Screening of phytocompounds, molecular docking studies, and in vivo antiinflammatory activity of heartwood aqueous extract of Pterocarpus santalinus L.f.
Shanti Vasudevan C.N., Bibu John Kariyil, Athira Nair D., I’ma Neerakkal
1Department of Botany, Sacred Heart College, Thevara, Kochi 682013, Kerala, India
2Department of Veterinary Pharmacology & Toxicology, College of Veterinary and Animal Sciences, Pookode 673576, Wayanad, Kerala, India
ABSTRACT
KEYWORDS: Phloridzin; COX-1; COX-2; PGES-1; 5-LOX
1.Introduction
The body produces inflammation as a defense mechanism to protect from harmful stimuli.It forms an important part of the body’s immune system[1].Pyrexia is produced as a result of various biochemical reactions in response to infectious or inflammatory stimuli[2].The most common anti-inflammatory and antipyretic drugs (NSAIDs) inhibit the cyclooxygenase enzymes isoforms cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2) to varying degrees[3] and thereby stop or decrease the formation of the principal fever mediator, PEG-2.COX inhibition can cause various health complications like gastrointestinal bleeding, nephrotoxicity,and cardiovascular side effects[4].Reye’s syndrome (liver damage)may be caused due to long term usage of ibuprofen[5].The strategy of third-generation NSAIDs includes development of dual cycloxygenase–lipoxygenase and prostaglandin synthase inhibitors[6].
Flavonoids show anti-inflammatory effects due to their potential for inhibiting proinflammatory enzymes like COX-1, COX-2, and 5-lipoxygenase (5-LOX)[7].Several plants are used for treating inflammation[8] and fever since ancient times[9].Hence investigating the anti-inflammatory and antipyretic potential of such plants can prove to be useful in finding natural substitutes for synthetic drugs with less toxicity and side effects.
Pterocarpus
santalinus
(P
.santalinus
) L.f.(Fabaceae), commonly known as ‘red sanders’, was listed as endangered by the IUCN but reclassified as near threatened[10].It is an endemic species to southeastern mountain range of South India.In the traditional system of Ayurvedic medicine, the decoction prepared from the heartwood ofP
.santalinus
has various medicinal properties.Ayurvedic textMadanapala
Nighantu
suggestsjwaraghna
(antipyretic) property for the heartwood ofP
.santalinus
.It is reported to have antipyretic,anti-inflammatory, antihelminthic, anti-hemorrhoidal, antidysenteric,aphrodisiac and diaphoretic activities[11].Phytochemical studies on the bark ofP
.santalinus
have identified the presence of isoflavone, isoflavone glucosides, aurone glycosides,lignans, yellow or orange pigments, and terpenoids.The heartwood contains isoflavone glucosides and two antitumour lignans,viz
.,savinin and calocedrin[12].Little information is available on the phytochemical and pharmacological studies of aqueous extract of heartwood ofP
.santalinus
.In this context, this study was aimed to identify the phytochemical components in the aqueous extract ofP
.santalinus
heartwood by high-performance liquid chromatography-mass spectrometry (HPLC-MS) and to investigate the possible mechanism behind traditionally known anti-inflammatory and antipyretic action using molecular docking experiments.The binding affinity of the major component detected in the extract was evaluated with COX-1,COX-2, prostaglandin E synthase-1 (PGES-1) and 5-LOX.To validate the anti-inflammatory potential of the aqueous extract ofP
.santalinus
heartwood, anin
vivo
experiment was carried out using carageenan-induced paw edema model in Wistar Albino male rats.2.Materials and methods
2.1.Plant material collection and extraction
The heartwood samples ofP
.santalinus
