Real-world treatment attrition rates in advanced esophagogastric cancer
Erica S Tsang, Howard ] Lim, Daniel J Renouf, Janine M Davies, Jonathan M Loree, Sharlene Gill
Abstract
Key Words: Esophagogastric cancer; Gastric cancer; Treatment attrition; Systemic therapy; Treatment outcomes; Real-world evidence
INTRODUCTION
Gastric cancer is the fourth most common type of cancer, while esophageal cancer remains the eight most common type of cancer[1]. Locally advanced unresectable and metastatic disease in both sites have dismal outcomes, with 5-year survival rates measuring less than 4%[2,3]. The current standard of care for advanced esophagogastric cancer is systemic chemotherapy with a first-line platinum and fluoropyrimidine combination[4]. Rates of second-line treatment have been reported to be between 14% and 75%[5,6].A retrospective Italian study early in the ramucirumab era examined rates of second-line treatment and reported a range of 7%-41%; however, this was limited by a small number of patients who received ramucirumab, which has now been established as a standard second-line regimen[7].
Over the last decade, multiple agents have also been tried in later line settings, including ramucirumab, irinotecan, trifluridine/tipiracil, and immunotherapy[8-11]. Current trials such as the CCTG GA.3 trial focus on examining the role of regorafenib in the later line setting (NCT02773524). It is important to gauge the real-world use of multiple lines of therapy for patients with metastatic esophagogastric cancer, beyond a clinical trial population.
The objectives of this study were to characterize the attrition rates between lines of therapy for patients with unresectable locally advanced or metastatic esophagogastric cancer (EGC), and to identify prognostic factors for improved survival.
MATERIALS AND METHODS
In British Columbia (BC), Canada, BC Cancer is the provincial institution responsible for overseeing cancer-related care for 4.4 million residents, including the development of cancer therapy guidelines, provision of radiation therapy, and funding for all approved systemic therapies. The BC Cancer Provincial Pharmacy Database provides and holds records for all provincially funded systemic therapies across six cancer centres in BC.
We sought to characterize the use and attrition rates between lines of therapy for patients with advanced EGC, defined as either esophageal, gastroesophageal junction, or gastric cancer. We identified patients who received at least one cycle of systemic therapy for advanced histology-confirmed EGC between July 1, 2017 and July 31, 2018 from the BC Cancer Provincial Pharmacy Database. These dates were chosen based on the funding and availability of ramucirumab and paclitaxel after May 2017, and to allow for sufficient follow-up. Clinicopathologic data, treatment details, and survival outcomes were extracted by chart review. Patients who continued on treatment were censored at the date of last contact. Given the focus on systemic therapy, data regarding radiotherapy for palliative purposes was not explicitly included. This study was approved by the BC Cancer Research Ethics Board.
Statistical analysis
Baseline clinicopathologic characteristics and delivery of multiple lines of therapies were reported using descriptive statistics, with differences in variables analyzed using the chi-square or Wilcoxon rank-sum tests where appropriate. Overall survival (OS) was calculated from the date of diagnosis of advanced disease to date of death or date of last follow-up. Univariable and multivariable logistic regression analyses exploring factors associated with improved survival were performed with the Cox proportional hazards model. Variables included in the Cox proportional hazards models were selected based on known prognostic factors and those significant on univariable analysis (P<0.05). OS was estimated using the Kaplan-Meier method, with the logrank test used to compare differences. All tests were two-sided, and aPvalue of < 0.05 was considered statistically significant. Stata version 15.1 was used for all statistical analyses (Stata, College Station, TX, United States).
RESULTS
Of 245 patients who received at least one line of therapy, median age was 66 years (IQR 58.2-72.3) and 186 (76%) were male. Baseline Eastern Cooperative Oncology Group (ECOG) performance status was 0-1 in 80%, and site of primary was gastricvsGEJ (36%vs64%). Histologies included adenocarcinoma (78%), squamous cell carcinoma (8%), and signet ring (14%), with 31% HER2 positive. 72% presented with de novo disease, and 25% had received previous chemoradiation. Further clinicopathologic characteristics are detailed in Table 1.
