9-Hydroxy-6,7-dimethoxydalbergiquinol suppresses hydrogen peroxide-induced senescence in human dermal fibroblasts through induction of sirtuin-1 expression
Seok-Hee Lim, Bing Si Li, Ri Zhe Zhu, Byung-Min Choi
Department of Biochemistry, School of Medicine, Institute of Biotechnology, Wonkwang University, 460 Iksandae-ro, Iksan, Jeonbuk 54538, Republic of Korea
ABSTRACT
KEYWORDS: 9-Hydroxy-6,7-dimethoxydalbergiquinol;Hydrogen peroxide; Senescence; Sirtuin-1; Human dermal fibroblasts
1.Introduction
Cellular aging, known as senescence, is a state of irreversible cellcycle arrest characterized by a specific set of altered cell behaviors.The gradual accumulation of senescent cells contributes to tissue dysfunction and the aged phenotype.Cellular senescence can be induced by a wide variety of factors, and the age-related accumulation of reactive oxygen species (ROS) is a forceful modulator of oxidative damage and inflammation[1].Cells contain many antioxidants to minimize fluctuations in ROS; however, once the ROS generation exceeds the antioxidant ability of the cells, the excess ROS induces oxidative stress[1].Previous studies have shown that hydrogen peroxide (HO) is a potential candidate for efficiently mimicking the oxidative stress that occurs in the aging population[2,3].Experimental evidence has proved that a sublethal concentration of HOcan induce premature senescence in different types of cells[2,4,5].
Recently, sirtuin-1 (SIRT1) has attracted the most attention.Scientific studies have found that SIRT1, the human orthologue of yeast Sir2,can regulate multiple important signaling pathways, including DNA repair, apoptosis, mitochondrial biogenesis, cell stress responses, and inflammation[6]; extend the lifespan, and alleviate senescencein
vivo
andin
vitro
[7-10].For instance, SIRT1 overexpression significantly decreases the level of acetylated p53 (ac-p53) and the level of its prosenescence effector p21 under sublethal oxidative stress in human intervertebral disc cartilage endo-plate cells[9].SIRT1 can mediate the expressions of p53 and p16 by deacetylation to delay the progress of senescence, as observedin
vivo
[11].SIRT1 up-regulation prevents ultraviolet B-induced damage by inhibiting Akt phosphorylation[10].However, the inhibition of SIRT1 regulates increased SA-β-gal activity, induces a senescent phenotype, and promotes endothelial dysfunction[7].For hundreds of years,Dalbergia
odorifera
(D
.odorifera
) has been commonly used to treat blood, rheumatic pain, swelling, and necrosis[12].Previous studies have reported thatD
.odorifera
exhibits antioxidant, anti-inflammatory, anti-osteoporotic, and neuroprotective effects in diverse cell types[13-15].In a previous study, 9-hydroxy-6,7-dimethoxydalbergiquinol (HDDQ), a phenolic compound isolated from the heartwood ofD
.odorifera
, induces the expression of heme oxygenase-1, an important antioxidant protein, and exhibits protective effects against lipopolysaccharide-stimulated inflammation in BV2 microglial cells[16,17].Besides, polyphenols could be helpful to slow the aging process and improve age-related diseases[18].However, the antiaging effect of HDDQ remains unknown.Therefore, in this study, we aimed to examine the role of HDDQ as an antioxidative SIRT1 inducer and its role in the regulation of HO-induced senescence in human dermal fibroblasts (HDFs).2.Materials and methods
2.1.Preparation of HDDQ
HDDQ (No NNMBP026) was kindly provided by Wonkwang-Oriental Medicines Research Institute.HDDQ was dissolved in DMSO before use and the final vehicle content was less than 0.01% in each experiment.
