Enteroscopy in children and adults with inflammatory bowel disease
Giovanni Di Nardo, Gianluca Esposito, Chiara Ziparo, Federica Micheli, Luigi Masoni, Maria Pia Vlla, Pasquale Parisi, Maria Beatrice Manca, Flavia Baccini, Vito Domenico Corleto
Abstract Inflammatory bowel disease (IBD) includes Crohn’s disease (CD), ulcerative colitis and unclassified entities. CD commonly involves the terminal ileum and colon but at the time of diagnosis it can be confined to the small bowel (SB) in about 30% of the patients, especially in the young ones. Management of isolated SB-CD can be challenging and objective evaluation of the SB mucosa is essential in differentiating CD from other enteropathies to achieve therapeutic decisions and to plan the follow-up. The introduction of cross-sectional imaging techniques and capsule endoscopy (CE) have significantly expanded the ability to diagnose SB diseases providing a non-invasive test for the visualization of the entire SB mucosa. The main CE limitations are the low specificity, the lack of therapeutic capabilities and the impossibility to take biopsies. Device assisted enteroscopy (DAE) enables histological confirmation when traditional endoscopy, capsule endoscopy and cross-sectional imaging are inconclusive and also allows therapeutic interventions such as balloon stricture dilation, intralesional steroid injection, capsule retrieval and more recently stent insertion. In the current review we will discuss technical aspect, indications and safety profile of DAE in children and adults with IBD.
Key Words: Enteroscopy; Device assisted enteroscopy; Inflammatory bowel disease; Crohn’s disease; Small bowel disease; Endoscopic balloon dilation
INTRODUCTION
Inflammatory bowel disease (IBD) represents a group of chronic inflammatory disorders that involve the colon, small bowel (SB) and the entire gastrointestinal tract and include Crohn’s disease (CD), ulcerative colitis (UC) and unclassified entities[1,2]. CD is a chronic immuno-mediated inflammation that most commonly involves the terminal ileum and colon, but at the time of diagnosis, it can be confined to the SB, as seen in approximately 30% of CD patients, especially young patients[2-8]. Isolated SBCD can be challenging to diagnose and manage for several reasons. First, the SB is less easily accessible by endoscopy, making it easy to miss a SB-CD diagnosis with conventional endoscopy contributing to a delay in diagnosis as observed in many patients with CD[5]. Second, SB ulcerations induced by infection (such as tuberculosis) or drugs can sometimes be difficult to differentiate from CD[1,8]. Third, compared with the other phenotypes, SB-CD is associated with an increased risk of relapse and stricture development[1,3,6,7]. Fourth, SB cancer associated with CD is a rare but difficult problem because only a minority of these cases are diagnosed preoperatively and at an early stage[9]. Finally, in the paediatric population, SB-CD has particular clinical relevance for its negative impact on growth and pubertal development[2,7]. Thus, objective evaluation of the SB mucosa is essential in differentiating CD from other enteropathies to make therapeutic decisions and to plan follow-up.
For many years, investigation of the SB has been a challenge because of its anatomy, location, and relative tortuosity. The introduction of cross-sectional imaging techniques such as computed tomography (CT) and magnetic resonance imaging (MRI), enterography/enteroclysis and SB ultrasound, have enhanced SB assessment with great accuracy in evaluating transmural and extraluminal disease, but subtle mucosal changes can still be missed[8,10,11].
Capsule endoscopy (CE) and device-assisted enteroscopy (DAE) have significantly expanded the ability to diagnose SB diseases. CE provides a non-invasive test for the visualization of the entire SB mucosa, which can aid in the diagnosis of SB-CD and monitoring the therapeutic response. The main CE limitations in IBD patients are a low specificity, a lack of therapeutic capabilities and the inability to perform biopsies[12,13].
DAE enables histological confirmation when other modalities, such as traditional endoscopy, CE and cross-sectional imaging, are inconclusive and allows therapeutic interventions, such as balloon stricture dilation, intralesional steroid injection, capsule retrieval and, more recently, stent insertion[12-14].
In the current review, we will discuss the technical aspects, indications and safety profiles of DAE in children and adults with IBD.
TECHNICAL ASPECTS
DAE is a generic term for any endoscopic technique that includes assisted progression (i.e., balloons, overtubes or other devices) and includes double-balloon enteroscopy (DBE), single-balloon enteroscopy (SBE), balloon-guided endoscopy (BGE) and spiral enteroscopy (SE). Table 1 shows the main characteristics of the currently available DAE systems.
