Anti-senescence and anti-wrinkle activities of 3-bromo-4,5-dihydroxybenzaldehyde from Polysiphonia morrowii Harvey in human dermal fibroblasts
Su-Hyeon Cho, Eun-Yi Ko, Soo-Jin Heo, Seo-Young Kim, Juhee Ahn, Kil-Nam Kim✉
1Chuncheon Center, Korea Basic Science Institute, Chuncheon 24341, Republic of Korea
2Bio research Center, Dermapro, Jeju 63309, Republic of Korea
3Jeju Marine Research Center, Korea Institute of Ocean Science & Technology, Jeju 63349, Republic of Korea
4Department of Medical Biomaterials Engineering, College of Biomedical Sciences, Kangwon National University, Chuncheon 24341, Republic of Korea
ABSTRACT
KEYWORDS: Polysiphonia morrowii Harvey; 3-bromo-4,5-dihydroxybenzaldehyde; Oxidative stress; Human dermal fibroblast;Anti-senescence activity; Anti-wrinkle activity
1.Introduction
Recent changes in the standard of living and the development of medical technology have been accompanied by an aging population as the lifespan has been extended.Due to the entry of an aging society, there is a growing interest in improving the quality of life and health, and not simply extending life expectancy.Accordingly,anti-aging markets are rapidly increasing, and anti-aging research is progressing actively[1,2].Aging refers to a process that increases the risk of death due to changes in the body that occur through the passage of time.When aging occurs, there is a reduction of the adaptive capability to cope with stress, with adverse effects on physiological functions and increased susceptibility to diseases[3-6].The theory of aging is divided into two categories.The first of them is intrinsic aging; the theory of genetic etiology that proposes that there is information preserved in the genes determining the lifespan, and that aging progresses according to such information.On the other hand, extrinsic aging is the theory of environmental etiology, which proposes that aging progresses due to the influence of various environmental and aging aggressors.In particular, the reactive oxygen species (ROS) produced by UV and respiration are considered to be the most important factor of aging[7,8].Among ROS, hydrogen peroxide (HO) is widely used in aging research because it is known to cause the senescence of cells[9-11].
The human skin comprises the epidermis and dermis.Dermal fibroblasts are generated by the extracellular matrix (ECM), which produces elastin fiber and collagen in tissue and represents the extracellular part of a multicellular structure[12,13].Skin aging,caused by intrinsic and extrinsic factors, is a complex biological process[14].Wrinkle formation is a representative feature of skin aging.It is caused by declined skin elasticity and degradation of the ECM including collagen, which is generated by fibroblasts in the dermis[14,15].Especially, the important factors affecting wrinkles include the dissipation of cytokines and collagen and the activation of matrix metalloproteinases (MMPs) and human neutrophil elastase[12,14-16].Oxidative stress is also a crucial factor of wrinkle formation[14,17].The production of ROS promotes the expression of MMPs and the subsequent degradation of the collagen matrix system in the dermis[14].Therefore, in order to prevent and treat wrinkle formation, it is important to identify compounds that can promote collagen synthesis and inhibit MMPs and elastase.
Marine algae possess several bioactive compounds with nutraceutical potential and are used as food and as a therapeutic agent for preventing and treating diseases[18-20].3-Bromo-4,5-dihydroxybenzaldehyde (BDB) is a natural bromophenol compound isolated from the red algaPolysiphonia
morrowii
(P
.morrowii
)Harvey.Several previous studies have reported that BDB derived fromP
.morrowii
Harvey has diverse physiological activities such as antioxidant, anti-inflammatory, and antiviral activities[18,21,22].However, the possible anti-senescence and anti-wrinkle activities of BDB have not been determined.Therefore, this study was conducted to study the potential of BDB, isolated fromP
.morrowii
Harvey, as an anti-senescence and anti-wrinkle agent.2.Materials and methods
2.1.Cell culture
Human dermal fibroblasts (HDF) were obtained from Korean Cell Line Bank (KCLB, Korea).The cells were grown at 37 ℃ in a 5% COhumidified incubator using Ham’s F-12 Nutrient mix (Gibco/BRL, Canada) and Dulbecco’s Modified Eagle Medium (Welgene,Korea) mixture (1:3) supplemented with 10% fetal bovine serum(Welgene, Korea) and 1% antibiotic (Gibco/BRL, Canada).