There was a high level of treatment attrition, with 50% of patients (n= 122) receiving only one line of therapy. Distribution across subsequent lines was: two lines (n= 83, 34%), three lines (n= 34, 14%), and four lines (n= 6, 2%). Patients who received at least two lines of therapy were younger (median age 62.2vs67.6 years,P<0.05) and demonstrated a longer and improved response to first-line systemic therapy compared to those who only received one line of treatment (median duration 5.0vs2.7 mo,P<0.05; 50% complete or partial response (40%vs32%,P<0.05).
In terms of systemic therapy regimens, ramucirumab and paclitaxel was the most common second line treatment (62%), with an objective response (complete or partial response) observed in only 16% of patients (Table 2). In the third-line setting, 5-fluorouracil and irinotecan was the most common regimen (35%). Fourth-line regimens were largely 5-fluorouracil based, with one of six patients receiving nivolumab.
Kaplan-Meier survival analysis demonstrated improved survival with increasing lines of therapy (median OS 7.7vs16.6vs22.8vs40.4 mo,P<0.05; Figure 1). On multivariable Cox regression, improved survival was associated with better baseline ECOG and increased lines of therapy (P<0.05; Table 3).
DISCUSSION
In this population-based analysis, we demonstrate high treatment attrition rates among patients with advanced EGC, with only half of patients proceeding to receive two or more systemic therapy regimens. Survival improved with the application of multiple lines of therapy and a good baseline ECOG performance status.
Our findings of high levels of attrition are consistent with other retrospective studies reported in the literature, with a number of these employing electronic health record (EHR) data. Leet al[12]examined EHR data from the Flatiron Health database, and found a 75% rate of first-line, 32% rate of second-line, 14% rate of third-line, and6% rate of fourth-line therapies.We note that this was primarily in a United States community-based practice setting, compared to our Canadian single-payer system. In a similar EHR-based analysis, Barziet al[13]described a 60% rate of second-line therapy use.Previous retrospective EHR-based studies have reported an approximately 50% rate of second-line therapies[14,15].In a Japanese single institution study, Uenoet al[16]reported a 26% rate of third-line systemic therapy.

Table 1 Baseline characteristics of 245 patients with advanced esophagogastric cancer who received at least one line of systemic therapy
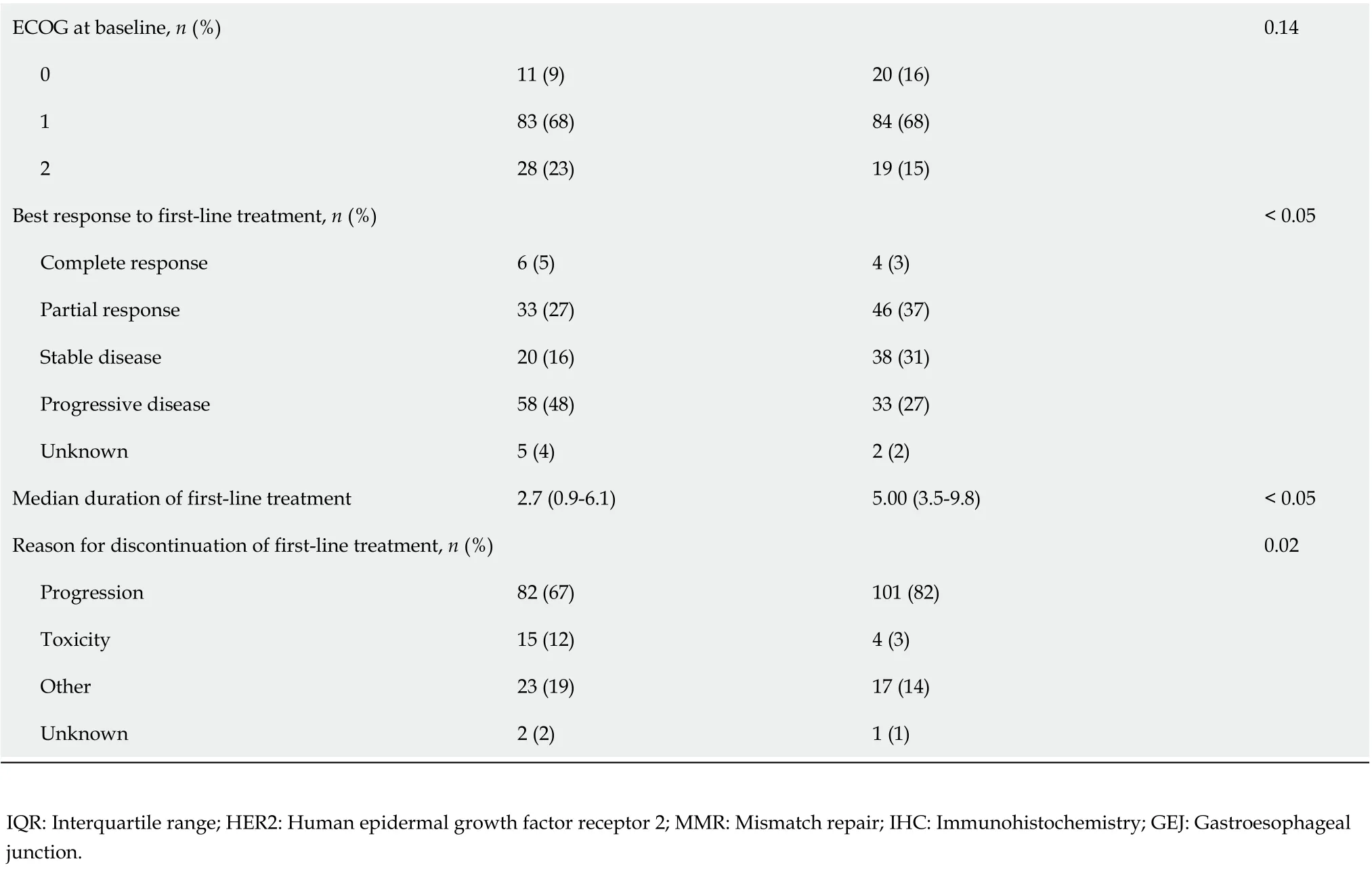