The DBE system was presented for the first time in Japan in 2001, and the first paediatric report was described in 2003. The DBE system is constituted of a highresolution enteroscope with a latex balloon attached to the end of the enteroscope and a second balloon attached to the tip of a polyurethane overtube. Both balloons are inflated and deflated using an external pressure-controlled pump system. Currently, three DBE systems are available. The P type is the thinnest; however, the small diameter of its working channel (2.2 mm) limits the possibility of performing advanced therapeutic procedures. The T type has an outer diameter of 9.4 mm and a working channel diameter of 3.2 mm. The main advantage of the short type (working length of 152 cm) is the compatibility with all conventional devices; it can also be used for endoscopic retrograde cholangiopancreatography in patients with altered anatomy and difficult or failed colonoscopy[14-18].
SBE was introduced in 2007, and in contrast to DBE, there is no balloon at the tip of the enteroscope; therefore, handling of the balloon control unit is simplified. The enteroscope is a high-resolution video endoscope, the overtube and balloon are made of silicon, and a control unit with a safety pressure setting that controls the balloon inflation and deflation[14-18].
DBE and SBE use the push-and-pull method, however, the number of balloons makes a slight difference in the insertion technique between the two balloon assisted enteroscopy (BAE) systems.
Two operators are generally needed to perform DBE. For the antegrade approach, the endoscope and overtube are advanced to the duodenum beyond the major papilla, at which point the balloon located on the overtube is inflated to hold the small bowel tightly. The enteroscope is then advanced to the distal side of the SB, and its balloon is inflated to prevent slippage of the scope backward. The balloon on the overtube is then deflated, and the overtube is advanced towards the tip of the enteroscope. The balloon on the overtube is then reinflated. The enteroscope-overtube is then withdrawn to fold the SB along the overtube. This process can be repeated until the maximal insertion point or the target lesion is reached[16].
SBE is usually performed by two operators, but it may be easier than DBE to perform with a single-operator. The enteroscope and overtube are advanced similar to the DBE insertion technique. However, the enteroscope tip must be angled during advancement of the overtube and only after the angulation of the tip, the overtube is advanced towards the tip of the enteroscope, and the overtube balloon is then inflated. The enteroscope-overtube is then withdrawn to fold the SB along the overtube. The overtube balloon is left inflated and the enteroscope is advanced from the overtube tip. This cycle of forward advancement and withdrawal is repeated until the maximal insertion point or target lesion is reached[16].
Retrograde insertion is more difficult than antegrade insertion, even in experienced hands. For both techniques (DBE and SBE), the enteroscope and overtube are advanced to the caecum. After inflation of the overtube balloon, the enteroscopeovertube is withdrawn to decrease the ileo-colic angle. With the overtube balloon inflated, the enteroscope is then passed through the ileocecal valve, and endoscope balloon is inflated within the ileum to hold the position. The overtube is then advanced into the ileum with the balloon deflated. For SBE, although the insertion technique is the same, backward slippage of the tip of the enteroscope to the caecum during insertion of the overtube is frequent because of the lack of a balloon at the enteroscope tip, which would enable holding the position[16].
DBE and SBE have the same procedural technique in children and adults. BAE is suitable and safe for children aged>3 years and weight>14 kg. However, due to a smaller abdominal cavity, thinner intestinal walls and a narrower intestinal lumen, BAE requires a greater level of skill in younger children[18].
BGE is performed by using an on-demand through-the-scope (TTS) balloon insertedin the working channel of any endoscope. The balloon is then inflated, allowing anchoring in the SB, and progression is obtained with repeated push-and-pull maneuvres by sliding the endoscope over the catheter. The balloon catheter can be removed to perform therapeutic interventions. The main limitation of this technique is the low stability of the endoscope during the therapeutic procedure due to the lack of an anchoring balloon[14,15]. To overcome this drawback, a colonoscope with an integrated latex-free balloon at the bending section has recently been developed (see Table 1). These colonoscopes, combined with a disposable advancing balloon (AB) applied through the instrument channel, provide the assembly to perform deep SB double-balloon endoscopy. The balloon endoscopes as well as the AB devices are controlled simultaneously by the inflation system. The feasibility and safety of BGE using the NaviAid AB device has recently been evaluated in children with IBD[19].