2.2.Chemicals and reagents
Antibodies were used as the following description: Acetyl-p53(ac-p53, Cell Signaling.2525), p21 (Santa Cruz Biotechnology, sc-397), p16 (BD Pharmingen, 511325GR), phospho-Rb (p-Rb, Cell Signaling, 8180), β-actin (Santa Cruz Biotechnology, sc-1616), Sirt1(Merck Millipore, 07-131), phospho-Akt (p-Akt, Cell Signaling,9271), and Akt (Santa Cruz Biotechnology, sc-8312).TheSIRT1
siRNA (sc-40987) was bought from Santa Cruz Biotechnology.5-Bromo-4-chloro-3-indolyl-β-D
-galactosidase (X-Gal,11680293001) and hydrogen peroxide solution (HO, 7722841)were bought from Sigma-Aldrich.2.3.Cell culture and H2O2 treatment
HDFs (KCLB, Korea) were bought from Korea Cell Line Bank.The cells were grown in low-glucose Dulbecco’s modified Eagle’s Medium (DMEM, Thermo Fisher, 11885) containing 10% fetal bovine serum (FBS, RMBIO, FBS-BBT), 1% penicillinstreptomycin (Gibco, 10378016) and stored in a humidified incubator (37 ℃, 5% CO).HDFs were seeded in a 60 mm dish or 6-well plate and cultured for 24 h.Cells were pre-treated with various concentrations HDDQ for 24 h at a humidified incubator(37 ℃, 5% CO).A new medium, which contained 200 μM HO,was used for 2 h then grew in normal medium for 3 d.HDFs were treated with 200 μM HOat time different timepoints (5-240 min).
2.4.Cell grouping and treatment
The HDFs were divided into seven groups: normal control group cultured in complete medium (10% FBS), HDDQ (0-40 μM, 24 h)group 1, HDDQ (40 μM, 0-24 h) group 2, HDDQ (0-40 μM, 24 h)with HO(200 μM, 2 h treatment, 72 h incubation),SIRT1
siRNA(80 nM, 18 h) and HDDQ (40 μM, 24 h) with HO(200 μM, 2 h treatment, 72 h incubation) or with HO(200 μM, 1 h treatment),LY294002 (0-10 μM, 30 min) with HO(200 μM, 1 h treatment).2.5.3-(4,5-dimethylthiazol-2-yl) -2,5-diphenyltetrazolium bromide (MTT) assay
Cell viability was performed as described previously[19].HDFs (7×10) were seeded in a 96-well plate and were treated with HDDQ at concentrations of 0-160 μM.Then, we added a total of 20 μL of MTT(abcam, ab211091) in each well according to the manufacturer’s protocol.The absorbance was measured at a wavelength of 490 nm using a SpectraMax M3 instrument (Molecular Devices, California,USA).
2.6.Senescence-associated β-galactosidase (SA-β-gal)staining
The proportion of SA-β-gal positive cells was determined as Debacq-Chainiauxet
al
.[20].HDFs were washed twice with phosphate-buffered saline (PBS) and fixed with 3.7% formaldehyde in PBS for 10 min at room temperature.After washing with PBS,cells were incubated with β–galactosidase reagent [1 mg/mL 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-gal), 40 mM citric acid/sodium phosphate buffer, pH 6.0, 5 mM potassium ferrocyanide/potassium ferricyanide, 150 mN NaCl, 2 mM MgCl]at 37 ℃.The cells were washed twice with PBS, then we counted aging cells (blue) and total cells under fluorescence microscopy using a Zeiss microscope (Zeiss, Oberkochen, Germany).2.7.Western blotting assay
Western blotting assay was performed as described by Chenget
al
.[21].HDFs were harvested, washed with ice-cold 1×PBS, and resuspended in pre-cold 1×RIPA buffer with protease inhibitors.Protein concentration was quantified according to BCA method.Samples (50 μg) were separated by 12% SDS and were transferred to PVDF membranes.The membranes were incubated in first antibody overnight and then incubated in secondary antibody at 4 ℃ for 2 h.The first antibodies were used anti-ac-p53, anti-p21, antip16, anti-p-Rb, anti-SIRT1, anti-p-Akt, anti-Akt and anti-β-actin.The secondary antibodies were used goat anti-mouse IgG-HRP,mouse anti-rabbit IgG-HRP, donkey anti-goat IgG-HRP.Protein bands were detected using the ECL system (Amersham Pharmacia Biotech, Piscataway, NJ).2.8.SIRT1 activity assay
The HDFs were treated with different concentrations of HDDQ(0-40 μM) for 24 h, or treated with 40 μM HDDQ at different timepoints (0-24 h).Then HDFs were harvested, washed with ice-cold 1×PBS, and lysed with NETN buffer (1 mM EDTA, 20 mM Tris-HCl, pH 8.0, 100 mM NaCl, 0.5% NP-40).A SIRT1 fluorometric drug discovery kit (Enzo Life Sciences, BML-AK511)was used to detect SIRT1 activity.Briefly, lysates were incubated with SIRT1 assay buffer (45 min, 37 ℃), and then stopped by SIRT1 Developer (15 min, room temperature).At last, the sample was analyzed by a Spectra M3 instrument at 355 nm/460 nm.
