Use of artificial intelligence in improving adenoma detection rate during colonoscopy: Might both endoscopists and pathologists be further helped
Emanuele Sinagra, Matteo Badalamenti, Marcello Maida, Marco Spadaccini, Roberta Maselli, Francesca Rossi,Giuseppe Conoscenti, Dario Raimondo, Socrate Pallio, Alessandro Repici, Andrea Anderloni
Abstract Colonoscopy remains the standard strategy for screening for colorectal cancer around the world due to its efficacy in both detecting adenomatous or precancerous lesions and the capacity to remove them intra-procedurally. Computeraided detection and diagnosis (CAD), thanks to the brand new developed innovations of artificial intelligence, and especially deep-learning techniques, leads to a promising solution to human biases in performance by guarantying decision support during colonoscopy. The application of CAD on real-time colonoscopy helps increasing the adenoma detection rate, and therefore contributes to reduce the incidence of interval cancers improving the effectiveness of colonoscopy screening on critical outcome such as colorectal cancer related mortality. Furthermore, a significant reduction in costs is also expected. In addition, the assistance of the machine will lead to a reduction of the examination time and therefore an optimization of the endoscopic schedule. The aim of this opinion review is to analyze the clinical applications of CAD and artificial intelligence in colonoscopy, as it is reported in literature, addressing evidence, limitations, and future prospects.
Key Words: Colonoscopy; Artificial intelligence; Adenoma detection rate; Pathology; Endoscopy; Computer-aided detection and diagnosis
INTRODUCTION
Colorectal cancer (CRC) is a major healthcare issue all over the world. It is the third most common cancer in males and second in females, and the fourth cause for cancer death worldwide[1-3]. As of now, almost 60%-70% of recognized cases in symptomatic patients are diagnosed at an advanced stage[4]. Early stage detection would offer better outcome in terms of decreasing the disease burden[5].
Globally, colonoscopy remains the standard strategy for CRC screening due to its efficacy in both detecting adenomatous or pre-cancerous lesions and the possibility to remove them intra-procedurally[5,6]. However, the identification as well as the classification of lesions are strictly operator-dependent, human errors in this setting increases the chances of progression to CRC[6]. Although uncertain, indirect evidence highlights that endoscopists might fail to detect lesions even when within the endoscopic field of view[7-11], therefore increasing the occurrence of interval colorectal cancer, defined as a CRC diagnosed within 60 mo after a negative colonoscopy.
Computer-aided detection and diagnosis (CAD), thanks to the brand new developed innovations of artificial intelligence (AI), and especially deep-learning techniques, leads to a promising solution to human biases in performance by guarantying decision support during colonoscopy[11]. The aim of this opinion review is to analyze the clinical applications of CAD and AI in colonoscopy, as it is reported in literature, addressing evidence, limitations, and future prospects.
WHAT IS AI
CAD systems by the use of advanced AI techniques represent an innovative technology that will likely lead to a paradigm move in the field of diagnostic colonoscopy[12,13]. Since endoscopy is generally related to computer vision technology, this technique allows computers to “see” and decipher visual content[14]. Through processes of machine learning, and more recently deep learning, AI systems can be trained to recognise “normal” characteristics by connecting a gold standard to suitable images[14](Figure 1).
Deep learning is a recent machine learning method that utilizes a deep neural network automatically extracting specific features from data without human power if a very high numbers of learning samples are available[15,16].
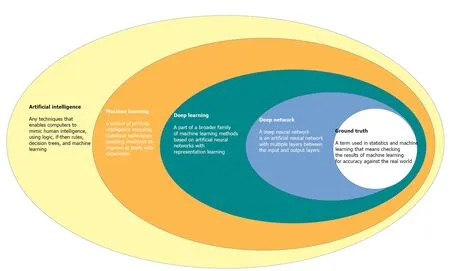
Figure 1 Interlacing of the main concept involved in the filed of artificial intelligence.
Initial AI development is based on creating an algorithm that uses highly accurate datasets, coded in an independently organized manner by a group of specialists[16]. This process is called the “ground-truth” setting, where the computer is prepared to distinguish between ‘abnormal’ and ‘normal’ tissue[16]. Once an algorithm is made, deep convoluted neural networks use vast datasets to enable the algorithm to become more skilled[16]. This intelligence can extend to the algorithm becoming agnostic to manual data input and it can learn by developing its own rules and classifiers[16,17]. AI is expected to have at least 2 major roles in colonoscopy practice: Polyp detection (CADe) and polyp characterization (CADx). CADe has the potential to decrease the polyp miss rate, contributing to improve adenoma detection, whereas CADx can improve the accuracy of colorectal polyp optical diagnosis, leading to reduction of unnecessary removal of non-neoplastic lesions, potential implementation of a resectand-discard strategy, and proper application of advanced resection techniques[17-20].