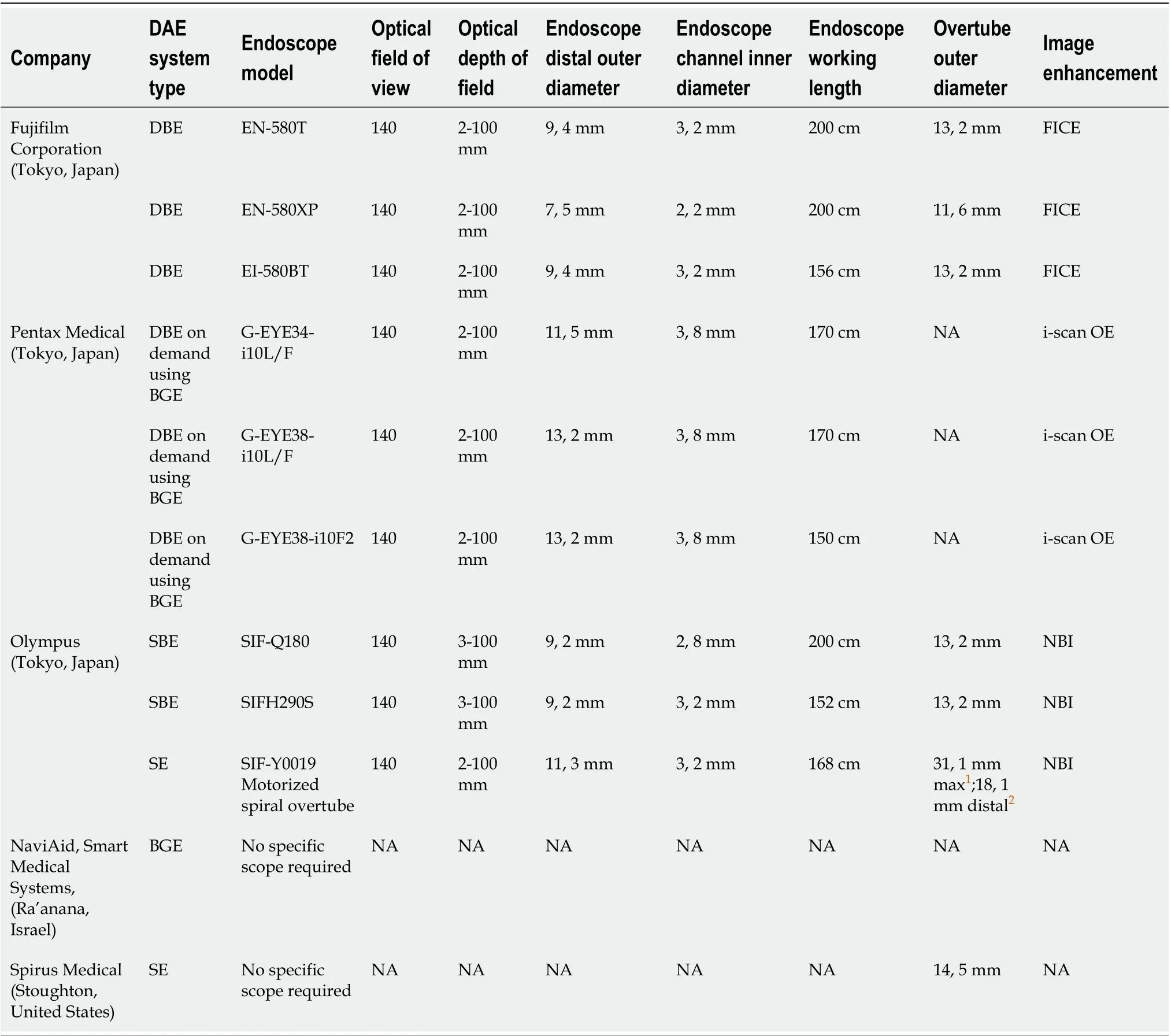
Table 1 Technical characteristics of the currently available device-assisted endoscopes
SE involves the use of an overtube with a raised spiral ridge. The SB is pulled and pleated onto the overtube by continuous rotation of the spiral, and advancement into the SB is allowed by clockwise rotation of the overtube during insertion and anticlockwise rotation during withdrawal. SE overtubes have been replaced by a new motorized spiral enteroscopy (NMSE) system. NMSE system is composed of a reusable endoscope with an integrated motor permitting the rotation of a short spiral overtube placed on the insertion tube portion of the endoscope and a motor control unit with a foot pedal and visual force gauge. The foot pedal activates the drive motor located in the endoscope handle, which controls the rotational direction and speed of a coupler located in the middle of the insertion tube of the endoscope. The bowel is pleated or unpleated on the insertion tube of the endoscope with clockwise or anticlockwise rotation, respectively. After reaching the required depth of insertion, the endoscope will be withdrawn using motorized anticlockwise spiral rotation[14,20]. Preliminary data shows that NMSE offers advantages over traditional methods, in particular concerning the duration of the procedure and the relative ease of use; otherwise, it has similar diagnostic and therapeutic yields as both SBE and DBE[20,21]. Nevertheless, data on SE in IBD patients are not yet available, and the large diameter of the SE overtube makes this technique unsuitable for children.