2.9.Cell counting
Cells (7×10) were pre-treated with 40 μM HDDQ for 24 h and then exposed in HOat different timepoints (0-72 h).We used trypsin to harvest cells and counted them on a hemocytometer by an inverted microscope (Olympus, Tokyo, Japan).
2.10.SIRT1 siRNA transfection
Eighty nMSIRT1
siRNA and negative control were transfected into HDFs using Lipofectamine 2000 (Invitrogen, 12566014) according to the manufacturer’s protocol.After 18 h transfection, HDFs were treated with HDDQ (40 μM, 24 h) before HOtreatment and then SA-β-gal staining, SIRT1 activity assay, and Western blotting were performed.2.11.Statistical analysis
All data were repeated at least three independent times and were expressed as mean ± standard deviations (SD).One-way analysis of variance was used for data comparisons within multiple groups.P
<0.05 was considered statistically significant.3.Results
3.1.HDDQ inhibits H2O2-induced senescence in HDFs
In this study, we evaluated the effect of HDDQ on the viability of HDFs to determine its cytotoxic potential.Up to a concentration of 40 μM, no cytotoxic effects could be detected using the MTT assay (Figure 1).To investigate the anti-aging effect of HDDQ,we induced senescence in the HDFs by exposure to HO200 μM for 2 h and subsequently cultured them for 3 d.We found that the addition of HDDQ dramatically reduced the HO-induced senescent cells (Figure 2A and 2B).Additionally, pre-treatment with HDDQ significantly attenuated ac-p53, p21, and p16expression and increased the expression of p-Rb upon HOexposure (Figure 2C).Furthermore, the addition of HOreduced the proliferation of the HDFs, and the HDDQ treatment recovered the cell proliferation(Figure 2D).3.2.HDDQ increases SIRT1 expression in HDFs
As shown in Figure 3A, HDDQ induced the expression of SIRT1 in a dose-dependent manner.The maximal induction of SIRT1 was achieved at a dose of 40 μM HDDQ (Figure 3A).The induction of SIRT1 expression by HDDQ (40 μM) was detected as early as 12 h following HDDQ treatment; the augmentation lasted for an additional 12 h (Figure 3B).SIRT1 activity was found to increase over the same HDDQ dose range (Figure 3C) and to reach a maximum level after 24 h of HDDQ treatment (Figure 3D).
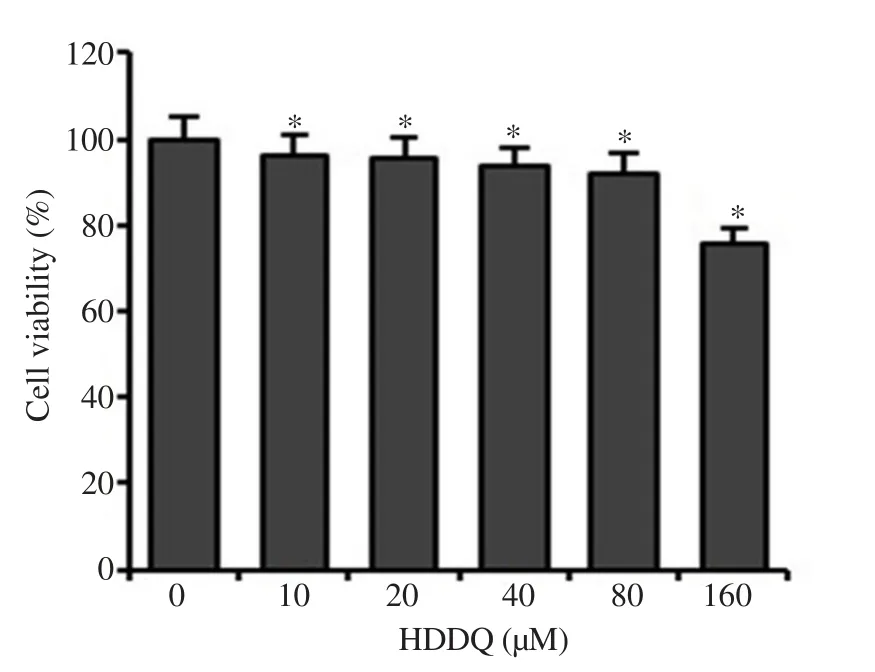
Figure 1.Concentration-dependent effect of 9-hydroxy-6,7-dimethoxydalbergiquinol (HDDQ) on cell viability.Cell viability was determined by MTT assay.Data represents mean values of triple experiments.Significance versus control: *P<0.05.
