and concentrated HSO(1:1 v/v)were mixed and the appearance of red color indicated the presence of quinones.
2
.4
.8
.Test
for
carbohydrates
A total of 5 mg of plant extract was dissolved in distilled water treated with 0.2% of anthrone reagent and a few drops of concentrated HSO.The appearance and dark green color indicated the presence of carbohydrate[21].
2
.4
.9
.Test
for
carboxyl
group
Ten drops of 10% NaHCOwere added in the solution and formation of CObubbles was considered as a positive reaction[22].
2
.4
.10
.Test
for
phenols
A few drops of 1% FeClwere added in the stock solution (1 mL) and the appearance of dark greenish-blue color indicated the presence of phenolic components[23].
2.5.Bacterial strains and growth conditions
The bacterial strains used in this study wereC
.violaceum
(CV026;a mini-Tn5 mutant ofC
.violaceum
31532 that cannot endogenously synthesize AHLs but responds to exogenous C4 and C6 AHLs) andP
.aeruginosa
(PAO1; C4 and 3-Oxo-C12 HSL producer)[24].All the bacterial strains were cultured in LB medium at 30 ℃ for 24 h.The culture medium used for CV026 was supplemented with hexanoyl homoserine lactone (C6-HSL; Sigma-Aldrich, St Louis, MO, USA)[25].2.6.Determination of minimum inhibitory concentration(MIC)
The MIC of COAF was determined by the broth micro-dilution method (CLSI).The COAF (1.0 g) was serially diluted 2-fold in Mueller-Hinton broth (300, 150, 75, 37.5, 18.5, 9.25, 3.375, and 3.125 mg/mL).Next, each dilution of COAF was added to the wells(100 µL/well) of a 96-well microtiter plate.Each dilution of COAF was incubated with 100 µL of PAO1 (1×10CFU/mL) suspension in Mueller-Hinton broth for 20 h at 37 ℃.The MIC of COAF was defined as the lowest concentration of the extract that visibly inhibited bacterial growth.Additionally, the effect of sub-MICs of COAF on the growth of PAO1 was determined.The absorbance of untreated and COAF-treated cultures were measured at 600 nm using a Biotek Spectrofluorimeter (Biotek, USA)[26].The sub-MICs of COAF were selected for the assessment of anti-virulence and antibiofilm activity in the test strain.
2.7.QSI effect of COAF on reporter strain
The QSI effect of COAF was estimated by agar well diffusion assay using CV026[24,25].The bacteria were cultured in LB broth for 24 h at 28 ℃.To prepare the LB agar plates (1.5% agar), 15 mL of medium was poured into each plate.Next,C
.violaceum
was inoculated (100 µL/plate) into the LB soft agar (0.5% agar) and poured on the solidified LB agar plates.The wells with a diameter of 10 mm were bored in the LB agar medium.Next, sub-MICs (100µL) of COAF were added to individual wells.The assay plates were incubated at 28 ℃ for 48 h.Inhibition of pigment production by the indicator strain around disk was considered as positive for QS interference.The inhibition of QS was calculated as follows: r2–r1(in mm); where r2 is the total growth inhibition (QSI) zone radius(TI) and r1 is the clear zone radius (GI).QSI zone with a diameter<10 mm was considered moderate activity, whereas that with a diameter >10 mm was considered a potent effect[13].2.8.Violacein inhibition assay
The effect of sub-MICs of COAF on violacein production was analyzed using CV026.Violacein was extracted from CV026 and quantified photometrically following the method of Blosser and Gray (2000)[27] with minor modifications proposed by Husainet al
.[28].Briefly, the COAF was subjected to qualitative analysis to evaluate its QSI potential against CV026.C
.violaceum
synthesizes the violetcolored pigment violacein in response to the QS signal moleculeN
-hexanoyl-L