The approach (oral, anal or both) is determined on clinical judgement, crosssectional imaging (CT/MRI - enterography) and CE. Usually, the oral approach is the first choice due to its lower technical difficulty[16,17,22]. Both oral and anal approaches are used if inspection of the whole intestine is clinically needed. An ink tattoo or clip is left at the deepest point of insertion achieved during the first approach.
Overnight fasting of 12 h for solid food and 4 h for clear liquids before starting the procedure is enough for the oral approach, the same bowel cleansing suggested for colonoscopy required for retrograde enteroscopy. General anaesthesia is recommended for long procedures or for patients in whom sedation is not appropriate (i.e., paediatric and high-risk patients). Fluoroscopy is not always needed, except when adhesions or massive SB-CD involvement is expected and when enteroscopy is aimed to perform stricture dilation[16,17,22]. Insufflation of CO2is recommended due to its capability to allow deeper SB intubation of the scope and minimize postprocedural discomfort[22,23].
ENTEROSCOPY IN ADULT PATIENTS WITH SUSPECTED IBD
Five studies evaluated the impact of DAE on adults with suspected IBD (Table 2)[24-28].
In a retrospective German study, 16 adult patients with clinical suspicion of isolated SB-CD underwent DBE after negative gastroscopy and colonoscopy. Abnormal SB findings were detected in 7 patients (44%) but pathognomonic histological findings of CD were found in only one case (6%). However, a diagnosis of CD was confirmed in 11 out of 16 (69%) patients taking into account clinical, endoscopic and radiological features[24].
Navaneethanet al[25]retrospectively reviewed a BAE registry, which included DBE and SBE procedures performed on adult patients, to assess the diagnostic yield and clinical impact of BAE in suspected SB-CD. They identified 22 patients with suspected SB-CD and inconclusive results from conventional upper and lower gastrointestinal endoscopy, radiological cross-sectional imaging studies, and CE. These patients underwent BAE, which provided a histological diagnosis of CD in 6 patients (27.3%) and non-steroidal anti-inflammatory drug-induced enteropathy in 3 patients (13.6%), whereas no lesions were found in 13 patients (59.1%). One newly diagnosed CD patient underwent successful balloon dilation of a jejunal stricture without complications. The authors also evaluated the agreement rate between CT or MRI enterography and BAE findings which was quite low (36.4%).
In a multicenter retrospective study, 43 adult patients with suspected CD based on abnormal cross-sectional imaging or CE were evaluated by DBE. The diagnostic yield reached 79%. SB-CD diagnosis was confirmed in 17 patients, and DBE examination was normal in 12 cases. The remaining 5 patients received alternative diagnoses, such as NSAID ulceration, stricture/solitary ulcer, anastomotic ulcer, or Meckel’s diverticulum. Another main outcome measure of this study was a comparison of DBE and CE findings. Overall, only 46% of lesions were confirmed on DBE. In 33 (77%) patients, BAE modified clinical management by the exclusion of CD in 11 patients, confirming CD in 17, diagnosing stenosis in 2, nonspecific ulcer in 2 and stopping the NSAID treatment in 1[26].
Tunet al[27]retrospectively evaluated the impact of DBE and histology on the diagnosis and management of 100 adult patients with suspected SB-CD for whom, based on clinical and laboratory data and after colonoscopy and radiological imaging studies or CE, a diagnosis of CD was not achieved. Abnormal macroscopic DBE findings were detected in 60 patients (ulcers,n= 47; stricture,n= 11; abnormal mucosa,n= 2), and biopsy samples was obtained. Twenty-three showed no histological abnormalities despite positive macroscopic appearances on DBE, whilst among the remaining 37 patients, according to histological examination, the diagnosisof CD was confirmed in 8 patients (22%), and 15 (41%) had histology suggestive of CD.
Nevertheless, combining clinical, biochemical, endoscopic, and histological findings, 45% of all patients received treatment for CD. After a median follow-up period of 27 mo, the diagnosis of CD was confirmed in 38% of DBE-positive patients[27].
The diagnostic yield and the impact on clinical outcome of the use of SBE for suspected SB-CD were evaluated in an Italian retrospective study, which included 13 adult patients. The diagnostic yield was 39%, detecting four patients with active ileitis and one with ileal stricture. For the remaining eight patients, a new diagnosis of CD was reached in 4 patients (8%) and excluded in the other 4 patients (8%)[28].
ENTEROSCOPY IN CHILDREN WITH SUSPECTED IBD
Five studies, two on SBE, two on DBE and one on BGE, evaluated the impact of DAE on children with suspected IBD[19,29-32](Table 3).
