Acclimatization of microalgae Arthrospira platensis for treatment of heavy metals in Yamuna River
Nilesh Kumar,Shriya Hans,Ritu Verma,Aradhana Srivastava*
University School of Chemical Technology,Guru Gobind Singh Indraprastha University,New Delhi 110078,India
Received 23 December 2019;accepted 1 July 2020
Available online 30 September 2020
Abstract Bioaccumulation and biosorption in microalgae are effective approaches for the removal of heavy metals(HMs)from river water.The objective of this study was to investigate the potential for use of acclimatized microalgae in the removal of HMs from the Yamuna River water as an acclimatizing medium.An active culture of Arthrospira platensis(A.platensis)was acclimatized to HMs up to a concentration of 100 mg/L.It was gradually exposed to increasing concentrations of HMs in five subsequent batches with a step increase of 20 mg/L to acclimatize live cells in the simulated Yamuna River water.The presence of high levels of HMs in the Yamuna River water caused growth inhibition.An empirical growth inhibition model was developed,and it predicted high threshold concentrations of HMs(210.7-424.5 mg/L),producing a positive specific growth rate of A.platensis.A.platensis also showed high average removal efficiencies of HMs,including 74.0%for Cu,77.0%for Cd,50.5% for Ni,76.0% for Cr,76.5% for Pb,and 63.5% for Co,from HMs-enriched Yamuna River water.The findings demonstrated that the maximum specific removal amounts of Cu,Cd,Ni,Cr,Pb,and Co were 54.0,58.0,39.0,62.8,58.9,and 45.3 mg/g,respectively.The maximum yields of the value-added products chlorophyll and phycocyanin were 2.5 mg/g(in a batch of 40 mg/L for Cd)and 1 054 mg/g(in a batch of 20 mg/L for Cu),respectively.Therefore,acclimatized A.platensis was proven to be a potential microalga not only for sequestration of HMs but also for production of valuable pigments.
© 2020 Hohai University.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Keywords:Yamuna River;A.platensis;Heavy metals;Acclimatization;Biosorption;Pigments
1.Introduction
The Yamuna River contains a tremendous amount of untreated and unchecked industrial effluents from approximately 350 industrial units,out of which more than 40 are situated in its routes within Delhi(CPCB,2006).Hazardous heavy metals(HMs)such as Cu,Cd,Ni,Cr,Pb,and Co are discharged from thermal power plants,and paint,electroplating,and textile factories.HMs accumulate in water bodies and cause an imbalance in microflora and aquatic life.Biological treatment processes are preferred due to their versatility,flexibility,robust nature,the capability of tolerating effluents in varied concentration,selectivity for HMs over alkaline earth metals,and cost-effectiveness(Lyon et al.,2015).Microalgae-based HM removal process has emerged as an economical,renewable approach possessing low-energy requirements,less sludge formation,CO2sequestration,and low greenhouse gas emissions(Udaiyappan et al.,2017;Zeraatkar et al.,2016).Microalgae can grow phototropically,mixotrophically,and heterotrophically,causing various metabolic activities to produce valuable products by utilizing pollutants,namely HMs,and organic and inorganic compounds,as nutrient sources from wastewater(Leite et al.,2019;Randrianarison and Ashraf,2017;Gao et al.,2016).They survive even at a very high concentration of HMs and other potentially toxic pollutants and facilitate the process of biosorption for removing HMs(Bilal et al.,2018).Microalgae also have the potential to sequester a high concentration(330 mg/L)of hazardous chemicals like p-cresol from industrial effluents and produce valuable products(Surkatti and Al-Zuhair,2018),and even the dead biomass alleviates HMs from wastewater.Moreover,the uptake mechanism for living biomass is much more complex than that of non-living biomass for two reasons:(1)the environmental conditions such as temperature,pH,and initial HM concentration;and(2)the presence of different binding groups on the living algae surface like polysaccharides,transport proteins,OH-,SH-,COO-,,and other charged groups(Kaplan,2013;Carolin et al.,2017).The difference in protein components in terms of their affinity and specificity governs the mentioned reasons and affects biosorption capacity for different strains of microalgae species(Chojnacka,2010).Porphyra leucosticta has exhibited specific removal amounts of 31.5 mg/g and 36.6 mg/g of Cd and Pb,respectively,present in fertilizer industry effluent using biological enrichment and precipitation(Ye et al.,2015).Chlorella pyrenoidosa and Scenedesmus obliquus have been found to sequester 79.0%-91.0% and 76.0%-91.3% of Cu,respectively,in an aqueous solution(0-20 mg/L)using nonmetabolic absorption(Zhou et al.,2012).Species of Scenedesmus isolated from a local habitat(Tamil Nadu,India)were also reported high HM removal efficiencies of 73.2%-98.1%of Cu(4.9 mg/L),75.3%-99.0% of Pb(3.2 mg/L),and 81.2%-96.0% of Cr(12.8 mg/L)from tannery wastewater using phycoremediation(Ajayan et al.,2015).Freshwater Chlorella vulgaris and marine Chlorella salina,when grown in sewage diluted with seawater and well water respectively,resulted in HM(Zn,Cu,Co,Ni,and Cr)removal efficiencies of 13.6%-100.0%(El-Sheekh et al.,2016).Chlorella vulgaris had greater removal efficiencies than Chlorella salina at higher concentrations(36.8 mg/L of Zn2+,81.0 mg/L of Cu2+,3.84 mg/L of Co2+,21.0 mg/L of Ni2+,and 27.0 mg/L of Cr3+).Extensive research has also been conducted on Spirulina platensis for the removal of HMs from different aqueous solutions.Dead biomass of Spirulina showed the highest removal efficiency of 87.7%of Cd at a pH value of 8.0,a biosorbent amount of 2.0 g,a temperature of 26.0°C,and 60.0 mg/L of Cd concentration after 90 min of contact time with the variation of the mentioned operating parameters(Al-Homaidan et al.,2015).Biosorption of Cu2+and Ni2+by dead