-homoserine lactone (HHL) produced by the autoinducer synthase CviI.HHL binds to its receptor CviR, which induces the production of violacein.The overnight culture (10 µL) ofC
.violaceum
[adjusted to an optical density (OD) at 600 nm to 0.4] was added into wells of sterile microtiter plates containing 2 mL of LB broth.The culture was incubated with various concentrations of COAF (0.25-2 mg/mL) at 30 ℃ for 16 h.The culture (1 mL) was centrifuged at 13 000 rpm for 10 min to precipitate the insoluble violacein.The culture supernatant was discarded and 1 mL of DMSO was added to the pellet.The solution was vortexed vigorously for 30 s to completely solubilize violacein.The mixture was centrifuged at 13 000 rpm for 10 min to remove the cells.The violacein-containing supernatant (200 µL) was added to 96-well flat-bottom microplates(Polylab, India) in quadruplets.The absorbance of the solution was measured using a microplate reader (Thermo Scientific Multiskan Ex) at a wavelength of 585 nm.The inhibition of violacein production by COAF was measured as follows: % inhibition =[(OD– OD)/OD]×100; where ODand ODare the OD of control and treatment groups, respectively.2.9.Effect of COAF on QS-regulated virulence factors
The effect of sub-MICs of COAF on the virulence factors ofP
.aeruginosa
, such as LasB elastase, pyocyanin, swarming motility,and exopolysaccharide (EPS) production was assessed as described previously by Husainet
al
.[29,30].In the LasB elastolytic activity assay, the bacterial culture was treated with COAF for 16 h at 37 ℃.The treated and untreated culture supernatants (100 µL each)were incubated with 900 µL of elastin Congo red (ECR, Sigma,USA) buffer (100 mM Tris, 1 mM CaCl, pH 7.5) containing 20 mg of ECR at 37 ℃ in a shaker incubator for 3 h.The insoluble ECR was removed by centrifugation.The absorption of Congo red in the supernatant was measured at 495 nm.The LB medium without COAF was included as a negative control.In the pyocyanin production assay, the culture supernatant (5 mL) of PAO1 treated with or without sub-MICs of COAF was first extracted with chloroform (3 mL).Next, the mixture was extracted with 0.2 mol/L HCl (1 mL).The absorbance of the solution was measured at 520 nm.For assaying swarming motility, the test cultures (PAO1 and CV026) were point inoculated at the center of a medium (1% tryptone, 0.5% NaCl, and 0.3% agar) containing sub-MICs of COAF overnight.The diameter of the swarm area was measured.For estimating EPS production, PAO1 was cultured in the presence of sub-MICs of COAF.The culture was centrifuged to obtain the supernatant.The supernatant was filter sterilized and incubated with chilled absolute ethanol overnight to precipitate EPS at 4 ℃.2.10.Biofilm inhibition assay
The effect of COAF on biofilm formation was measured using the microtiter plate assay described by O’Toole and Kotler[31].Briefly,1% overnight culture (0.4 OD at 600 nm) of test pathogens was incubated with 1 mL of fresh LB medium containing sub-MICs of COAF.The bacteria were allowed to adhere and grow without agitation for 24 h at 30°℃.The medium containing the free-floating planktonic cells was removed.The biofilms were gently rinsed twice with sterile water.The surface-attached cells (biofilm) were stained with 200 µL of 0.1% crystal violet (CV) (Hi-media, Mumbai, India)solution for 15 min.The CV solution was discarded completely and 200 µL of 95% ethanol was added to solubilize CV from the stained cells.The biofilm biomass was then quantified by measuring the absorbance at 470 nm in a microplate reader (Thermo Scientific Multiskan Ex, India).