2.2.Measurement of cell viability
HDF was seeded in a 96 well plate at a concentration of 1.5 × 10cells/mL for 24 h.Then, the cells were treated with 25, 50, and 100 μM of BDB for 72 h at 37 ℃.The cells were incubated with 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide(Amresco, USA) dissolved in phosphate buffered saline (PBS) for 3 h.After the supernatant was removed, and the formazan was dissolved in dimethyl sulfoxide (Sigma-Aldrich, USA).The absorbance was read at 540 nm using a spectrophotometer (Molecular Devices,USA).
2.3.Senescence-associated β-galactosidase (SA-β-gal)staining
HDF was seeded in a 24 well plate at a concentration of 1.5 × 10cells/mL for 24 h.Then, the cells were pre-treated with 25 and 50 μM of BDB and treated with 400 μM of HO(Sigma-Aldrich, USA)for 72 h at 37 ℃.The cells were washed once with PBS (Gibco/BRL, Canada) and fixed using 4% formaldehyde (EMS, USA) for 15 min.After the cells were washed twice, the cells were incubated with SA-β-gal staining solution containing 1 mg/mL 5-bromo-4-chloro-3-indolyl β-D
-galactopyranoside (X-gal; Sigma-Aldrich, USA), 5 mM potassium hexacyanoferrate (Ⅱ) trihydrate (Sigma-Aldrich, USA),5 mM potassium hexacyanoferrate (Ⅲ) (Sigma-Aldrich, USA),and 2 mM MgCl(Biosesang, Korea) in 40 mM citric acid/sodium phosphate buffer (pH 6.0) (Sigma-Aldrich, USA) for 24 h at 37 ℃ in a CO-free atmosphere in the dark.The SA-β-gal stained cells were observed using an inverted microscope (Olympus, Japan).2.4.Measurement of intracellular ROS production
HDF was seeded in a 96 well plate at a concentration of 8 ×10cells/mL for 24 h.Then, the cells were treated with 25 and 50 μM of BDB for 2 h, and 400 μM of HOwas added for 30 min at 37 ℃.The cells were then treated with 5 μg/mL of 2’,7’-dichlorodihydrofluorescein diacetate (Sigma-Aldrich, USA) for 30 min at 37 ℃ in the dark.Fluorescence intensity was detected at an excitation wavelength of 485 nm and an emission wavelength of 535 nm, using a spectrophotometer (Molecular Devices, USA).
2.5.Western blotting analysis
HDF was seeded in a 60 mm dish at a concentration of 1.0 × 10cells/mL for 24 h.The cells were treated with 50 μM of BDB for 2 h, and then with 400 μM of HOfor 6 h at 37 ℃.After the media were removed, the cells were washed two times with PBS.RIPA buffer (Sigma Aldrich, USA) was treated for 10 min at 4 ℃.The cell lysates were collected, vortexed 6 times for 1 h, and centrifuged at 15 000 rpm at 4 ℃ for 20 min.Afterward, protein concentration was assessed by Protein Assay Dye Reagent Concentrate (Bio-Rad, USA), protein samples were subjected to electrophoresis and transferred to polyvinylidene flouride membranes (Thermo Fisher Scientific, USA).The membranes were blocked for 2 h at room temperature.The following primary antibodies were used for overnight incubation at 4 ℃: anti-glutathione peroxidase 1(GPX1), anti-catalase (Cell signaling Technology, USA), and antiβ-actin (Santa Cruz Biotechology, USA).The following secondary antibodies were used for 2 h at room temperature: anti-rabbit IgG,HRP-linked antibody, anti-mouse IgG, and HRP-linked antibody(Cell signaling technology, USA).The bands were detected using the SuperSignal West Femto Trial kit (Thermo Fisher Scientific, USA).