HOW COULD AI HELP IN POLYP DETECTION
Adenoma detection rate (ADR) is defined as the proportion of patients with at least one colorectal adenoma detected at first-time screening colonoscopy, among all the patients examined by an endoscopist[21]. The benchmark for ADRs is 25% overall (30% in males, 20% in females)[22]and it is reported that a 1% increase in the ADR is associated with a 3% decrease in interval CRC incidence[20]. A recent systematic review with Meta-Analysis reported that about 25% of colorectal neoplasm are missed at screening colonoscopy[18], resulting in an unacceptable variability in the key quality indicator ADR, among endoscopists[19,20].
The rationale on the working process and therefore on the aid of AI CADe system is the Deep Learning properties. CADe systems are fed with data derived from video recordings of colonoscopies that are post-hoc revised by experts. Among them, a Convolutional Neural Network (GI-genius, Medtronic) for instance was trained and validated (99.7% sensitivity, 0.9% of activation noise due to false-positive frames)[23]using a video library of 2684 Lesions that were confirmed at histology, in a highquality clinical trial[24]. This CAD system collects input from the digital frames of the standard endoscopic processor and outputs the coordinates of a signal box only when instance of the target lesions are recognized in the image. Real-time assessment is conceived thanks to the fact that this input-output process, leading to the CADe signal on the endoscopy display, is not perceivable as a matter of time by the endoscopist, because it appears immediately (1.52 ± 0.08 μsi.e.,1.52 millionth of a second)[25].
The average video recording of a 30 min colonoscopy is made of approximately 50000 frames, corresponding to 25-30 frames per second, and one polyp may be recognizable even only in few frames, explaining how failure in polyp recognition is likely to occur, irrespectively of the endoscopy setting. In this way this cutting-edge new technology could provide the essential help in decreasing the CRC incidence and related mortality. In fact, CADe systems have been recently introduced in the real-time endoscopic setting to overcome the colossal miss lesions rate. The outcomes reported by differentmono- and multi-centric randomized clinical trials (RCTs) seem to be stronglypromising: The overall ADR of these studies was significantly higher while aided by CADe systems as shown in Table 1[25-29]. Repiciet al[25]conducted in 2019 a multicentered study in which 700 consecutive 40 to 80 years old patients were randomized 1: 1 in CADe arm and Control. ADR was significantly higher in the CADe arm 54.8%vs40.4%. They also reported the Adenoma Per Colonoscopy, that still was higher in the CADe group (1.05 ± 1.55vs0.7 ± 1.19)[25]. Wanget al[26]in 2019 performed an open, non-blinded trial, in which 1058 consecutive patients were prospectively randomized to undergo diagnostic colonoscopy with (n= 522) or without (n= 536) assistance of a real-time automatic polyp detection system. Thanks to the aid of AI, ADR increased from 20% to 29%[26]. Wanget al[26]in 2020 performed a double blind monocenter RCT enrolling 1010 patients that were randomized in a CAD aided arm (n= 508) and a Control one (n= 502), reporting an higher ADR in the CAD assisted arm: 34.1%vs28%. Gonget al[28]in 2020 performed and RCT in which 704 patients were randomly allocated with the ENDOANGEL CADe system (n= 355) or unassisted (control) colonoscopy (n= 349). ADR was significantly higher in the CADe group: 16.7%vs8.2%. Liuet al[29]enrolled 1026 patients in a RCT and randomized them in a Control arm (n= 518) and a CADe assisted one (n= 508), also in this case ADR was reported to be significantly higher on the CADe assisted group: 39.2%vs24%. This type of AI could also help in standardizing colonoscopy regardless the operator and the endoscopic setting by eliminating the subjective biases.