In a study previously published by our group, 16 paediatric patients with suspected CD and unspecific findings after extensive assessment with upper and lower GI endoscopy, MRE and CE were assessed by SBE. SBE provided a histological diagnosis of CD in 12 patients and eosinophilic enteropathy in 2 patients, no lesions were found in the remaining 2 patients. Moreover, SBE allowed dilation of SB strictures identified on MRI in 2 suspected CD patients[29].
de Ridderet al[30]evaluated the diagnostic yield of SBE for paediatric CD. In this study, patients were evaluated directly by two-route SBE, not preceded by conventional endoscopy or CE, and in 8 out of 14 patients with suspected CD, the diagnosis was confirmed after SBE.
Urset al[31]performed DBE in 3 patients with suspected CD after CE examination indicating mucosal abnormalities. DBE led to a CD diagnosis in 2 out of 3 patients, and CD was excluded in the other child due to the lack of mucosal lesions and a normal histology.
The study from Uchidaet al[32], evaluated the efficacy and safety of DBE in 8 children with suspected CD after inconclusive gastroscopy, colonoscopy and an SB-contrast study. DBE confirmed a CD diagnosis in 2 out of 8 patients, led to an alternative diagnosis in four patients and did not find mucosal lesions or histological abnormalities in the remaining 2 patients.
Recently, Broideet al[19]evaluated the feasibility and safety of BAE using a NaviAid AB device in children with suspected IBD. Technical success was achieved in 95.23%and 85.7% of the anterograde and retrograde approaches, respectively. Moreover, the total procedure time was significantly shorter and the learning curve was faster than with BAE, as its operation is intuitive and simple. Unfortunately, the diagnostic yield of this technique was not assessed. In the 15 patients with suspected IBD, 3 patients were diagnosed with UC and 3 patients with CD; the remaining 9 patients showed no intestinal abnormalities.

Table 3 Available study on device assisted enteroscopy in pediatric inflammatory bowel disease
According to the previously described literature and our previously published algorithm (Figure 1), in children with suspected IBD, we suggest the following approach. DAE is recommended when conventional endoscopy, imaging of the SB and CE are inconclusive and tissue sampling and/or therapeutic procedures would alter disease management. DAE should be the preferred primary endoscopic procedure only if a stricture or an easy-to-reach lesion (i.e., proximal SB wall thickness) is suspected at imaging. This is reasonable due to DAE diagnostic and therapeutic possibilities and to the high capsule retention risk[6,18].
ENTEROSCOPY IN SUSPECTED AND ALREADY KNOWN IBD ADULT PATIENTS
Three studies evaluated the impact of DAE on both suspected and already known IBD adult patients (Table 2)[33-35].
Kondoet al[33]analyzed a total of 1444 cases of DBE performed for various indications collected in a multicenter database to investigate the efficacy of DBE for the diagnosis and treatment of CD on adult patient. A total of 50 known and 25 newly diagnosed patients with CD were included. Active inflammatory lesions (ulcer and erosion/redness) were found in 51.2% of the symptomatic patients, but they were also detected in 33.3% of the asymptomatic CD patients. Overall, the treatment was altered for 53.3% of the patients, resulting in introduction of anti-TNF antibody (20.4%) or other medication (17.5%), dose reduction (1.9%), endoscopic balloon dilation (EBD, 7.8%), and surgical treatment (5.8%).
In 2011, Möschleret al[34]published the results derived from a large prospective German database that reported data from 2245 DBE examinations carried out on 1765 adult patients over a 2-year period. Overall, 193 patients (11%) with known or suspected CD underwent endoscopic examination of the SB, showing pathological findings in 91 of them, with a diagnostic yield of 47%.
In a retrospective study, Christianet al[35]evaluated the diagnostic and therapeutic yields of SBE using solely a retrograde approach. Overall, 136 retrograde SBE procedures were considered. Twenty-nine (21.3%) were performed on adult patients with suspected or known CD. Twelve new diagnoses of CD were established, with a diagnostic yield of 41.4%, and the therapeutic yield was 17.2%.

Figure 1 Suggested algorithm in children with suspected inflammatory bowel disease (adapted by reference 6). GI: Gastrointestinal; MR: Magnetic resonance; SB: Small bowel.
ENTEROSCOPY IN ADULT PATIENTS WITH KNOWN IBD
Four studies evaluated the impact of BAE on adults with known IBD (Table 2)[25,26,28,36].
Mensinket al[36]assessed the clinical impact of endoscopic evaluation of the SB by DBE for patients with known CD and clinical suspicion of SB activity. They retrospectively analyzed 52 DBE procedures performed in 40 adult patients. Twentyfour patients (60%) showed macroscopically active inflammation of the SB, and 18 of them (75%) had to switch therapy with persistent clinical improvement in 83% of patients after a mean follow-up of 13 mo. In particular, amongst the 18 patients, 11 introduced anti-TNF therapy, 2 switched from infliximab to adalimumab, 1 introduced steroid therapy, 2 underwent surgical resection, and 2 underwent balloon dilation.