2.6.Measurement of intracellular collagen content
HDF was seeded in a 24 well plate at a concentration of 5 × 10cells/mL, incubated for 24 h, and then treated with 25 and 50 μM of BDB.After 24 h, the supernatant was collected and stored at 20 ℃ until testing.The amount of procollagen in the medium was evaluated at 450 nm using Procollagen TypeⅠC-Peptide ELISA Kit(TaKaRa, Japan).The degree of collagen production was normalized by total protein content and compared with transforming growth factor β1 (TGF-β1; Sigma-Aldrich, USA) at 0.4 nM as a positive control.Media without test materials were used as solvent control.
2.7.Measurement of intracellular collagenase (MMP-1)activity
HDF was seeded in a 24-well plate at a concentration of 5 × 10cells/well for 24 h.Then, the cells were then treated with 25 and 50 μM of BDB for 48 h.The collagenase activity was determined at 450 nm using the Human MMP-1 ELISA Kit (Abcam, USA).MMP-1 activity was evaluated by total protein content and compared with TGF-β1 as a positive control.Media without test materials were used as solvent control.
2.8.Measurement of elastase activity
The cells were seeded in 100-mm dishes at a concentration of 5 ×10cells/well and lysed with 0.2 M Tris-HCl (pH 8.0; Merck, USA)buffer containing 0.2% Triton X-100 (Sigma-Aldrich, USA).The cell lysate was centrifuged at 10 000 rpm at 4 ℃ for 30 min, and the supernatants were used as the fibroblast enzyme solution.An 80 μL of each enzyme solution (100 μg/80 μL 0.2 M Tris-HCl, pH 8.0)was dispensed into 96-well plates, and 10 μL of each concentration of BDB into 96-well plates.After the addition of 10 μL of 10 mMN
-succinyl-tri-L
-alanyl-p
-nitroanilide (STANA; Sigma-Aldrich,USA), a further incubation was performed for 90 min at 37 ℃.The release ofp
-nitroaniline was measured at an absorbance of 504 nm.Phosphoramidon (10 μM) (Sigma-Aldrich, USA) was used as a positive control.We used 0.2 M Tris-HCl (pH 8.0) buffer without test materials as solvent control.2.9.Protein quantification
Protein concentration was evaluated by the Bradford method using bovine serum albumin (BSA; GenDEPOT, USA) as a standard solution.To create a BSA curve, different concentrations of BSA (0,5, 10, and 20 μg/mL) were obtainedvia