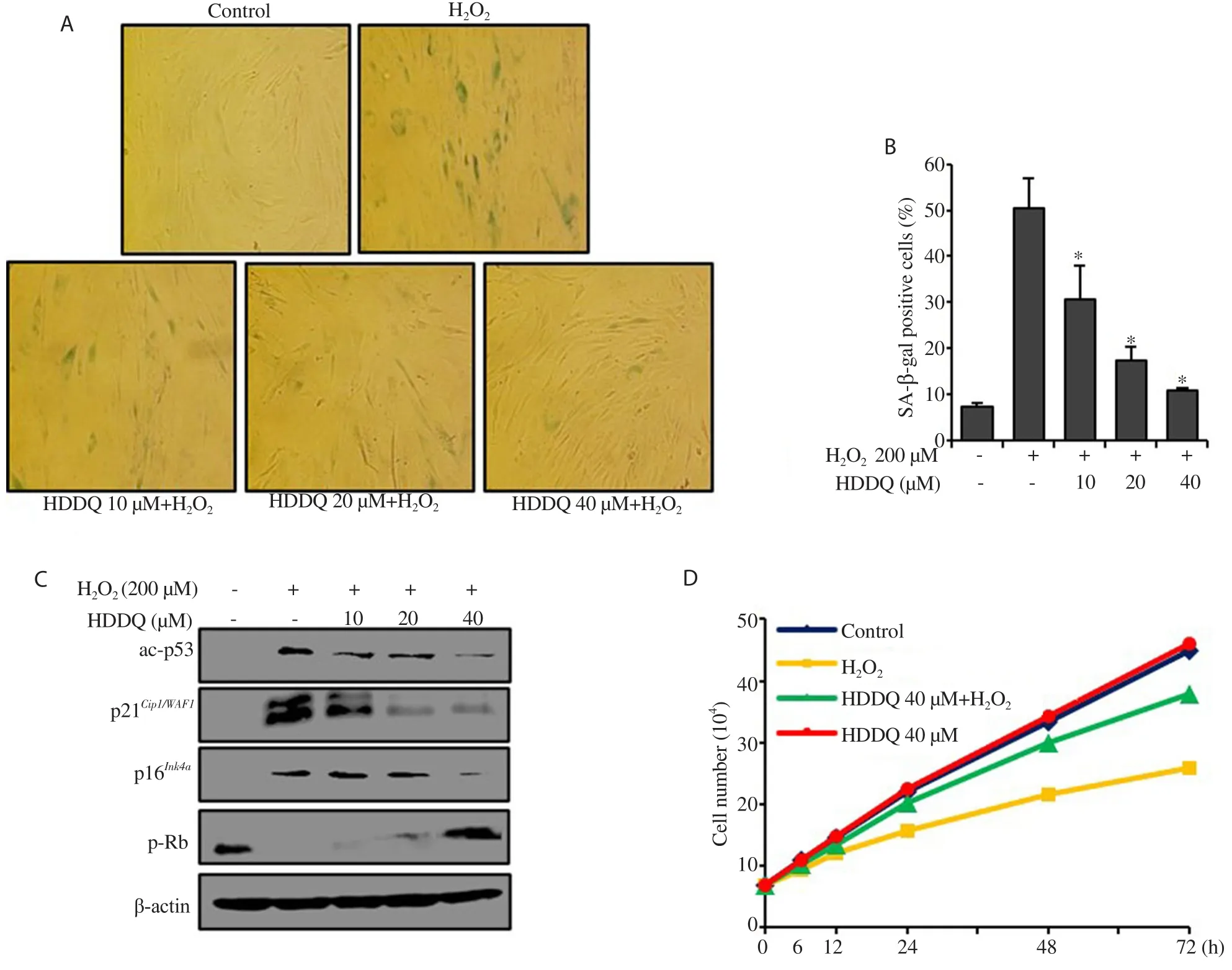
Figure 2.Effect of HDDQ on H2O2-induced senescence in human dermal fibroblasts (HDFs).HDFs were treated with HDDQ (10-40 μM, 24 h) followed with H2O2 treatment.(A): Representative images of SA-β-gal staining of HDFs.(B): The percentage of senescent cells.(C): The protein level detected by Western blotting assay.(D): Cell growth curve examined after H2O2 addition.Data represents mean values of triple experiments.Significance versus H2O2-treated cells:*P<0.05.