In the study from Navaneethanet al[25], BAE was performed in 43 adult patients with an established diagnosis of CD whose main indications were disease activity and extent assessment in uninvestigated CD, anaemia or obscure gastrointestinal bleeding, confirmation and treatment of SB strictures diagnosed on radiological examination, and evaluation of activity and extent of CD in postoperative patients. BAE had impact on clinical management of 23 patients (53.4%): 18 patients (41.8%) had active inflammation with ulcers or strictures, which led to a switch of medical therapy or surgery, 5 (11.6%) with documented stenosis in the absence of active ulcers underwent EBD to treat obstructive symptoms. Overall, thirteen patients (30%) required surgery: Two due to lack of other therapeutic strategies, five as a result of medical treatment escalation failure, five based on patient’s choice, and one due to bowel perforation after BAE. Finally, the authors found that the agreement rate between CT or MRI enterography and BAE findings were higher (75.6 %) in already diagnosed CD patients.
In the study by Rahmanet al[26], the authors analyzed the diagnostic yield and the clinical impact of DBE on the management of 38 adult patients with known CD and clinical suspicion of SB disease activity. In this setting, the diagnostic yield was 87% (33/38 patients), revealing an active disease in 11 patients (29%), 5 with CD stricture (13%), 3 (8%) with functional obstruction due to fixed/angulated bowel, 3 (8%) with anastomotic ulcers, and 2 (5%) with nonspecific ulceration. DBE examination was normal in 9 cases (23.6%). Therefore, DBE resulted in a clinical management change for 82% of patients with known CD. Thirteen patients (34%) needed an increase or a switch of therapy or surgery, and three patients (8%) underwent endoscopic dilation without any related complications. One patient (1.2%) underwent surgery due to perforation consequent to diagnostic DBE and directly related to an ulcer at an anastomosis site.
Holleranet al[28]evaluated the diagnostic yield and the impact on clinical outcome of the use of SBE for 39 patients with established SB-CD. In this setting, the diagnostic yield was significantly higher than for adult patients with suspected CD (77%vs39%,P< 0.01). The most frequent findings were ileal or anastomotic strictures in 38% and 26%, respectively, and active ileitis in 21% of patients. SBE had an immediate clinical impact on 69% (n= 33) of patients, including stricture dilation in 27%, adjustment of medications in 48%, and referral for surgical resection in 6%. Long-term follow-up (mean duration of 11 mo, range of 3-22 mo), performed in 34 patients (65%) of the 52 patients, showed a significant change in the mean Harvey-Bradshaw index score from 6.6 to 4.2 after the procedure (P< 0.0001).
Regarding the therapeutic role of enteroscopy in adult patients with IBD complications, several studies evaluated the clinical impact of EBD using BAE for SB stricture in CD (Table 4)[25,28,33,37-45]. Among them, Hiraiet al[45]conducted, to our knowledge, the largest multicenter study currently available, which prospectively enrolled 112 patients with symptomatic SB strictures related to CD to clarify the efficacy and safety of EBD. Ninety-five patients (85%) were included, and balloon dilation was successful in 89 (94%) of them. The primary endpoint related to shortterm outcomes was an improvement of symptoms, which was achieved in 66 patients (70%). The dilation diameter was significantly larger (15.20 ± 1.70 mmvs13.65 ± 2.59 mm,P= 0.03) in the short-term symptomatic improvement group than in the no improvement group. There were no other significant differences in the groups’ baseline characteristics or stricture features.
The long-term outcomes of EBD for SB stricture in CD adult patients were extensively evaluated by two retrospective Japanese studies[43,44].
Hiraiet al[43]evaluated 65 CD patients with obstructive symptoms caused by endoscopically manageable SB strictures (stricture length ≤ 5 cm, not associated with fistulae, abscesses or deep ulcers, and without severe curvature of the stricture) with clinical success in 80.0% of patients (52/65). During the observation period after the initial EBD (mean 41.8 ± 24.9 mo), seventeen patients (26.2%) underwent surgery. The cumulative surgery-free rate was 79% and 73% at 2 and 3 years, respectively, and it was significantly higher among successful EBD cases (P< 0.0001). Moreover, the cumulative re-dilation-free rate was 64% at 2 years and 47% at 3 years. No significant differences in terms of concomitant treatment or initial dilation method were detected between patients with and without the need for re-dilation.