stepwise dilution in distilled water, and then 20 μL of each concentration of BSA was put into 1 mL of Bradford solution for 5 min.The absorbance of the mixture was detected at 595 nm.The absorbance of the cell lysate was measured in the same way, and the total protein content of the cell lysate was determined using the BSA standard curve.2.10.Statistical analysis
The data are expressed as mean ± standard deviation (SD) analyzed by one-way ANOVA with Tukey post test.P
< 0.05 was considered as statistical significance.All statitstical tests were performed using GraphPad PRISM software version 8.0 (GraphPad Software, USA).3.Results
3.1.Cytotoxicity of BDB
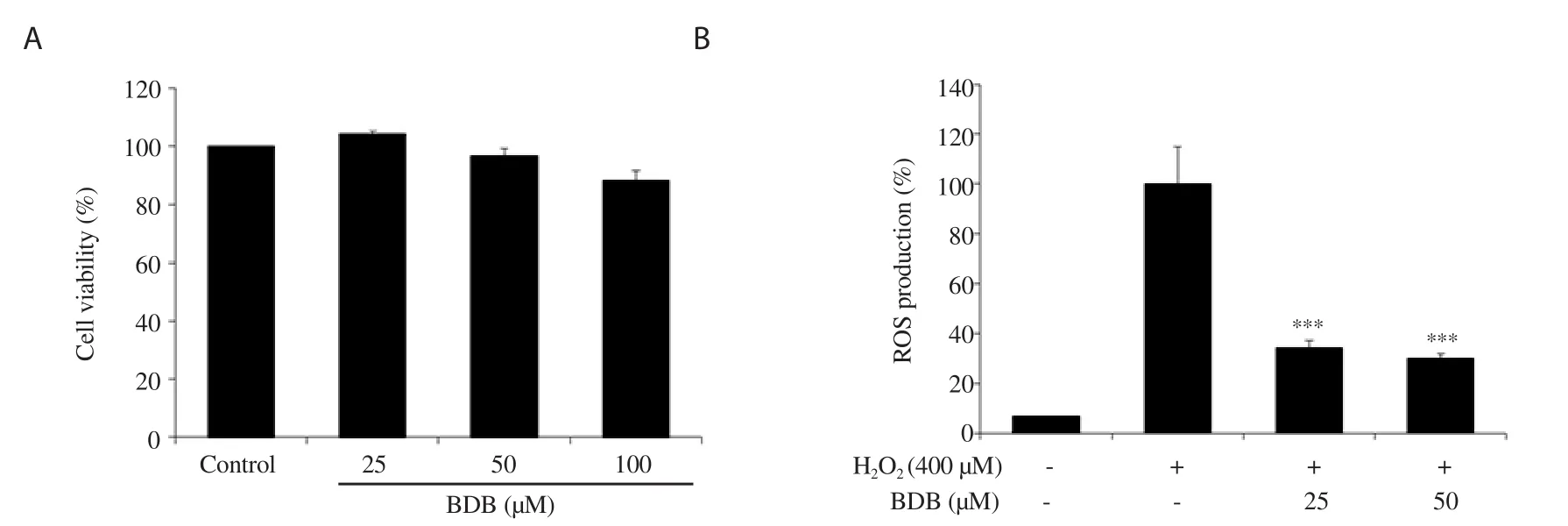
Figure 1.Effect of 3-bromo-4,5-dihydroxybenzaldehyde (BDB) on (A) cell viability and (B) H2O2-induced ROS production in human dermal fibroblasts (HDF).All results are expressed as mean ± SD of more than three individual experiments; ***P < 0.001 compared with the H2O2-stimulated group.
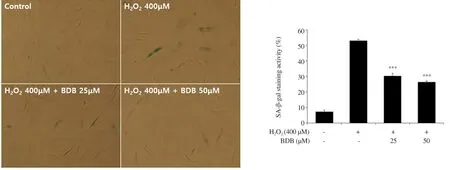
Figure 2.Effect of BDB on senescence-associated β-galactosidase (SA-β-gal) activity against H2O2-induced aging in HDF.All results are expressed as mean ±SD of more than three individual experiments; ***P < 0.001 compared with the H2O2-stimulated group.
We first examined the cytotoxicity of BDB in HDF and found that it was not cytotoxic at the concentration up to 50 μM (Figure 1A).Moreover, in our preliminary experiments, we used HOat 400 μM, and it appeared to induce cellular senescence with the low cytotoxicity (data not shown).Thus, non-cytotoxic doses were used to determine the anti-senescence effect of BDBvia
regulation of antioxidant enzyme activities, and 400 μM of HOwas used to cause oxidative stress and aging.3.2.Anti-senescence activity
ROS production was reduced to 34.16% and 30.06% in the groups treated with 25 and 50 μM of BDB, respectively.The results showed that BDB confers protection against oxidative stress in HO-stimulated HDF (Figure 1B).
In addition, we measured SA-β-gal activity to confirm that BDB has an inhibitory effect on cellular senescence induced by HO.After treatment with 400 μM of HO, SA-β-gal activity was increased to 52.87% and induced morphological enlargement of HDF.Thus, HOcan induce cellular senescence by increasing the expression of the senescence marker SA-β-gal.However, SA-β-gal activity was reduced to 30.08% and 26.19% in the cells treated with 25 and 50 μM, respectively (Figure 2).