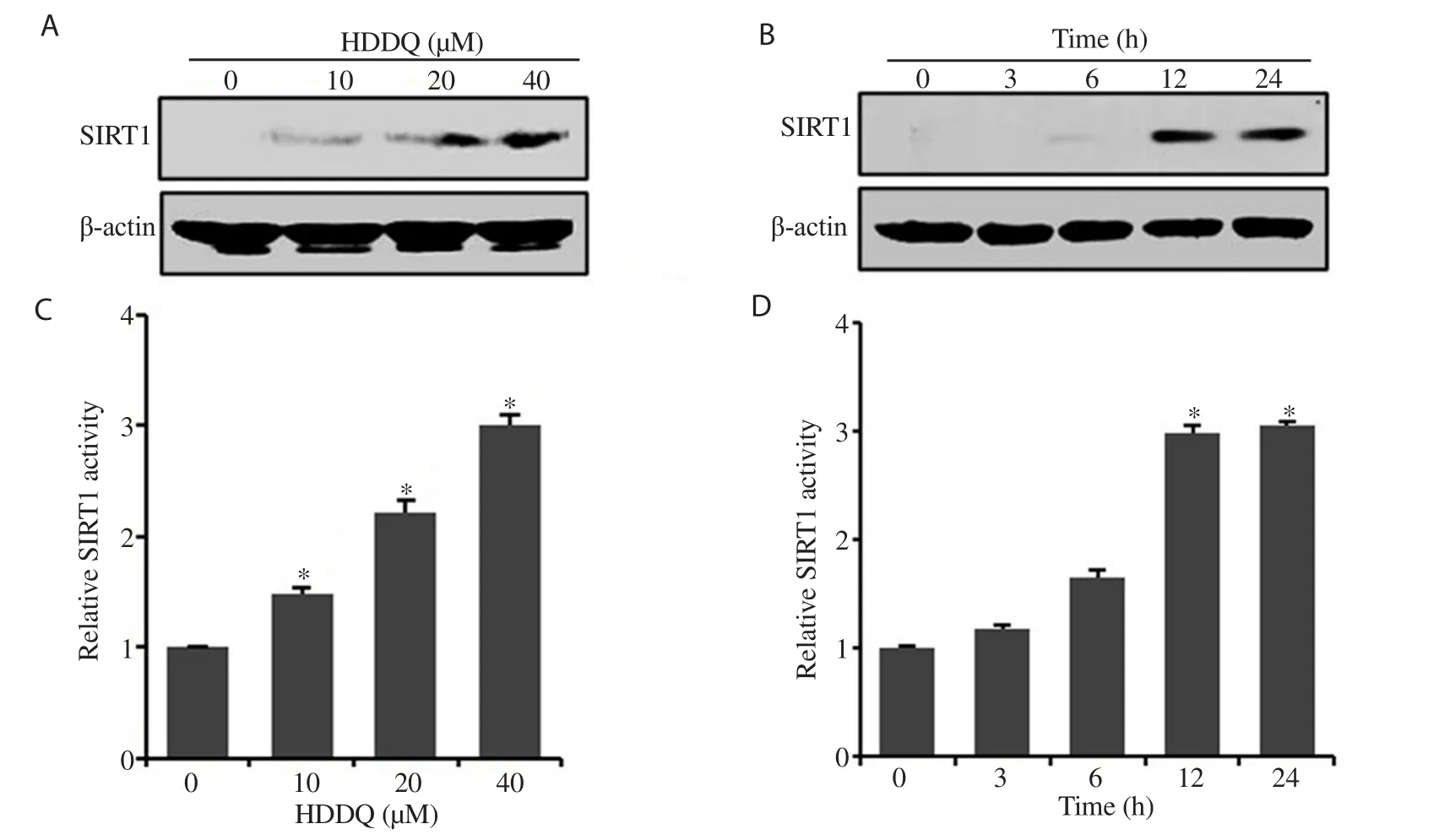
Figure 3.Effects of HDDQ on sirtuin-1 (SIRT1) expression in HDFs.A and C: HDFs were treated with HDDQ (10-40 μM, 24 h).B and D: Cells were treated with HDDQ (40 μM, 3-24 h).The protein level (A and B) and SIRT1 activity (C and D) were detected with Western blotting assay and SIRT1 fluorometric kit, respectively.Data represents mean values of triple experiments.Significance versus control: *P<0.05.