were collected, identified,authenticated, and submitted at Kerala Forest Research Institute,Peechi, Kerala (A voucher specimen - Accession No:16686).The plant sample was washed, dried under shade and ground to a fine powder in an electric blender.The aqueous extract was prepared in distilled water using a Soxhlet apparatus by continuous heat application.The extract was further concentrated in a rotary evaporator (Hanvapor HS-2005V, Hahnshin Scientific) and lyophilized.2.2.HPLC-MS analysis of aqueous extract of P.santalinus heartwood
To identify the phytocomponents present in the aqueous extract ofP
.santalinus
heartwood, high-resolution LC-Q-ToF-MS analysis was carried out at Sophisticated Analytical Instrument Facility,IIT Bombay, India.The analysis was performed using Agilent Technologies, USA, Model:1290 Infinity nano HPLC (Binary) with Chipcube (Microfluidic column), 6550 iFunnel Q-TOFs.The solvent system was used as follows: solvent A: water + 0.1% formic acid and solvent B: 90% acetonitrile + 10% water + 0.1% acetonitrile; a gradient started with 95% solvent A and ended with 100% solvent B.The flow rate was maintained at 0.3mL/min and injection volume was 5 µL.The column used was hypersil GOLD column (C18 100 mm × 2.1 mm, 3-micron).The sample was run in both positive and negative ionization modes from 140m
/z
to 1 000m
/z
.The analysis was performed using the following tuning parameters: gas temperature (250 ℃), gas flow (13 L/min), nebulizer (35 psig), and nozzle voltage (1 000 V).Total LC running time was 30 min.The MS spectra of the analyzed sample was searched against the Metlin database to find the probable compounds present in the sample.2.3.Molecular docking studies
Ibuprofen and paracetamol, widely used standards for inflammation and pyrexia respectively, were docked into the binding pockets of COX-1, COX-2, PGES-1 and 5-LOX.The most abundant compound with known anti-inflammatory and antipyretic effect in the aqueous extract ofP
.santalinus
heartwood was also docked with the same enzymes to compare its binding affinity with the standards used.Molecular docking simulations were carried out using AutodockVina[13].Crystal structures of COX-1 (PDB ID 2OYE), COX-2 (PDB ID 3LN1), PGES-1 (PDB ID3DWW), and 5-LOX (PDB ID3V99) were obtained from PDB and the structures of ibuprofen, paracetamol and phloridzin were downloaded from PubChem database.The predicted docked poses of ibuprofen,paracetamol and phloridzin against COX-1, COX-2, PGES-1, and 5-LOX were analysed in Discovery studio visualizer.2.4.In vivo experiment
The anti-inflammatory activity of the extract was tested using carageenan-induced rat paw edema model as described previously with slight modifications[14].A total of twenty-four Wistar Albino male rats weighing 100-200 g were used in this study.Rats were randomly divided into four groups of six rats each.GroupⅠ,GroupⅡ, Group Ⅲ, and Group Ⅳ received distilled water (control),ibuprofen (30 mg/kg BWp
.o
.), 200 and 400 mg/kg BWp
.o
.of the aqueous extract ofP
.santalinus
heartwood, respectively.After 1 h, 0.1 mL of 1% (w/v) carageenan was injected in the subplantar tissue of the right hind paw of each rat.The thickness of hind paw was measured at 1-hour interval for 4 h using vernier callipers.The percentage inhibition (PI) was calculated according to the below equation[14].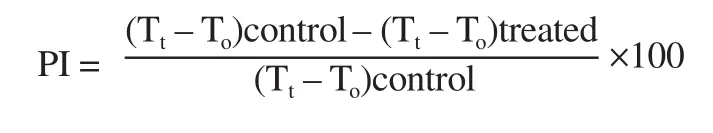
where T= mean paw thickness at 0 h; T= mean paw thickness at a particular time interval.
2.5.Statistical analysis
Measurements of paw thickness were expressed as mean ±standard error of the mean (SEM).Statistical analysis of results was performed by one-way analysis of variance (ANOVA) followed by multiple Tukey’s comparison tests.P
<0.05 was considered statistically significant.2.6.Ethical statement
The experiment was performed in accordance with the CPCSEA norms and approved by the Institute of Animal Ethical Committee of the College of Veterinary and Animal Sciences, Thrissur, Kerala,India where the study was conducted [Approval Order No.Acad(3)/6554/04 of Kerala Veterinary & Animal Science University, dated 27/09/18].