As we found that HOtreatment induced the generation of ROS in HDF, we further evaluated the expression levels of the antioxidant enzymes GPX1 and catalase in HDF exposed to 400 μM of HOin the presence or absence of BDB.After treatment with 400 μM of HOfor 6 h, the expression of GPX1 was inhibited compared with the control.However, treatment with 50 μM of BDB in HO-stimulated HDF significantly increased the expression of GPX1(Figure 3), while the expression levels of catalase, unlike GPX1 expression, were not significantly different (data not shown).
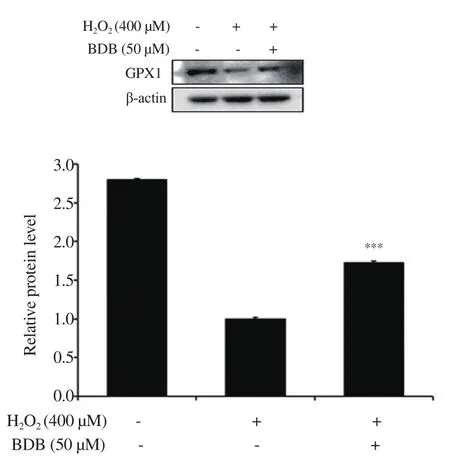
Figure 3.Effect of BDB on the expression of glutathione peroxidase 1(GPX1) in H2O2-stimulated HDF.All results are expressed as mean ± SD of more than three individual experiments; ***P < 0.001 compared with the H2O2-stimulated group.
3.3.Anti-wrinkle activity
We evaluated the effect of BDB on typeⅠcollagen content, MMP-1 and elastase activities in HDF.The intracellular collagen content was significantly increased to 148.2% and 153.9% in the groups treated with 25 and 50 μM, respectively, compared with the control group (Figure 4).Moreover, MMP-1 content was significantly suppressed in the groups treated with 50 μM of BDB.Interestingly,TGF-β1 treatment induced greater inhibition (Figure 5).In addition,the elastase activity was slightly reduced, which showed significant differences compared with the control group (Figure 6).
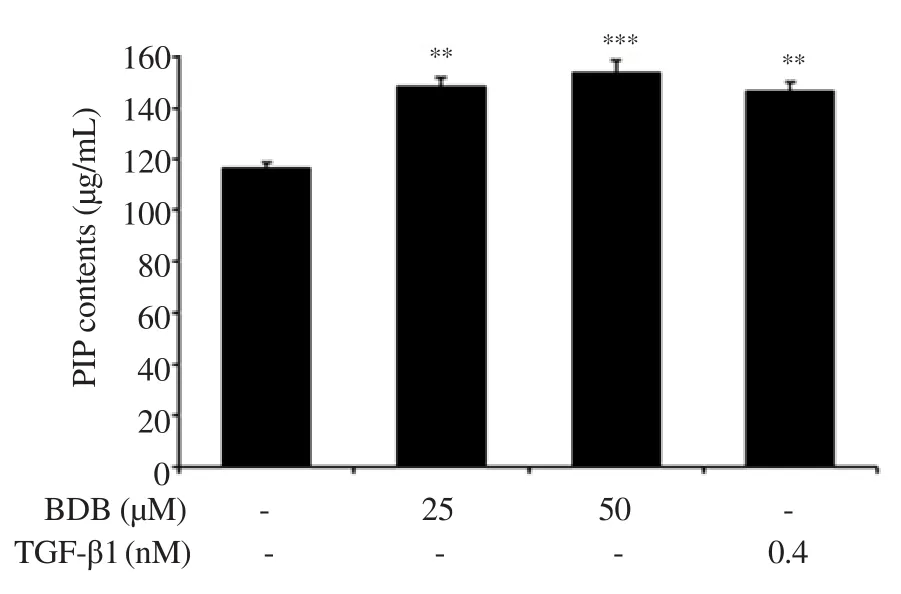
Figure 4.Effect of BDB on the intracellular collagen content in HDF.All results are expressed as mean ± SD of more than three individual experiments; **P < 0.01 and ***P < 0.001 compared with the control group.TGF-β1: transforming growth factor β1; PIP: Procollagen typeⅠC-peptide.