2.11.Statistical analysis
Growth and biofilm-inhibition data were analyzed by SigmaPlot 12.0 and Minitab (Minitab, Inc.) software version 14.0 for Windows.All the experiments were performed in triplicates.Result data were presented as mean ± standard deviation (SD) and differences were considered as significant atP
<0.05.One-way ANOVA was used to compare the effects of different fractions.The difference between control and test groups was analyzed using LSD test.3.Results
3.1.Phytochemical screening
Results of phytochemical investigation of different fractions ofC
.olitorius
are summarized in Table 1 and it showed that polyphenolic,flavonoids, triterpenes, tannins, coumarins, quinones, steroids,carbohydrates, and amino acids were the main chemical components.3.2.Assessment of MIC
Different fractions ofC
.olitorius
were prepared in petroleum ether,chloroform, ethyl acetate,n
-butanol, and aqueous and evaluated for QS modulatory activity against CV026 and PAO1.Out of all 5 fractions, aqueous fraction displayed dose-dependent anti-QS potential and was the most effective with inhibitory effect on the bacteria growth at 0.8 mg/mL.Pigment inhibition was also observed at higher concentrations, which is a determinant of anti-QS activity and accompanied by growth inhibition of CV026 and PAO1.Ethylacetate fraction showed anti-QS activity only at 2 000 mg/mL.Then
-butanol fraction inhibited pigments at 500 and 700 mg/mL and it also inhibited the growth of bacteria at a similar concentration.Petrolum ether and chloroform fractions showed no inhibition of QS at 200-1 800 mg/mL.Results of preliminary anti-QS activity screening by the agar well diffusion method showed that aqueous fraction had the highest activity and it was selected for further study.The MIC value of COAF against PAO1 was 2.4 mg/mL.The sub-MICs of COAF were selected for the assessment of anti-virulence and anti-biofilm activity in the test strain.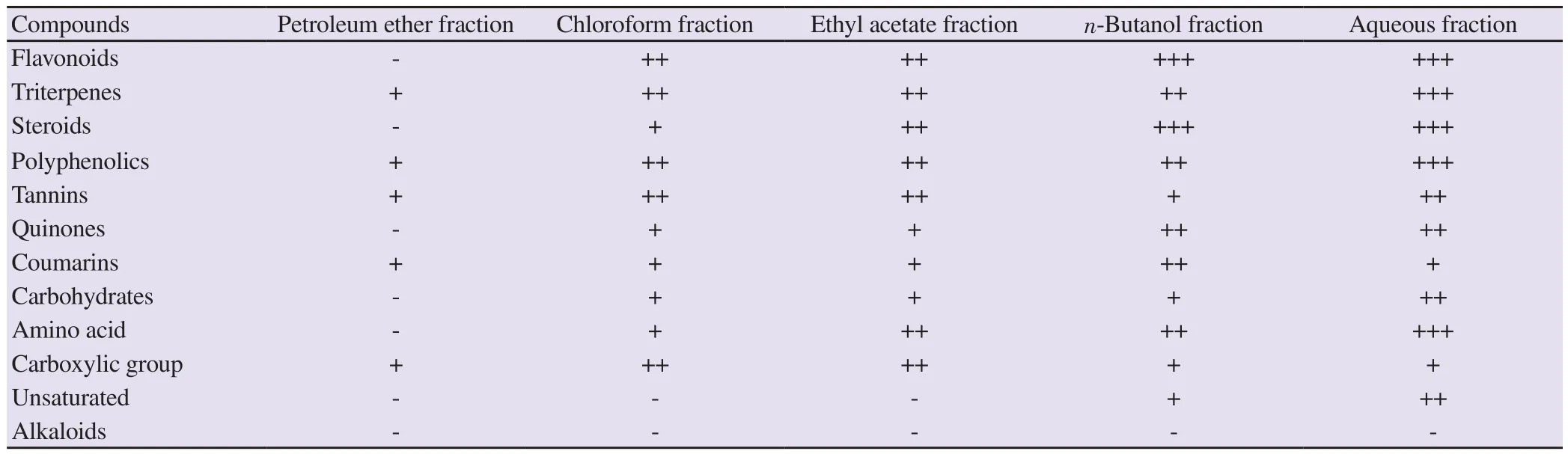
Table 1.Phytochemical screening results of different fractions of Corchorus olitorius.