3.Results
3.1.Secondary metabolites identified from the aqueous extract of P.santalinus heartwood
The compounds identified using HPLC-MS analysis are presented in Table 1.Phloridzin was detected both in positive and negative ionization mode.In negative ionization mode, compound 3,phloridzin, at retention time 4.775, showed the highest intensity(Figure 1).Its MS spectra showed the molecular ion peak –ve ESI−m
/z
435.128 and the fragment ionsm
/z
435, 190, 221, 315 (Figure 2).3.2.Molecular docking
Binding energy scores and amino acid interactions for ibuprofen,paracetamol, and phloridzin docked to COX-1, COX-2, PGES-1,and 5-LOX are shown in Table 2.Two-dimensional binding site interaction models are shown in Figure 3.Phloridzin had a higher affinity with COX-1, COX-2, PGES-1, and 5-LOX than the standard drugs ibuprofen and paracetamol.For phloridzin, the highest binding energy score of -8.7 kcal/mol was observed with COX-2.In addition,ibuprofen showed a better affinity with COX-1, COX-2, and PGES-1 than paracetamol.
3.3.Anti-inflammatory activity
Anti-inflammatory effect of the aqueous extract ofP
.santalinus
heartwood on carageenan-induced paw edema is presented in Table 3.The PI values obtained for the standard drug ibuprofen at a dose of 30 mg/kg BW from 1 h to 4 h were 45.5%, 68.5%, 82.4% and91.8%, respectively.The PI values obtained for the aqueous extract ofP
.santalinus
heartwood at the same time interval at doses of 200 and 400 mg/kg BW were 20.3%, 25.8%, 35.1%, 41.8% and 27.6%,38.9%, 45.6%, 53.5%, respectively.Thus, the aqueous extract ofP
.santalinus
heartwood at doses of 200 and 400 mg/kg BW inhibited paw edema in a dose-dependent manner.But the effect was slow when compared to the standard drug ibuprofen.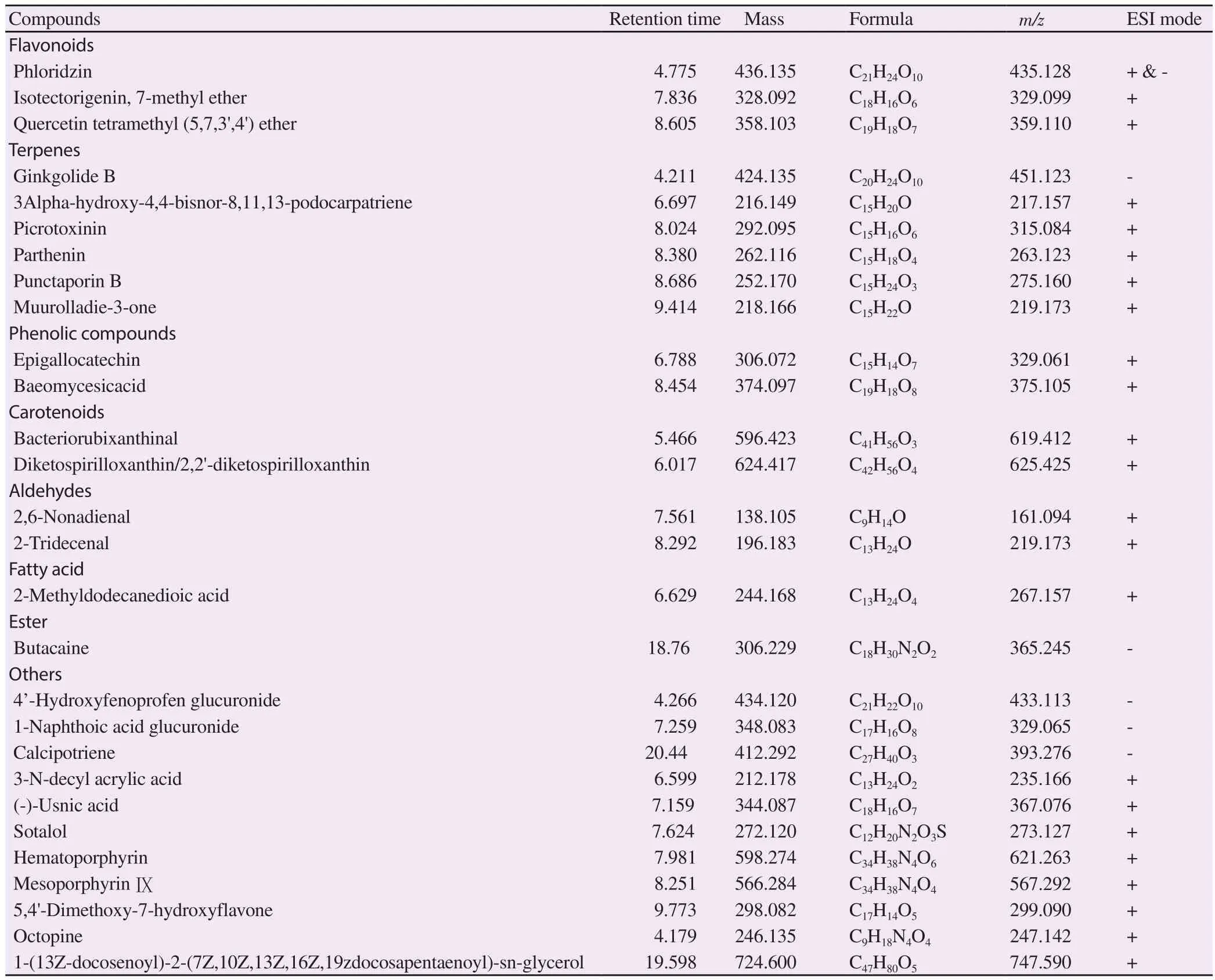
Table 1.Secondary metabolites identified from the aqueous extract of Pterocarpus santalinus heartwood and their retention time, m/z value and elemental composition.