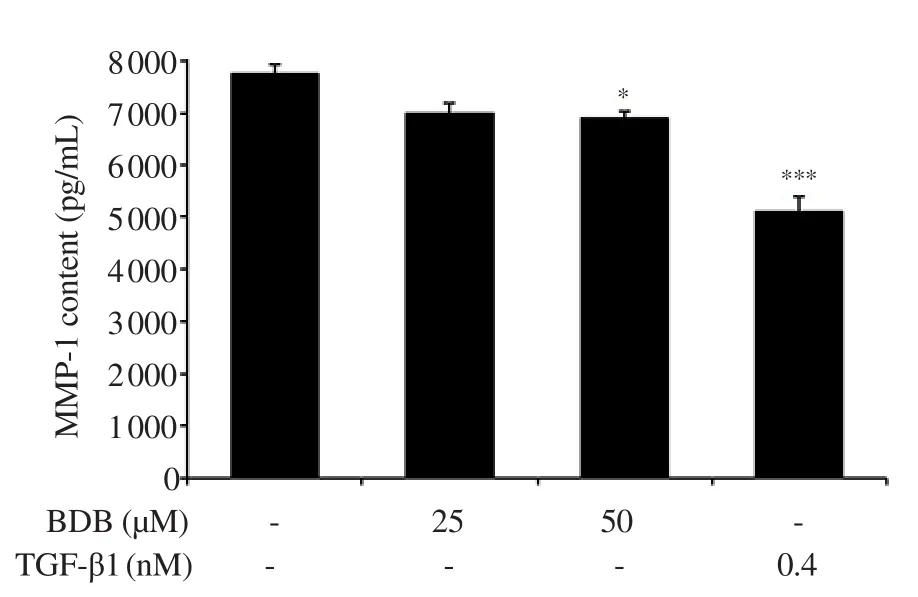
Figure 5.Effect of BDB on matrix metalloproteinase-1 (MMP-1) activity in HDF.All results are expressed as mean ± SD of more than three individual experiments; *P < 0.05 and ***P < 0.001 compared with the control group.
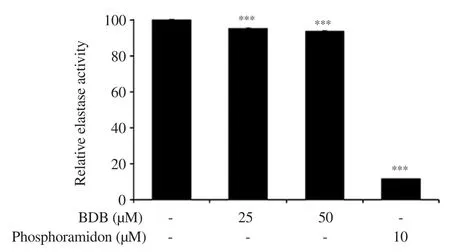
Figure 6.Effect of BDB on elastase activity in HDF.All results are expressed as mean ± SD of more than three individual experiments; ***P < 0.001 compared with the control group.
4.Discussion
The overexpression of ROS causes injury to the cells of organisms through oxidative damage to DNA, RNA, proteins, and lipids,and hinders the repair of damaged nuclear and mitochondrial DNA, contributing to cellular imbalance[23-25].Eventually, ROS causes cellular and tissue damage and finally various diseases such as Parkinson’s disease, cancer, inflammatory conditions, and aging[26-28].In previous studies, it has been demonstrated that HOaccelerates cellular senescence by inducing oxidative stress[29,30].For that reason, in this study, we evaluated the anti-senescence effect of BDB on HO-induced premature senescence in HDF.As a result, BDB was found to inhibit the HO-induced intracellular ROS production when compared with the HO-treated group.This result demonstrates that BDB can suppress the HO-induced oxidative stress in HDF.