HOW COULD AI HELP IN POLYP CHARACTERIZATION
In order to reduce the incidence and related mortality of CRC, there is also a major factor other than missing-lesions that need to be demolished: The accuracy of characterizing and classifying small (< 5 mm) polyps by trained endoscopists is reported to be < 80%[30]. Furthermore, the resection of non-neoplastic/hyperplastic polyps, that are almost never evolving in a malignant form, represent a financial burden, an unnecessary intervention performed on an healthy tissue, and both the resection or/and the observation of these irrelevant polyps represent an unnecessary waste of time[31]. Recently, CADx system AI for polyp characterization and diagnosis, optical biopsy and histology prediction classificationhas been developed by multiple groups that used computational analysis to predict polyp histology[32-34]. CADx are applications of AI where the system helps the physician in interpreting the content of a medical image by assigning a label to the image itself. Consequently, AI systems that support the real time optical biopsy by providing the classification of a colon polyp into a category (e.g.,adenoma, hyperplastic,etc.) are CADx. The Convolutional Neural Networks that are able to perform such task are called image classifiers, and the research in this field has been extremely active in the past five years. Both accuracy, sensitivity, specificity, positive predictive value and negative predictive value (NPV) as well as feasibility of these systems have been recently described: Aiharaet al[35]initially published a prospective study of 32 patients with 102 colorectal lesions, with the use of CAD to differentiate neoplastic from non-neoplastic lesions using a CADx system that enables “real-time” color analysis of colorectal lesions when applied to autofluorescence endoscopy to perform color-tone sampling aiming to the reduction of unnecessary treatments for non-neoplastic lesions; all lesions greater than 5 mm were endoscopically removed and lesions less than 5 mm were biopsied. They concluded that lesions with a green/red ratio of less than 1.01 were neoplastic while those above that cut-off were considered non-neoplastic, with sensitivity, specificity and NPV of 94.2%, 88.9% and 85.2% respectively[35]. Kuiperet al[36]used a system called WavSTAT for real-time optical diagnosis based on laser-induced autofluorescence spectroscopy on 87 patients with 207 small (< 10 mm) colorectal lesions. During colonoscopy, the endoscopists tried to differenciate real-time adenomasvsnon-adenomas as a low or high confidence call. Then, all lesions were analyzed using the system. Histopathology was used as the reference standard. The accuracy and NPV of WavSTAT were 74.4% and 73.5% respectively for WavSTAT alone, while they were 79.2% and 73.9% respectively combining WavSTAT with high resolution endoscopy[36]. The study concluded that it did not fulfill the American Society of Gastrointestinal Endoscopy (ASGE) performance thresholds for the assessment of diminutive and small lesions. Rathet al[37]used the WavSTAT4 optical biopsy forceps system designed by Spectrascience Inc, San Diego, California, United States. For prediction of histology using laser-induced autofluorescence spectroscopy on 27 patients with 137 diminutive(≤ 5 mm) polyps[37]. The accuracy was 84.7% along with sensitivity of 81.8%, specificity of 85.2% and NPV of 96.1%. They concluded that this new WavSTAT4 systemhad the potential to meet the ASGE thresholds for the “resect and discard” strategy. Kominamiet al[38]used a narrow band imaging (NBI) magnification colonoscopy system for CAD real-time image recognition system with a support vector machine outputof colorectal polyps histology on 41 patients with 118 colorectal lesions[38]. Concordance between the endoscopists and CAD was 97.5%. The accuracy, sensitivity, specificity, positive predictive value and NPV were 93.2%, 93%, 93.3%, 93% and 93.3% respectively, concluding that this CAD system may satisfy the Preservation and Incorporation of Valuable Endoscopic Innovations committee recommendations of ASGE for the resect and discard strategy. Moriet al[39]used × 520 ultramagnifying colonoscopes providing microvascular and cellular visualization of colorectal polyps after application of NBI and methylene blue staining modes to assess the performance of real-time CAD. To assist with CAD and characterization in 325 patients with 466 diminutive polyps. The accuracy was 98.1% with a NPV of 96.4% and 96.5% for methylene blue staining modes and NBI modes respectively. They concluded that their CAD system can achieve the thresholds of preservation and incorporation of valuable endoscopic innovations for diminutive, non-neoplastic rectosigmoid polyps[39]. Study data are reported in Table 2. Given the novelty of this type of AI, there is still a lack of Randomized Clinical Trials that would be needed to define the exact efficacy of CADx system.

Table 1 The overall adenoma detection rate of these studies was significantly higher while aided by computer aided detection systems
Anyhow, these technologies are rapidly evolving andare expected also to lead to a reduction of the costs related to unnecessary polypectomy, “resect and discard” and perhaps, in the future, to substitute completely the pathological examination of the resected tissue, therefore defining the specific histological characteristic and related malignancy potential as well as potential consequent follow ups immediately, during colonoscopy itself. In addition, most of the proposed AI are aiming to create a system which is able to write automatic endoscopic report. The main advantages of developing this aspect would increase the accuracy of the report itself that nowadays can stillbe compromised by the subjectivity bias of different endoscopists both in describing location and characteristics of the polyps, such as dimensions or pit patternetc.In this way, we would expect that this technology would help standardizing characterization real-time of the lesions, and the endoscopic reports as well. The automatic report would also help the endoscopist to avoid time wasting in the writing of the report and could therefore also increase the rate of endoscopic exams and interventions performed by the digestive endoscopic unit.