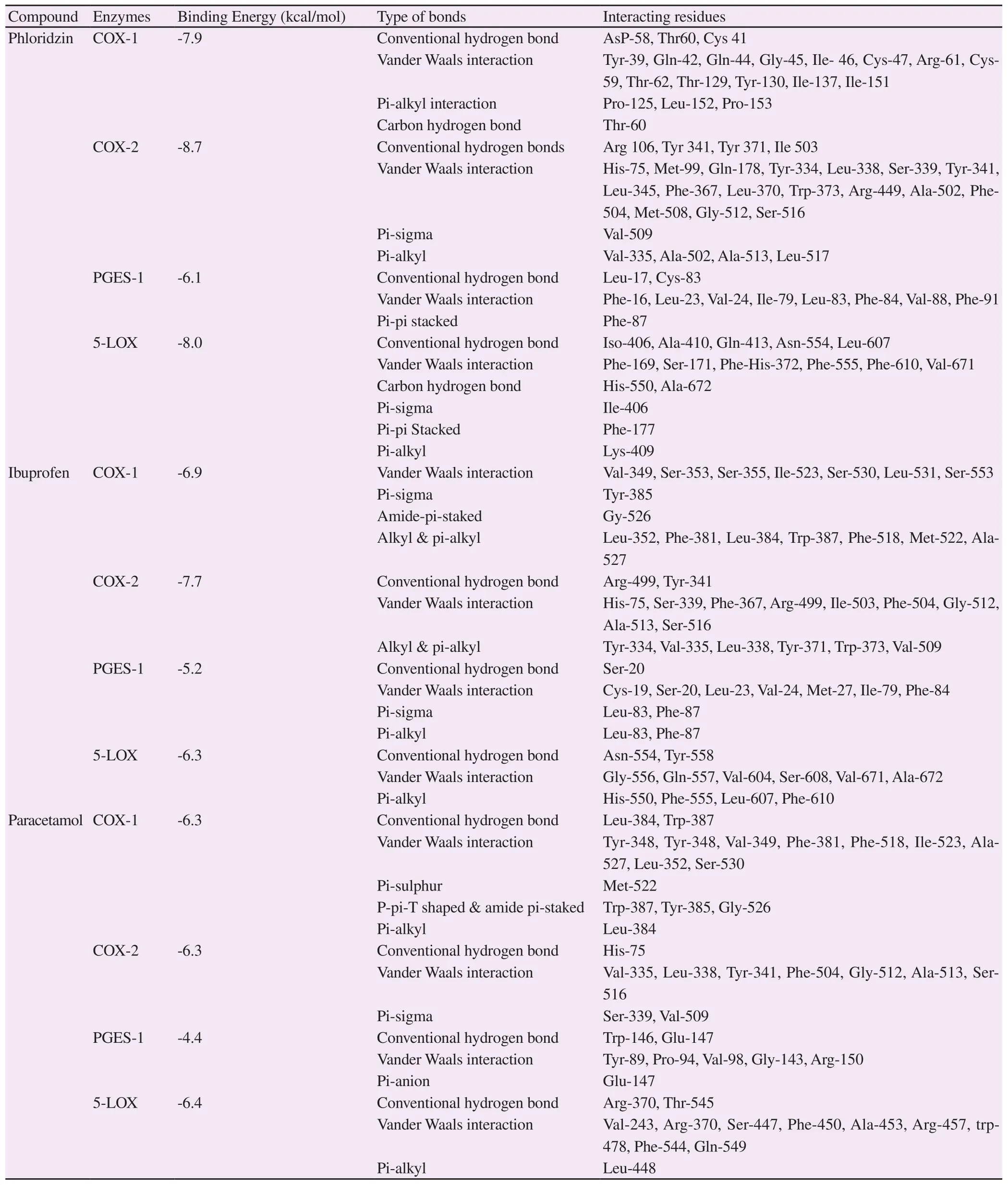
Table 2.Binding energy and amino acid interactions for ibuprofen, paracetamol, and phloridzin docked to selected targets.