SA-β-gal, the β-galactosidase activity, is widely used as a biomarker of cellular senescence[31,32].It can be detected at pH 6.0 in cultured cells and mammalian tissues during replicative or induced senescence, and it is of lysosomal origin[31,33,34].A previous study reported that the higher the induction of the senescence in cells, the greater the expression level of SA-β-gal[31,32].In the present study,SA-β-gal activity was remarkably increased in the HOgroup.Treatment with BDB significantly reduced its activity, demonstrating that BDB can alleviate HOinduced cellular senescence.In previous studies, the enzymatic antioxidant defense system was shown to be related to aging[35,36].GPXs are a family of enzymes that reduce HOto water, or lipid hydroperoxides to the corresponding alcohols[37,38].GPX1 is a critical factor in the removal of HOand the control of ROS in the metabolism of aerobic organisms[39].Therefore, the expression level of GPX1 was evaluated in this study.From the results, BDB was found to significantly increase GPX1 expression when compared with that of the HO-treated group.However, the expression of antioxidant enzyme catalase which was also determined in the study was not significantly altered.Our result demonstrates that BDB inhibits oxidative stress-induced cellular senescence by promoting the antioxidant enzyme GPX1.
TypeⅠcollagen is the main component of ECM and the most plentiful protein in skin connective tissue[40].It retains the structure of skin with other types of collagen (Ⅲ, Ⅴ, and Ⅶ), elastin,fibronectin, proteoglycans, and other ECM proteins[41].The degradation of typeⅠcollagen is related to MMPs.MMPs is a family of zinc-dependent endoprotease with the ability to decompose all the constituents of the ECM[41,42].In particular, MMP-1 is mainly associated with dermal collagen degradation during the skin aging process[43].Therefore, we evaluated the typeⅠcollagen content, and the activities of MMP-1 and elastase in BDB-treated HDF.Intracellular collagen content was significantly increased as compared with that of the control group.Moreover, MMP-1 content was significantly lowered in the BDB-treated group and elastase activity was suppressed.Collectively, our results suggest that BDB exhibits anti-wrinkle activity through the promotion of intracellular collagen content, and the inhibition of MMP-1, and elastase activities in HDF.
In conclusion, the present study showed that BDB inhibited premature senescence by reducing ROS productionvia
the restoration of the GPX antioxidant enzyme in HO-stimulated HDF.Moreover, BDB promoted typeⅠcollagen content and suppressed MMP-1 activity in HDF.These results demonstrate the potential of BDB, providing a basis for further studies regarding its use in the management of aging-related diseases and wrinkle formation.Conflict of interest statement
The authors declare that there is no conflict of interest.
Funding
This research was supported by Korea Basic Science Institute (grant number C39260), National Research Foundation of Korea (NRF)funded by the Korea government (MSIT) (grant number: NRF-2019R1C1C1005608), and a research grant from the Korea Institute of Ocean Science and Technology (PE99822).
Authors’ contributions
JA and KNK supervised and designed the study.SHC, EYK, SJH,and SYK performed experimental analysis.SHC, EYK, JA, and KNK wrote the manuscript.
 Asian Pacific Journal of Tropical Biomedicine2021年2期
Asian Pacific Journal of Tropical Biomedicine2021年2期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Anti-inflammatory, anti-oxidative and anti-apoptotic effects of Heracleum persicum L.extract on rats with gentamicin-induced nephrotoxicity
- Screening of phytocompounds, molecular docking studies, and in vivo antiinflammatory activity of heartwood aqueous extract of Pterocarpus santalinus L.f.
- Borassus flabellifer L.crude male flower extracts alleviate cisplatin-induced oxidative stress in rat kidney cells
- Corchorus olitorius aqueous extract attenuates quorum sensing-regulated virulence factor production and biofilm formation
- 9-Hydroxy-6,7-dimethoxydalbergiquinol suppresses hydrogen peroxide-induced senescence in human dermal fibroblasts through induction of sirtuin-1 expression