Arthrospira platensis(A.platensis)resulted in removal efficiencies of 73.6 mg/g for Cd2+and 69.0 mg/g for Ni2+following the Langmuir isotherm during the first 60 min of contact time(Çelekliand Bozkurt,2011).Although dead biomass provided quick removal of HMs following set isotherms exhibiting a fixed maximum absorption capacity of biomass,live cells can continuously divide and grow to enhance the rate of sequestration of HMs.Live microalgae selectively and specifically allow HMs to concentrate within cells due to the immense metal binding capacity of HMs in reactive chloroplast,vacuoles,and mitochondria(Udaiyappan et al.,2017;Romera et al.,2007;Balaji et al.,2014;Schiewer and Volesky,2000).However,industrial effluents possess very high levels of HMs,which have never been used in previous studies.This level of HM exposure is detrimental to microalgae if not acclimatized(Hultberg et al.,2001;Heiss et al.,2003).
The present work evaluated the use of acclimatized microalgae for the removal of HMs(Pb,Cr,Ni,Co,Cu,and Cd)from the Yamuna River water.Live cells of A.platensis were used for theiracclimatizationtoHMswithconcentrationsrangingfrom20 to 100 mg/L.This was carried out by sequentially adding HMs to river water samples.The acclimatization of microalgae cells to HMs is a novel technique that enhances the affinity of microalgae for HMs uptake from simulated Yamuna River water.The stepincrease in the concentration of HMs induces stress conditions that trigger the synthesis of the metabolites such as glutathione and ascorbic acid.These metabolites bind to HMs to produce their complexes with proteins(pigments)via covalent linkages(Jozefczak et al.,2012).This clearly indicates no desorption of HMs as there is in dead cells,which is a fascinating finding.Overall,this acclimatization not only enhances the HMs uptake capacity but aids their further utilization to produce pigments.
2.Materials and methods
2.1.Materials
In this study,the Yamuna River water was used as the medium for the growth of microalgae A.platensis.Analytical grade chemicals were used.All HMs were supplemented in their salt form(CuSO4,NiCl2,PbS,CrCl3,CdCl2,and CoCl2)with the Yamuna River water.All aqueous solutions and reagents were prepared using double-distilled water.
2.2.Strain and its maintenance
A.platensis was sourced from Algae Research and Supply(Carlsbad,USA).Strain propagation,and its preservation and maintenance were carried out with the methodology described earlier by Verma et al.(2016,2018).Microalgal culture grown in the Yamuna River water without any added nutrients was used as inoculum for further studies.
2.3.Collection and pretreatment of Yamuna River water
The Yamuna River water was collected from the river bank opposite the Pragati Thermal Power Plant,in New Delhi,India(28°36′59.9′′N,77°15′23.5′′E)at 1:00 p.m.on August 18,2018.The river water was used as the growth media for microalgae without any external addition of nutrients.Composition of collected water was measured for nitrate using the ultraviolet spectrophotometric method,ammonia with absorbance at a wave length of 420 nm using the colorimetric method,and nitrite with absorbance at a wave length of 493 nm using the spectrophotometric method(Collos et al.,1999;Golterman,1991;Narayana and Sunil,2009).Other chemical characteristics involving Ca2+,Mg2+,Cl-,,,biological oxygen demand(BOD),chemical oxygen demand(COD),total Kjeldahl nitrogen(TKN),total coliform,and faecal matter were measured.The collected water was pretreated by allowing suspended solid particles in the water to settle gravitationally for one day.It was filtered and the liquid was autoclaved in 2-L bottles(15 min at 15 pound-force per square inch and 121°C).Then,it was treated by cooling and was left at room temperature for two days.This process was repeated three times to ensure that no microorganism remained in the media.This water was supplemented with phosphate buffer to bring the pH value to 9.0 for optimum growth of the strain(Verma et al.,2018,2019;Verma and Srivastava,2018)and used in further studies.
2.4.Acclimatization of A.platensis to HMs
Six HMs,Cu,Ni,Pb,Cr,Cd,and Co,were used for acclimatization of A.platensis and removal studies of HMs.Acclimatization was performed for adapting the microalgae cells to increasing HM stress in photoautotrophic cultivations.The concentration of each HM in the Yamuna River water medium was serially increased in subsequent batches for the acclimatization of A.platensis.This was done by adding HMs to make the final concentrations of 20 mg/L,40 mg/L,60 mg/L,80 mg/L,and 100 mg/L in 1-L Erlenmeyer flasks containing 300-mL sterile Yamuna River water.The first batch with 20 mg/L of each respective HM was inoculated with 10%(volume/volume)microalgae culture and incubated at 30°C for five days.Compressed and sterile air was supplied continuously via a glass wool air filter.Sunlight was used as a natural source of illumination.Samples were withdrawn after every 24 h for the estimation of dry cell weight,chlorophyll,phycocyanin,and HMs.10%of the culturesampledonthefifthdaywasusedasinoculumfor thenext batch containing 40 mg/L of HM for serial increase.The method was repeated in successive batches for each HM.
2.5.Analytical methods
Dry cell weight was analyzed using the gravimetric method(Verma et al.,2018).Atomic absorption spectroscopy(Model AAS4141,ECIL,India)was used for estimation of HMs as described in Mehan et al.(2018).Chlorophyll and phycocyanin contents were calculated with the methanol extraction method(Lee and Shen,2004)and the method of Bennett and Bogorad(1973)using Eqs.(1)and(2),respectively.