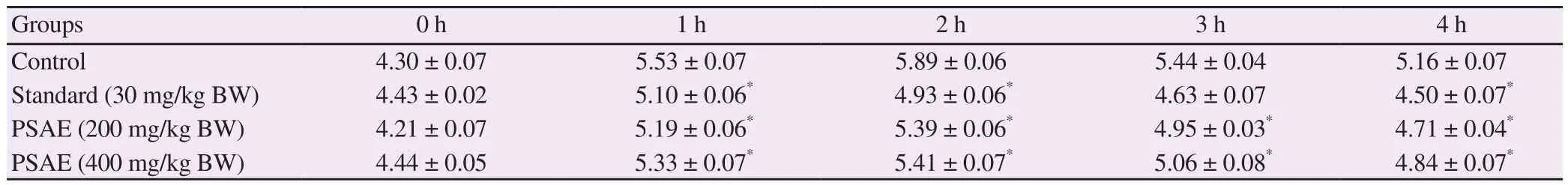
Table 3.Effect of aqueous extract of Pterocarpus santalinus heartwood on carrageenan-induced paw edema in rats.
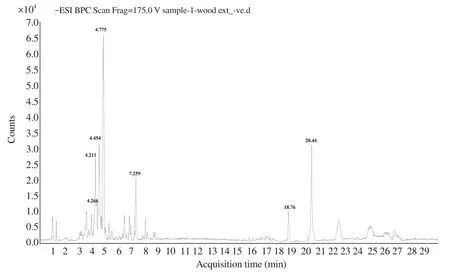
Figure 1.HRLC-ve ESI-MS-MS chromatogram of the aqueous extract of Pterocarpus santalinus heartwood.
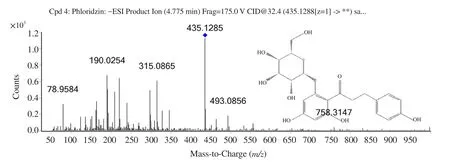
Figure 2.MS/MS spectra and spectrum peak list of phloridzin (-ve ESI ) with m/z 435.128.
4.Discussion
In the present study, active constituents of the aqueous extract ofP
.santalinus
heartwood were tentatively identified and the metabolite responsible for producing the anti-inflammatory and antipyretic effect was predicted.Flavonoids, terpenoid derivatives,phenolic compounds, and carotenoids prevailed among the extract.Quercetin tetramethyl (5,7,3’,4’) ether, epigallocatechin and 5,4’-dimethoxy-7-hydroxyflavone identified in the aqueous extract were in conformity with the earlier reports of similar compounds in the genusPterocarpus
[15-17].Among the compounds detected, phloridzin is reported to have anti-inflammatory[18] and antipyretic[19] activity.Phloridzin is a febrifuge like salicin.It was reported by De Koenick and Stass in the root bark of apple, pear, cherry and plum tree[20].Mode of action of phloridzin as an antimalarial agent is reportedin
vitro
cultures ofPlasmodium
falciparum
[21].Anti-inflammatory action of phloridzin by decreasing the synthesis of PGEand IL-8 is also reported[22].Phloridzin possesses structural features that interact with COX-1,COX-2, PGES-1 and 5-LOX indicating that it can be a potent inhibitor of these enzymes.The presence of phloridzin in the aqueous extract ofP
.santalinus
heartwood may be responsible for the antipyretic effect shown by the extract as reported in our previousin
vivo
study[23].In
vivo
study indicated that the aqueous extract ofP
.santalinus
heartwood showed a dose-dependent anti-inflammatory activity, but less effective than ibuprofen.The anti-inflammatory activity may be due to phloridzin content in the extract.Phloridzin showed more negative binding energyin
silico
, probably indicating the inhibition of COX-1, COX-2, and 5-LOX.Phloridzin showed more affinity with enzymes than the standards.However, the extract showed lower anti-inflammatory activity than the standards, which may be due to the synergistic effect of other compounds in the crude extract or lower concentration of phloridzin in the extract administered.The present molecular docking study supported the earlier reports of phloridzin being used against fever and inflammation.Its role in inhibiting cytokines responsible for inflammation process is also reported[24].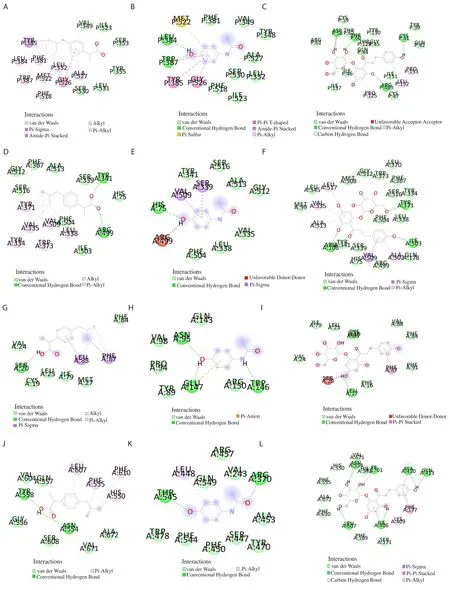