3.3.QSI activity of COAF
The COAF exhibited a potent QSI effect at high doses of 800 and 1 000 µg/mL.In the violacein bioassay, treatment with COAF at doses of 800 and 1 000 µg/mL generated a white halo with a diameter of 15 and 11 mm, respectively (Table 2).The higher inhibitions of violacein production by the supplementation of COAF were observed at doses of 2 000 µg/mL and 1 000 µg/mL (Figure 1).COAF displayed dose-dependent interference of QS activity by reducing violacein production in CV026.Growth curve analysis showed that sub-MICs ofC
.olitorius
did not cause significant changes in the growth ofC
.violaceum
(CV026).Thus, COAF inhibited the production of violacein without affecting the growth of CV026.The effect of COAF on PAO1 was evaluated and the results showed the dose-dependent effect of COAF of the virulence factors in PAO1.3.4.Pyocyanin inhibition
The production of pyocyanin was observed inP
.aeruginosa
after 24-48 h of growth.The pyocyanin level in the COAF-treated PAO1 group was significantly lower than that in the untreated group in a concentration-dependent manner.The higher inhibition of pyocyanin production was observed at 2 000 µg/mL (P
<0.01) and 1 000 µg/mL(P
<0.05), followed by 500 and 250 µg/mL (Figure 2).3.5.LasB elastase inhibition
Treatment with COAF at sub-MICs significantly and dosedependently decreased the LasB elastase activity in the culture supernatant of PAO1(Figure 2).The lowest inhibition of LasB elastase activity was observed in COAF at the dose of 250 µg/mL,whereas the maximum inhibition was observed in COAF at 2 000 µg/mL (P
<0.05).3.6.EPS and swarming motility inhibition
The supplementation of 2 000 µg/mL of COAF reduced EPS production more significantly (P
<0.01) in the treated cultures of PAO1 compared to control.The COAF showed a reduction of 10% and 40% in EPS at 250 and 1 000 µg/mL, respectively.Similarly,COAF significantly reduced flagella-mediated swarming of PAO1 in a dose-dependent manner.The treatment of COAF significantly impaired swarming migration of PAO1 by 56% and 61% at 1 000 and 2 000 µg/mL, respectively (Figure 2).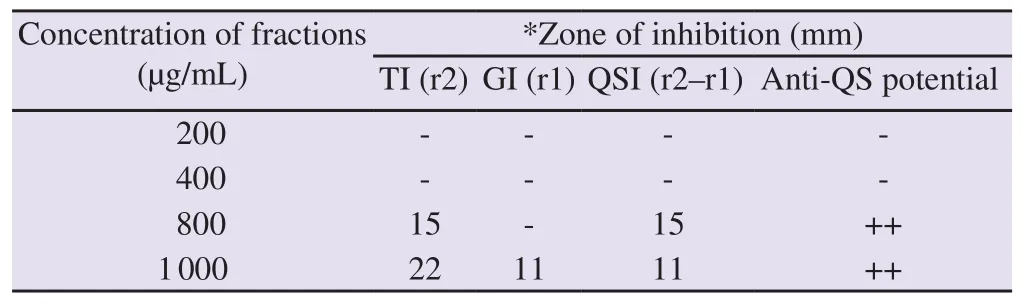
Table 2.Anti-QS activity of aqueous fraction of Corchorus olitorius at different concentrations.
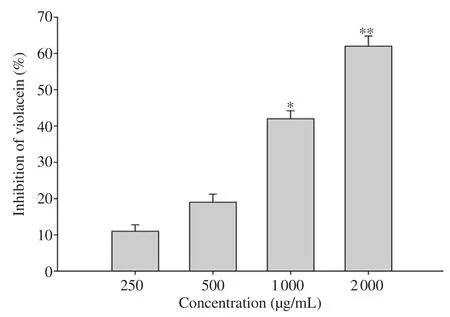
Figure 1.Effect of sub-minimum inhibitory concentrations of Corchorus olitorius aqueous fraction on the production of violacein.All data are presented as mean ± standard deviation.*P<0.05, ** P<0.01 compared with the untreated group.