ECOG at baseline, n (%)0.14 0 11 (9)20 (16)1 83 (68)84 (68)2 28 (23)19 (15)Best response to first-line treatment, n (%)< 0.05 Complete response 6 (5)4 (3)Partial response 33 (27)46 (37)Stable disease 20 (16)38 (31)Progressive disease 58 (48)33 (27)Unknown 5 (4)2 (2)Median duration of first-line treatment 2.7 (0.9-6.1)5.00 (3.5-9.8)< 0.05 Reason for discontinuation of first-line treatment, n (%)0.02 Progression 82 (67)101 (82)Toxicity 15 (12)4 (3)Other 23 (19)17 (14)Unknown 2 (2)1 (1)IQR: Interquartile range; HER2: Human epidermal growth factor receptor 2; MMR: Mismatch repair; IHC: Immunohistochemistry; GEJ: Gastroesophageal junction.
Similarly, improved survival outcomes have been associated with an increasing number of lines of therapy and improved baseline performance status[12,17]. Fanottoet al[18]described additional prognostic factors, including lower LDH levels and a lower neutrophil/Lymphocyte ratio at the initiation of second-line therapy.There was insufficient LDH data to examine this, and we did not find a similar association with the neutrophil/Lymphocyte ratio.
The survival outcomes and high level of treatment attrition highlight the lethality of EGC. Screening can play a role in detecting these cancers at an earlier stage. While this is feasible in countries with a high incidence rate such as South Korea and Japan, this is not readily available in Canada[19-21]. For patients already diagnosed with advanced disease, better risk stratification and more effective frontline therapies are urgently needed. The combined positive score is now used to identify patients who are more likely to benefit from immunotherapy, after the findings from KEYNOTE-181[22].Improved biomarkers for risk stratification and treatment selection are also being investigated. Despite the work done in molecular subtyping of gastric cancer, there are few actionable targets and significant intratumoral heterogeneity; thus, limiting the role for precision medicine in the upfront treatment setting[23,24]. Some markers, such asMEToverexpression, have been associated with poor prognosis and this continues to be studied as a potential actionable target[25,26]. In recent years, there has been a shift towards immunotherapy-based combinations, such as in the MORPHEUS umbrella trial[27]. It remains to be seen whether this can play an effective earlier-line strategy in patients with advanced EGC.
Limitations of our study include the retrospective nature, which limits causal inference regarding prognostic factors for improved survival. The limited sample size in a Canadian single-payer health system may limit generalizability. However, while our sample size of 245 patients is smaller than the United States nationwide EHR database, our data are manually curated and provide more granularity as a result. There is also selection bias and practitioner variability in defining ECOG performance status, and in offering a subsequent line of therapy. Finally, this study was done prior to the approval and availability of oral trifluridine/tipiracil in the third-line setting, which may influence treatment practices in this population.