3.3.Inhibition of SIRT1 abrogates the protective effect of HDDQ against senescence in HDFs via the p53-p21Cip1/WAF1 pathway and p16Ink4a-pRb pathway
As shown in Figure 4A and 4B, SIRT1 inhibition abrogated the effect of HDDQ on senescence-specific morphological changes and SA-β-gal activity.UnderSIRT1
siRNA transfection, HDDQ treatment did not increase the activity and expression of SIRT1(Figure 4C and 4D).In addition, the HDDQ-induced decrease in the expression of ac-p53, p21, and p16and increase in the expression of p-Rb were no longer observed when SIRT1 was inhibited (Figure 4C).These results indicated that SIRT1 could play a vital role in the protective effect of HDDQ against HO-induced cellular senescence.3.4.HDDQ-induced expression of SIRT1 alleviates H2O2-induced senescence in HDFs via inhibiting PI3K/Akt pathway
In our experiments, p-Akt expression in the HDFs was found to increase in a time-dependent manner upon HOtreatment and reach a maximum level after 60 min of HOtreatment (Figure 5A).As shown in Figure 5B, pretreatment with LY294002 (PI3K inhibitor)and HDDQ significantly attenuated the p-Akt expression induced by HO.In addition, cells pretreated with LY294002 and HDDQ reduced the SA-β-gal activity in response to HO(Figure 5C).However, SIRT1 inhibition bySIRT1
siRNA abolished the inhibitory effect of HDDQ on HO-induced p-Akt expression (Figure 5D).These data indicated that HDDQ prevented HO-induced senescence through the SIRT1/PI3K/Akt pathway.4.Discussion
Fibroblasts participate in skin maintenance and renewal[22].The skin fibroblasts play an important role in the induction of extracellular matrix components such as collagen and elastin.Therefore, inhibition of oxidative stress-induced senescence in skin fibroblasts is a potential treatment and prevention strategy for maintaining healthy youthful skin.
The heartwood ofD
.odorifera
is a perennial tree that mainly grows in southern China, such as Guangdong, Hainan, and Guangxi[23].It can reach a height of 10-15 m and has elliptical leaves and small yellow flowers that bloom in the summer[24].It is an important herbal and oriental medicine, named Jiangxiang in Chinese.Its main actions are dissipating blood stasis, regulating the flow of qi, and relieving pain in traditional Chinese medicine.HDDQ, a compound isolated fromD