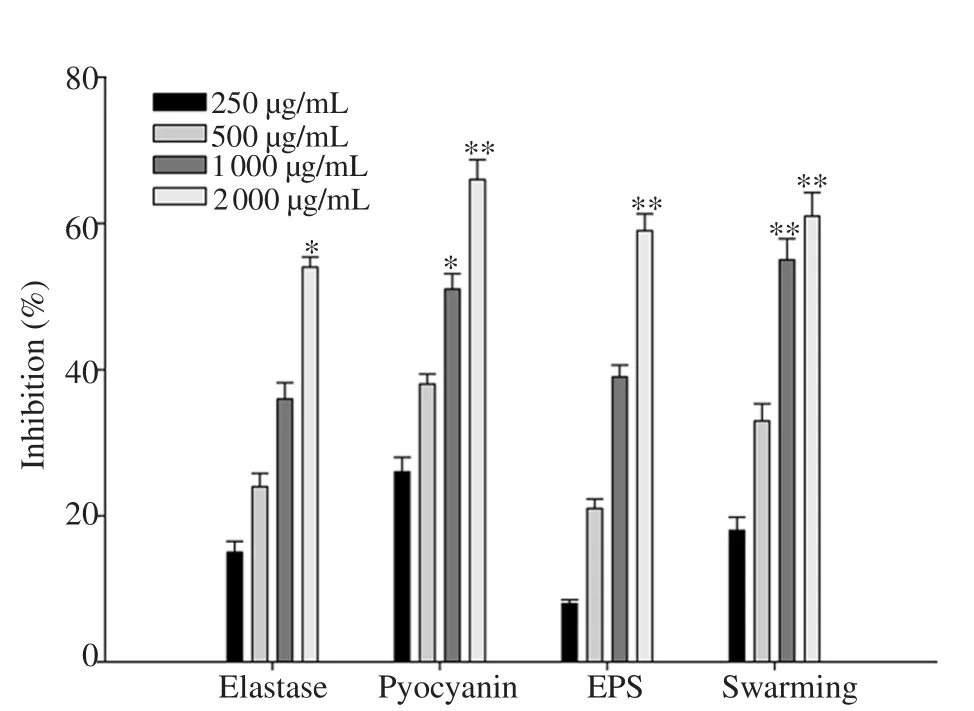
Figure 2.Effect of sub-minimum inhibitory concentrations of Corchorus olitorius aqueous fraction on quorum sensing-regulated virulence factors in Pseudomonas aeruginosa PAO1.All data are presented as mean ± standard deviation.*P<0.05, **P<0.01, compared with the untreated group.EPS:Exopolysaccharide.
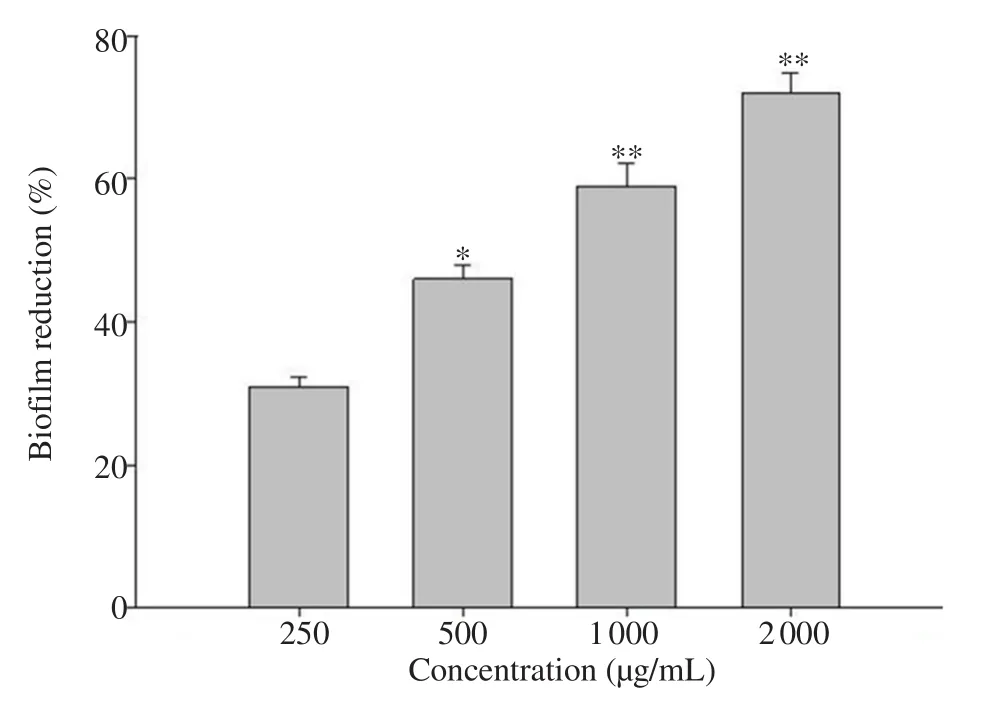
Figure 3.Effect of sub-minimum inhibitory concentrations of Corchorus olitorius aqueous fraction on biofilm formation by Pseudomonas aeruginosa PAO1.All data are presented as mean ± standard deviation.*P<0.05,**P<0.01, compared with the untreated group.