CONCLUSION
AI is an emerging technology which application is rapidly increasing in numerous medical fields. The several applications of AI in gastroenterology are showing promising results, especially in the setting of gastrointestinal oncology. Among these,the techniques able to increase the ADR will play a key role in reducing the CRC incidence and its related mortality caused by undetected or misclassified interval cancers. Furthermore, a significant reduction in costs is also expected. In addition, the assistance of the machine will lead to a reduction of the examination time and therefore an optimization of the endoscopic schedule.
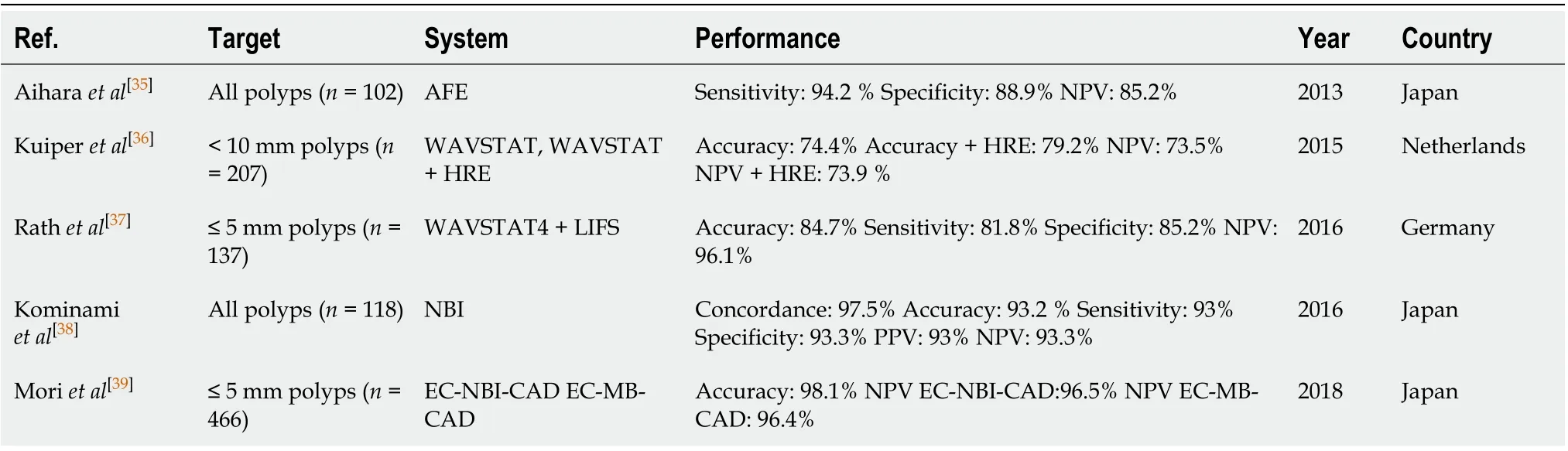
Table 2 Computer-aided detection and diagnosis system can achieve the thresholds of preservation and incorporation of valuable endoscopic innovations for diminutive, non-neoplastic rectosigmoid polyps
Moreover, the resect and discharge policy, supported by good sensitivity and specificity, will lead to decrease the direct costs of unnecessary polypectomies. Furthermore, greater diagnostic accuracy in identifying pre-neoplastic and neoplastic lesions will lead to a reduction in the secondary costs of the prevented neoplasms.
Despite the promising results, AI techniques for detection and identification of colorectal lesions, need to be furtherly investigated in the near future. In particular, since most of results come from trials performed in highly specialized centers, representing a limitation for the generalizability of results, they must be also confirmed in clinical practice.
Finally, the integration of AI in a human-base medicine setting has to be taken into consideration: AI is not conceived, nor now, not ever, to substitute the endoscopist, on the contrary, it seems to be an extremely helpful tool to be used from the endoscopist himself that, given his ability and skills, is the only one able to process and interpret all the AI information to make decisions on the patient management.
 World Journal of Gastroenterology2020年39期
World Journal of Gastroenterology2020年39期
- World Journal of Gastroenterology的其它文章
- Real-world treatment attrition rates in advanced esophagogastric cancer
- Metastatic pattern in esophageal and gastric cancer: Influenced by site and histology
- Relationships of early esophageal cancer with human papillomavirus and alcohol metabolism
- Dynamic contrast-enhanced magnetic resonance imaging and diffusion-weighted imaging in the activity staging of terminal ileum Crohn's disease
- Clinical assessment and management of liver fibrosis in non-alcoholic fatty liver disease
- Enteroscopy in children and adults with inflammatory bowel disease