More recently, Sunadaet al[44]analyzed data regarding 85 patients who underwent DBE-assisted EBD for SB-CD strictures and were then followed-up for a mean period of 41.9 mo (range, 0-141). The surgery-free rate after the initial EBD was 87.3% at 1 year, 78.1% at 3 years, and 74.2% at 5 years. Univariate analysis showed that the presence of an internal fistula beside the refractory stricture was significantly associated with the need for surgical intervention (hazard ratio: 5.50; 95%CI: 2.16-14.0;P= 0.01).
ENTEROSCOPY IN CHILDREN WITH KNOWN IBD
Five studies, two on SBE, two on DBE and one on BGE evaluated the impact of BAE in children with established IBD (Table 3)[19,29-32].
In our study, the SBE findings of 14 patients with longstanding CD and symptoms unexplained by conventional endoscopy led to the introduction of or to a change in therapeutic approach in 11 patients. Moreover, SBE allowed successful and safe dilation of SB strictures identified on MRE in 3 patients[29].
In the study of de Ridderet al[30]SBE findings led to a change in therapy in five out of six patients with established CD.
In the paper from Urset al[31], in the established CD group, 2 patients had adverse reactions to infliximab with poor response to adalimumab and 3 patients underwent DBE for disease evaluation and consideration of escalation of treatment. DBE led to a change of treatment in all 5 patients.
Uchidaet al[32]evaluated the efficacy and safety of DBE in 4 children with established CD. After DBE, one patient underwent balloon dilatation and a change in medical therapy, and in two patients, surgical resection was planned. In one patient, DBE was performed for assessment of intestinal lesions due to persistent abdominalpain, and only small erosion near the ileal stoma orifice was found; thus, therapy after DBE was not changed.

Table 4 Available studies on endoscopic balloon dilation using balloon-assisted enteroscopy for small bowel stricture in adult Crohn’s disease patients
Recently, Broideet al[19]evaluated the feasibility and safety of BAE using a NaviAid AB device in 16 children with known IBD (undetermined colitis = 7; CD = 9). In this group, 6 patients with undetermined colitis at baseline were confirmed to have active UC, and 1 patient exhibited mucosal healing. Eight of the 9 patients with known CD were confirmed to have active CD, and 1 patient exhibited mucosal healing.
According to the previously described literature and our previously published algorithm (Figure 2), we suggest the following approach for children with known IBD. DAE is recommended when endoscopic visualization and biopsies of the small intestine are needed to exclude an alternative diagnosis (lymphoma, tuberculosis or carcinoma) or to perform a therapeutic procedure including SB stricture dilation and removal of retained capsule. Endoscopists should keep in mind that in established CD adhesions may limit examination and that active stricturing CD significantly increases the perforation risk[6,18].
COMPLICATIONS
The most common complications related to DAE are perforation, bleeding, and pancreatitis, with an overall rate on adult patients of approximately 1%[34,46].
A large retrospective Japanese study identified 29068 patients who underwent diagnostic BAE, reporting 32 cases of perforation (0.11%). Nine hundred forty-two patients underwent a subsequent therapeutic BAE, but no perforations occurred in this group. Univariate logistic regression analysis showed that patients with IBD, irrespective of steroid therapy, had a significantly higher risk of perforation than patients without (8.6-fold and 2.5-fold, respectively)[47]. In most published studies, the perforation rate among CD patients who underwent EBD varied from 0% to 10% of subjects[25,28,33,37-41,44,45], although one small cohort reported a perforation rate of 20%[42](Table 4). Bleeding after balloon dilation of CD strictures occurs in approximately 2.5% of patients, and it only often requires conservative management[25]. Finally, pancreatitis has been reported to occur in 0.3% of patients, especially after procedures with an anterograde approach[34,48,49]. Adverse event rates for the different types of DAE have been shown to be similar[50].
In paediatric literature, major complications have been reported only for therapeutic procedures. A large retrospective study on 257 DBE procedures in children reported an overall complication rate of 5.4% (10.4% in patients under 10 years)[51]. No major complications related to either diagnostic or therapeutic procedures have been reported in the paediatric IBD setting.
CONCLUSION
This review analyzed the use of enteroscopy in children and adults with IBD.
Regarding the use of DAE for diagnostic purposes in adult patients, recent ECCOESGAR guidelines recommend its use for: (1) Patients with negative endoscopy and suspicion of CD on MRI or CE, if endoscopic and histological diagnostic confirmation is needed; and (2) Patients who need endoscopic intervention in the SB[52].