3.7.Effect of COAF on biofilm formation
The effect of COAF onPseudomonas
biofilm formation was evaluated using the tube assay method.Treatment with COAF at doses of 500 (P
<0.05), 1 000 (P
<0.01), and 2 000 (P
<0.01) µg/mL significantly decreased the biofilm biomass (Figure 3).This indicated that COAF could inhibit the biofilm formation of the target pathogens.4.Discussion
In several bacteria, virulence factors are controlled through QS, a cell density-dependent gene regulatory mechanism.Interference of QS circuits is considered as a potential strategy to attenuate bacterial pathogenicity.In the present study, ethanol extract ofC
.olitorius
was subjected to consequent fractionation with different organic solvents and evaluated for QS interference based on the reduction of violacein production inC
.violaceum
31532.Among all the fractions,the aqueous fraction showed the highest anti-QS potential.The aqueous fraction ofC
.olitorius
showed apparent inhibition of AHL-dependent pigment production, suggesting the interference of QS-regulated function.Further, interference of QS by the aqueous fraction is also evident as it reduced the violacein production in CV026 without affecting the growth ofC
.violaceum
.A study conducted onCuminum
cyminum
and clove oil has shown the same effect on violacein production[32,33].The QS inhibitor screening was carried out by usingC
.violaceum
which showed the appearance of purple-colored pigmentation (violacein) in the indicator organism.The analysis of inhibition of pigment production in CV026 is a rapid and simple method for screening QSI activity.Thus, based on the reduction in violaceum production and lack of growth inhibition of indicator organism CV026, the aqueous fraction was found to be the most active and was further tested againstP
.aeruginosa
PAO1 to evaluate wide spectrum anti-QS potential because this organism involves a different QS system.P
.aeruginosa
produces a number of QS-regulated factors such as elastase, protease, and chitinase[34].The hydrolytic enzymes (elastase and protease) of bacteria affect the proteins of host cells in the infected tissues and facilitate bacterial invasion and growth.In the current study, preincubation with the plant fraction resulted in dose-dependent inhibition of protease and elastase production.These results were in agreement with the literature where elastase, proteolytic, and chitinase activities ofP
.aeruginosa
were lowered after treatment with the extracts, fractions,and essential oils of plant origin by 2%-80%[35].A significant concentration-dependent decrease (P
<0.01) was noticed with COAF inC
.violaceum
(CV026) andP
.aeruginosa
(PAO1) strains, being higher in CV026 (62%).The COAF potently inhibited violacein production in the dose range of 800-1 000 µg/mL.Treatment with COAF at doses of 2 000 and 1 000 µg/mL inhibited violacein production by 62% and 42%, respectively.However,there was only 10%-20% of inhibition in violacein production by supplementations of COAF at 250 and 500 µg/mL.Similar inhibition of violacein production was recorded in extracts ofCapparis
spinosa
andCuminum
cyminum
[32,36].Another crucial virulence factor produced by QS-regulation is pyocyanin.It plays an important role in pathogenesis mainly in cystic fibrosis[37].In the current study,we evaluated the effect of COAF on the production of pyocyanin in PAO1.Treatment with COAF significantly decreased pyocyanin level in the PAO1 culture when compared to that in the untreated cultures.Similar decreases in pyocyanin levels were recorded in different extracts ofTerminalia
chebula
[38].In addition, treatment with COAF concentration-dependently inhibited elastase activity in PAO1.A significant inhibition of 55% (P
<0.05) in elastase activity was observed at 2 000 µg/mL.QS mediated by AHLs plays an important role in the formation of biofilm.The results of our study showed that the COAF at sub-MICs significantly inhibited the biofilm biomass (P