.odorifera
, has various biological activities.In this study, we attempted to explain the anti-aging effect of HDDQ against HO-induced senescence in HDFs and to explore the molecular mechanism underlying this effect of HDDQ, focusing on the up-regulation of SIRT1.SIRT1 can be up-regulated by various phytochemicals, including resveratrol, cilostazol, and ginsenoside Rb1, and it has been shown to prevent HO-induced senescence in different types of cells[5,25,26].We observed that HDDQ induced SIRT1 expression in HDFs and that HDDQ pretreatment resulted in dramatic resistance to HO-induced senescence.However,SIRT1
siRNA abrogated the protective effect of HDDQ on HO-induced senescence.Although the anti-inflammatory and antioxidant activities of HDDQ have been proved previously, our results indicate that the induction of SIRT1 expression by HDDQ may be an important mechanism behind this protective effect.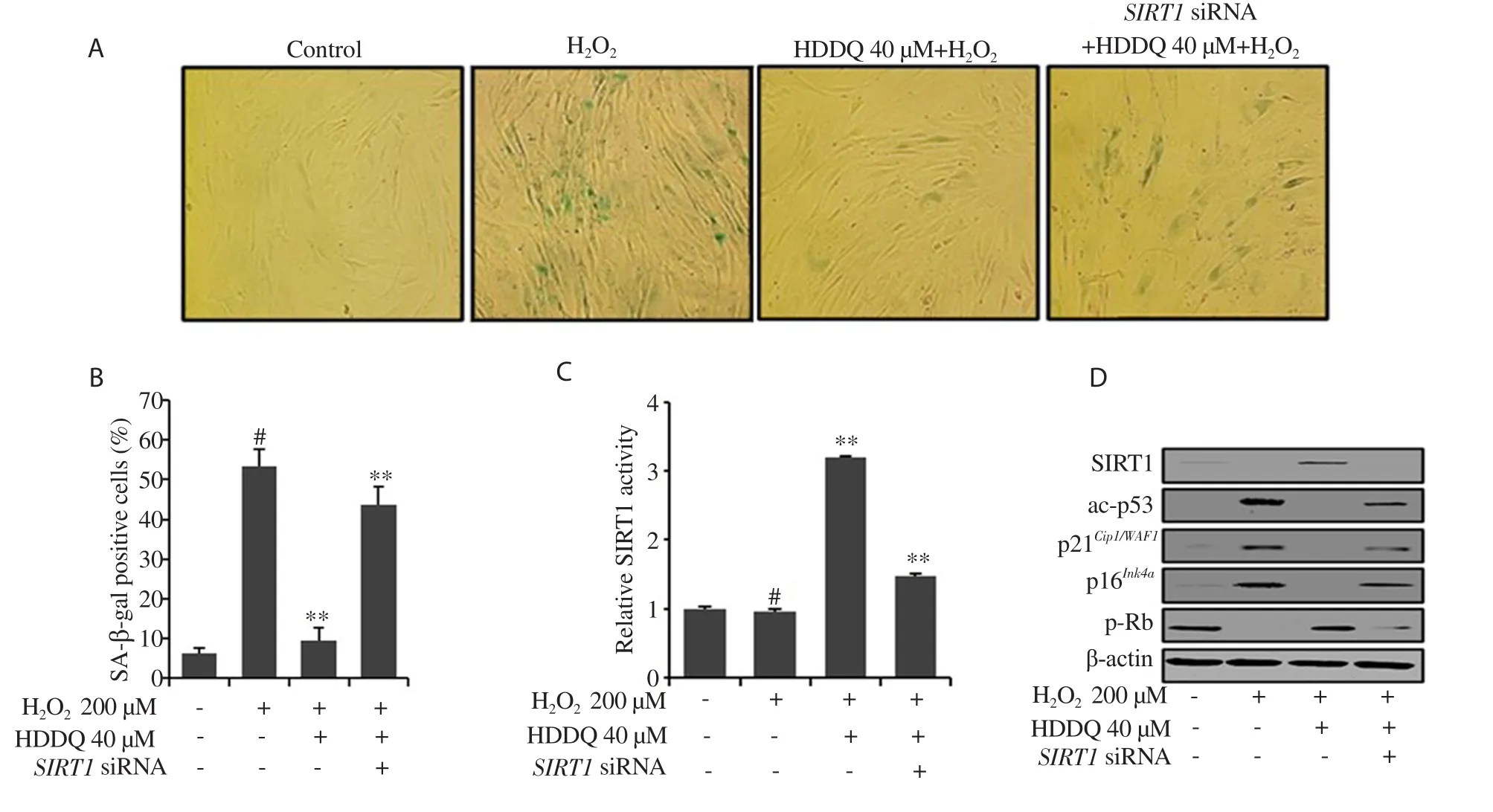
Figure 4.Inhibition of SIRT1 abolishes the effect of HDDQ in H2O2-induced senescence.HDFs were treated with SRIT1 siRNA (80 nM, 18 h) and then treated with HDDQ and H2O2.(A): Representative images of SA-β-gal staining of HDFs.(B): The percentage of senescent cells.(C): The SIRT1 activity.(D): The protein expression detected by Western blotting assay.Data presented mean values of three independent experiments ± SD.#P<0.05 vs.control; **P<0.05 vs.H2O2-treated cells.
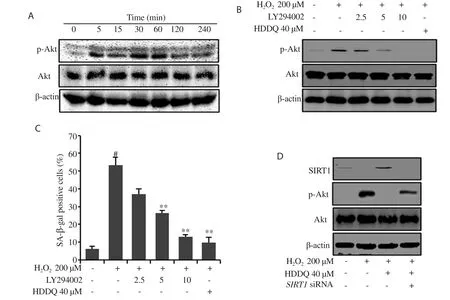
Figure 5.Activation of the PI3K/Akt pathway by H2O2 is inhibited in HDDQ-induced SIRT1 expression.(A) HDFs were incubated in 200 μM H2O2 and analyzed by Western blotting assay.(B-C) HDFs were pretreated with LY294002 and HDDQ before H2O2 exposure.Protein expression was detected by Western blotting assay (B).The percentage of SA-β-gal positive cells was calculated (C).(D) HDFs were treated with SRIT1 siRNA (80 nM, 18 h) and then treated with HDDQ and H2O2 as mentioned above.Protein expression was detected by Western blotting assay.Data represent mean values of three independent experiments ± SD.#P<0.05 vs.control; **P<0.05 vs.H2O2-treated cells.