Figure 3.Two-dimensional binding site interaction models.(A) ibuprofen with COX-1; (B) paracetamol with COX-1; (C) phloridzin with COX-1; (D)ibuprofen with COX-2; (E) paracetamol with COX-2; (F) phloridzin with COX-2; (G) ibuprofen with PGES-1; (H) paracetamol with PGES-1; (I) phloridzin with PGES-1; (J) ibuprofen with 5-LOX; (K) paracetamol with 5-LOX; (L) phloridzin with 5-LOX.COX-1 and COX-2: cyclooxygenase-1 and 2, PGES-1:prostaglandin E synthase-1, 5-LOX: 5-lipoxygenase.
The study revealed the presence of several metabolites in the aqueous extract ofP
.santalinus
heartwood.Molecular docking showed that phloridzin inhibits COX-1, COX-2, PGES-1 and 5-LOX with more affinity than ibuprofen and paracetamol.In
vivo
experiment proved anti-inflammatory effect of the extract.More studies, especially clinical trials, are needed to further confirm anti-inflammatory activity of the aqueous extract ofP
.santalinus
heartwood.Conflict of interest statement
The authors declare that there is no conflict of interest.
Acknowledgments
The corresponding author acknowledges UGC, Bangalore for granting Teacher Fellowship during the research work.Authors thank Sophisticated Analytical Instrument Facility, IIT Bombay for HRLCMS/MS service provided.The lab facility and animal house provided by the Dept.of Veterinary Pharmacology & Toxicology,College of Veterinary and Animal Sciences, Mannuthy, Thrissur,Kerala, India is duly acknowledged.
Authors’ contributions
SVCN carried out the experiments and wrote the entire manuscript.BJK supervised the animal study experiment.AND carried out the molecular docking experiment.IN gave overall direction and helped in interpreting the results.All the authors provided critical feedback.
 Asian Pacific Journal of Tropical Biomedicine2021年2期
Asian Pacific Journal of Tropical Biomedicine2021年2期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Anti-inflammatory, anti-oxidative and anti-apoptotic effects of Heracleum persicum L.extract on rats with gentamicin-induced nephrotoxicity
- Anti-senescence and anti-wrinkle activities of 3-bromo-4,5-dihydroxybenzaldehyde from Polysiphonia morrowii Harvey in human dermal fibroblasts
- Borassus flabellifer L.crude male flower extracts alleviate cisplatin-induced oxidative stress in rat kidney cells
- Corchorus olitorius aqueous extract attenuates quorum sensing-regulated virulence factor production and biofilm formation
- 9-Hydroxy-6,7-dimethoxydalbergiquinol suppresses hydrogen peroxide-induced senescence in human dermal fibroblasts through induction of sirtuin-1 expression