According to the studies considered in this review, as shown in Table 2, the diagnostic yields of DAE in adult patients with suspected CD and with known CD is 27%-79% and 53%-87%, respectively, and are higher if the indication for DAE is based on previous SB investigations that may identify suspected lesions and guide the choice of insertion route[25,26,28]. Meanwhile, the diagnostic yield of DAE drops drastically when the indication is placed exclusively based on non-specific abdominal symptoms[34]. Similarly the agreement rate between imaging and DAE findings appears to be higher in already diagnosed CD patients than in suspected CD patients (75.6%vs36.4% respectively)[25]. In published studies a significant impact of DAE on patient management, ranges from 17% to 82%, has been reported with a persistent clinical improvement reaching 83% after a mean follow-up of 13 mo in the study by Mensinket al[36](Table 2)[25-28,33,35].

Figure 2 Suggested algorithm in children with known inflammatory bowel disease (adapted by reference 6). GI: Gastrointestinal; MRI: Magnetic resonance imaging; SB: Small bowel; CE: Capsule endoscopy.
Endoscopic ballon dilation overall has a technical success rate from 72% to 100%, and a clinical success rate greater than 60%[25,28,33,37-45], although no standardized definitions of both these short-term outcomes has been clearly stated yet. The majority of studies evaluated clinical success basing on the obstructive symptoms reported by the patients often comparing the clinical improvement before and after dilation[40,45]. The most commonly used definition of technical success was a chance to get the successful inflation of a balloon catheter within the stenotic bowel segment and the subsequently endoscope passage through the dilated segment. Studies considered in this review showed that the mean stricture dilation diameter varied from 12.4 to 17 mm, up to a maximum of 20 mm, and the achievement of a larger dilation diameter seems to be related to a better short-term clinical improvement[45]. The recurrence of obstructive symptoms after EBD has been variously reported (14% to 78.5% of adult subjects) according to the time considered that in most cases was very short (less than fifteen months). However, in two studies the average duration of the observation period after initial EBD was greater than three years[43,44]. They showed the highest recurrence rates of obstructive symptoms (48% and 78.5%, respectively) but most patients underwent successful re-dilation with a high cumulative surgery-free rate (over 78% at three years). Although balloon dilation of CD strictures appears effective in the short term, it needs to take into consideration the high recurrence rate with the possibility of repeated endoscopic procedures and/or surgery. To our best knowledge, no RCT comparing surgeryvsballoon dilation has been conducted. As suggested by the ECCO guidelines[53], EBD or surgery are both suitable treatment options for patients with short (< 5 cm) strictures of the terminal ileum in CD, and the choice of treatment depends on local expertise and patient choice (very low level of evidence). Overall, DAE is a relatively safe procedure if it is performed in expert hands. However, needs deep sedation with the presence of anesthesiologist, may require a bidirectional approach, and has a high complication rate, making this technique not very widespread. Moreover, in children with CD EBD it could delay or avoid invasive surgery to dilate the stenosis and potentially positively affect the natural history of chronic disease all the more important the more it is in childhood.
The present review underlines several limitations related to the studies currently available in the literature. First, most of them are retrospective and include a small number of patients. Second, the studies are often extremely heterogeneous in terms of patient selection, including IBD and non-IBD patients. Third, there are no standardized definitions of the principal outcome measures, such as technical success and clinical efficacy. Fourth, observational period after therapeutic endoscopic procedures, such as EBD, are often too short, not allowing a long-term evaluation. Fifth, into the manuscripts some results are not always clearly stated but have been calculated on the basis of results by the authors of the review.
In conclusion, enteroscopy seems a promising technique especially in patients with suspected isolated SB-CD and inconclusive results from conventional studies (including ileocolonoscopy and radiological cross-sectional imaging) in whom histological diagnosis would alter patient management. Moreover, DAE could have many roles in patients with known CD, in terms of adjustment of medical therapy to obtain more lasting and deep clinical improvement, concomitant diagnosis and treatment of stenosing complications and accurate localization of lesions to allow targeted surgical intervention. Because mucosal healing is increasingly becoming a goal of therapy in CD, DBE may have a role in assessment response to therapy in the future in select cases. More standardized and wider studies are needed to confirm these evidences.
ACKNOWLEDGEMENTS
We thank Altobello Leo and Di Maio Adele for their tireless work to improve the care of pediatric patients.
 World Journal of Gastroenterology2020年39期
World Journal of Gastroenterology2020年39期
- World Journal of Gastroenterology的其它文章
- Use of artificial intelligence in improving adenoma detection rate during colonoscopy: Might both endoscopists and pathologists be further helped
- Real-world treatment attrition rates in advanced esophagogastric cancer
- Metastatic pattern in esophageal and gastric cancer: Influenced by site and histology
- Relationships of early esophageal cancer with human papillomavirus and alcohol metabolism
- Dynamic contrast-enhanced magnetic resonance imaging and diffusion-weighted imaging in the activity staging of terminal ileum Crohn's disease
- Clinical assessment and management of liver fibrosis in non-alcoholic fatty liver disease