SIRT1 is an NAD-dependent protein deacetylase that plays critical roles in numerous biological processes, including cell metabolism,cell proliferation, cell survival, and stress response[6].Recently,SIRT1 has been recognized as a major therapeutic target for aging and age-related diseases such as cardiomyopathy, sarcopenia, and cartilage end plate degeneration[9,27,28].Several studies on the regulation of SIRT1 have paid attention to the roles of the p53-p21 and p16-pRb pathways in various cell culture systems.Our results showed that activation of the p53-p21and p16pRb pathways by HOwas blocked by HDDQ-induced SIRT1 expression; however, when SIRT1 was knocked down bySIRT1
siRNA, the anti-aging protective effect of HDDQ was abolished.In agreement with our results, resveratrol and MHY2233 (a SIRT1 activator synthesized from 18 benzoxazole hydrochloride derivatives)have been found to attenuate cellular senescence through SIRT1 upregulation to block the p53-p21 and p16-pRb pathways[11,29].The PI3K/Akt pathway plays important regulating roles in both replicative and premature senescence[1].According to a recent report,Akt phosphorylation can increase the level of ROS by increasing oxygen consumption; thus, Akt-expression cells can be more sensitive to ROS-dependent premature senescence[30].In agreement with our result, HOtransiently induced Akt activation in HDFs and up-regulated the percentage of SA-β-gal-positive cells.Persistent oxidative stress in cells can directly stimulate PI3K, which leads to the downstream activation of Akt[31].We hypothesized that PI3K inhibition may reverse HOagainst premature senescence.In our study, we found that HDDQ and LY294002 significantly inhibited PI3K and the phosphorylation of Akt induced by HOand reduced the percentage of SA-β-gal-positive cells.These results suggested that blocking the activation of the PI3K/Akt pathway partially prevented HO-induced cellular senescence, and HDDQ decreased senescencevia
its inhibitory effect on the activation of the PI3K/Akt pathway.Previous studies have shown that SIRT1 is closely related to senescence and oxidative stress[5,26,31].We speculated that HDDQ-induced SIRT1 expression might influence the activation of the PI3K/Akt pathway by HO.We found that SIRT1 inhibition bySIRT1
siRNA recovered the HO-induced phosphorylation of Akt.In line with this finding, SIRT1 down-regulation by insulin-like growth factor-1 induces ac-p53 in a PI3K-dependent manner, leading to premature senescence[32].SIRT1 activation by resveratrol prevents UVB-induced damage by inhibiting Akt phosphorylation[10].Recently, we also examined the anti-aging effect of latifolin on HO-induced senescence in HDFs.Latifolin is also isolated fromD
.odorifera
.In this experiment, we replaced HDDQ with latifolin and followed the same treatment procedure[33].We found that latifolin also protected HDFs against HO-induced senescencevia
the p53-p21, p16-pRb, and PI3K/Akt pathways.Based on our data,there is a possibility that if HDDQ and latifolin are used together, the effect against HO-induced senescence could improve.In summary, we showed that HDDQ inhibited HO-induced premature senescence and up-regulation of SIRT1 expression played a vital role in the inhibition of the senescence phenotype in HDFs.Our results demonstrate that the HDDQ-induced expression of SIRT1 could contribute to the mechanism of cellular defense against oxidative stress-induced changes associated with aging.
Plant extracts-based products have been enjoying wide acceptability among consumers due to their immense medicinal benefits particularly on delaying aging and reducing aging-related diseases.D
.odorifera
is not only used in spices, cosmetics, but also used in the pharmaceutical industry.In this study, we report the potentials of HDDQ (D
.odorifera
extract) to protect against oxidative stressmediated skin aging.Thus, HDDQ could supplement the formulation of health care products for the population in oxidative stress-induced aging changes.Conflict of interest statement
We declare that there is no conflict of interest.
Funding
This study was supported by Wonkwang University in 2019.
Authors’ contributions
SHL and RZZ participated in the desiging of the study.SHL and BSL participated in experimental studies, data acquistion,data analysis, and statistical analysis.BSL participated in the manuscript preparation and editing.BMC and BSL participated in the manuscript review.All authors have read and approved the final manuscript, and agreed to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work is appropriately investigated and resolved.
 Asian Pacific Journal of Tropical Biomedicine2021年2期
Asian Pacific Journal of Tropical Biomedicine2021年2期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Anti-inflammatory, anti-oxidative and anti-apoptotic effects of Heracleum persicum L.extract on rats with gentamicin-induced nephrotoxicity
- Screening of phytocompounds, molecular docking studies, and in vivo antiinflammatory activity of heartwood aqueous extract of Pterocarpus santalinus L.f.
- Anti-senescence and anti-wrinkle activities of 3-bromo-4,5-dihydroxybenzaldehyde from Polysiphonia morrowii Harvey in human dermal fibroblasts
- Borassus flabellifer L.crude male flower extracts alleviate cisplatin-induced oxidative stress in rat kidney cells
- Corchorus olitorius aqueous extract attenuates quorum sensing-regulated virulence factor production and biofilm formation