<0.01)without affecting the growth of PAO1.A study reported that surface conditioning promotes the surface adhesion and formation of microcolonies[39].Our study revealed that treatment of COAF efficiently reduced the biofilm biomass formed by the bacterial strain.The possible interference in biofilm formation could be due to reducing surface adhesion or subsequent step of biofilm formation.The extract/fraction which has the ability to inhibit the QS is expected to affect the biofilm-forming capacity[40].The results of our study are in accordance with activity of extracts ofRhodomyrtus tomentosa
[41],Lagerstroemia
speciosa
[42], andSclerocarya
birrea
[43].Factors involved in biofilm formation ofP
.aeruginosa
such as EPS production and swarming motility were assessed in this study.Production of EPS is an essential condition required for biofilm maturation[44], a QS-regulated feature[45].Therefore, QS interference by plant extracts led to a reduction in EPS production[46].Our study also showed that treatment with 2 000 µg/mL COAF reduced EPS production and impaired swarming migration more significantly(P
<0.01).It has shown that flagella–driven motility is needed for surface attachment initiation in biofilm development.Therefore,flagellar synthesis inhibition by the plant extracts/fraction would assist the reduced swarming migration.Thus, this COAF might have indirectly influenced biofilm formation by bacterial strains at least in part by interfering AHL-mediated QS-system.Our results are in support of earlier studies conducted on various plant extracts, which demonstrated that several plant extracts have the ability to inhibit the biofilm formation by PAO1[40].Furthermore, this study indicated the presence of flavonoids,triterpenes, tannins, coumarins, quinones, carbohydrates, polyphenolic,and steroids in fraction that cause microorganism death by inhibiting DNA or RNA synthesis and flavonoids possibly involved in inhibition of extracellular microbial enzymes[47].
In conclusion, the irrational use of antibiotics has led to the emergence of antibiotic-resistant bacteria, which are a major threat to public health.The resistance to antibiotics is mediated by QS in the pathogenic bacteria.Thus, there is a need to develop novel inhibitors of QS, especially from natural sources.This study investigated the QSI activity of COAF.The effect of COAF on violacein pigment production in theC
.violaceum
biosensor system was evaluated.Treatment with COAF inhibited violacein production.This study demonstrated that the COAF inhibited biofilm formation, attenuated virulence factors, and inhibited swarming motility inP
.aeruginosa
.These results suggest that the COAF can be used to inhibit QS,biofilm formation, and virulence factors in pathogenic bacteria.Conflict of interest statement
We declare that there is no conflict of interest.
Funding
This research project was supported by a grant from the “Research Center of the Female Scientific and Medical Colleges”, Deanship of Scientific Research, King Saud University.
Authors’ contributions
HMAY and MA designed this research, carried out the extraction and fractionation of plant sample, WHBH and ZK performed the biological experiment, PA carried out statistical analysis.All authors revised and approved the paper.
 Asian Pacific Journal of Tropical Biomedicine2021年2期
Asian Pacific Journal of Tropical Biomedicine2021年2期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Anti-inflammatory, anti-oxidative and anti-apoptotic effects of Heracleum persicum L.extract on rats with gentamicin-induced nephrotoxicity
- Screening of phytocompounds, molecular docking studies, and in vivo antiinflammatory activity of heartwood aqueous extract of Pterocarpus santalinus L.f.
- Anti-senescence and anti-wrinkle activities of 3-bromo-4,5-dihydroxybenzaldehyde from Polysiphonia morrowii Harvey in human dermal fibroblasts
- Borassus flabellifer L.crude male flower extracts alleviate cisplatin-induced oxidative stress in rat kidney cells
- 9-Hydroxy-6,7-dimethoxydalbergiquinol suppresses hydrogen peroxide-induced senescence in human dermal fibroblasts through induction of sirtuin-1 expression