Table 2 Treatment details for patients who received at least two lines of systemic therapy

Unknown 3 (8)Fourth-line chemotherapy backbone [n = 6, n (%)]5-FU 1 (17)5-FU/oxaliplatin 2 (33)5-FU/irinotecan 1 (17)5-FU/cisplatin 1 (17)Nivolumab 1 (17)Best response to fourth-line treatment, n (%)Complete response 0 Partial response 0 Stable disease 1 (17)Progressive disease 5 (83)Median duration of fourth-line treatment (mo)2.57 (2.08-3.75)Reason for discontinuation of fourth-line treatment, n (%)Progression 6 (100)Toxicity 0 Other 0 Unknown 0 5-FU: 5-fluorouracil.
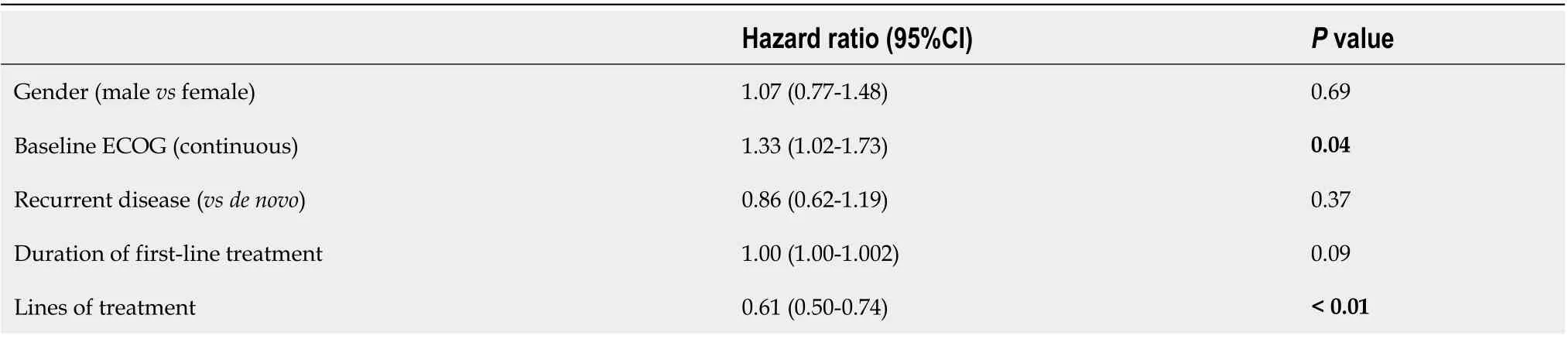
Table 3 Multivariable Cox regression analysis for overall survival
CONCLUSION
We present a population-based analysis of real-world use of later-line therapy in patients with advanced EGC. The steep attrition rates between therapies highlight the high symptom burden in this setting and the unmet need for more efficacious earlyline treatment options for patients with advanced EGC. Improved biomarkers may provide informed risk stratification in selecting later lines of treatment, and identifying patients who would derive greater benefit from multiple lines of therapy.

Figure 1 Overall survival by number of lines of treatment.
ARTICLE HIGHLIGHTS

Research conclusions
The steep attrition rates between therapies highlight the unmet need for more efficacious early-line treatment options for patients with advanced EGC.
Research perspectives
This real-world analysis demonstrating such steep attrition rates highlights the unmet need for more efficacious early-line treatment options.
 World Journal of Gastroenterology2020年39期
World Journal of Gastroenterology2020年39期
- World Journal of Gastroenterology的其它文章
- Use of artificial intelligence in improving adenoma detection rate during colonoscopy: Might both endoscopists and pathologists be further helped
- Metastatic pattern in esophageal and gastric cancer: Influenced by site and histology
- Relationships of early esophageal cancer with human papillomavirus and alcohol metabolism
- Dynamic contrast-enhanced magnetic resonance imaging and diffusion-weighted imaging in the activity staging of terminal ileum Crohn's disease
- Clinical assessment and management of liver fibrosis in non-alcoholic fatty liver disease
- Enteroscopy in children and adults with inflammatory bowel disease