where C1and C2are the contents of chlorophyll(mg/L)and phycocyanin(g/L),respectively;and D665,D650,D615,and D652are the optical densities recorded at wave lengths of 665 nm,650 nm,615 nm,and 652 nm,respectively.
2.6.Growth model in presence of HMs
The Monod model(Monod,1949)was adopted to express microalgae growth under substrate limiting conditions in the absence of HMs.The specific growth rate(μg)is

whereμmaxis the maximum specific growth rate in the absence of HMs,S(mg)is the substrate that is utilized for cell growth,and Ksis the substrate affinity(half-saturation constant).The observedμmax(μmax,obs)(d-1)in the presence of increasing concentration of HM is given in Fig.1,which states thatμmax,obsis a function of HM concentration(P)(mg/L).
The exponential decay model(Shuler and Kargi,2002)was adopted to depict the inhibited growth of microalgae:

where Pmis the threshold concentration of the inhibitor that represents no growth,and n is the empirical constant that varies according to the different operating conditions(temperature,pH,sunlight,and nutrient medium).

where Y is the fraction of specific growth rate retained in the presence of HMs.
This empirical model in its modified form was used for describing inhibition growth kinetics,in whichμg(in Eq.(3))is affected by the presence of varied HM concentrations.Eq.(4),which describes microalgae growth inhibition in the presence of different concentrations of HMs in the media,was fitted with the data of experimental findings using the curve-fitting tool in MATLAB(Code R2015b,MathWorks Inc.,USA).

Fig.1.Effect of heavy metal stress on A.platensis growth(all data presented have±3.0% standard deviation).
3.Results and discussion
3.1.Acclimatization of microalgae and effect of HM stress on A. platensis growth
Composition of collected water was measured as 3.4 g/L of nitrate,0.6 g/Lofammonia,and 0.004 3 g/L of nitrite,andHMsas 4.1 mg/L of Ni,8.5 mg/L of Cd,12.6 mg/L of Cu,9.4 mg/L of Pb,7.5 mg/L of Cr,and 5.4 mg/L of Co(all values within±2.0%standard deviation).Other chemical characteristics involving Ca2+,Mg2+,Cl-,,,BOD,COD,TKN,totalcoliform,and faecal coliform were measured as 7 mg/L,0.4 mg/L,4 mg/L,7 mg/L,0.02 mg/L,4-40mg/L,50-76 mg/L,1.20-20.30 mg/L,15.5×103to 89×107no./100 mL,and 2.54×103to 199×106no./100 mL,respectively.A.platensis grown in the Yamuna River water without any added HMs was used as the control batch,which produced a 0.44 g/g biomass yield on nitrate(YX/nitrate,whereXisthecellconcentration).Thisisdefinedastheratioofthe amount of biomass produced to the amount of nitrate utilized.Fig.1 shows the results of acclimatization studies.The positive maximum specific growth rate of A.platensis in all 30 batches confirmed its acclimatization to concentrations up to 100 mg/L.A declined maximum specific growth rate was observed with increased stress of HMs.Interestingly,in the case of Pb,there was an increasedμmax,obs,from 20 to 60 mg/L,and then a decline was observed with a further increase to 100 mg/L.The inhibition model was required for each batch of HMs except Pb.
Table 1 depicts the results of other 30 batches with different HMs in terms of YX/nitrateand removal efficiencies of HMs by biomass.The HMs removed is the amount of HMs that exist in the biotic phase.The maximum removal efficiency of Pb was 20 mg/L.This is probably due to the utilization of Pb for blocking cystine and impairing the activity of urease present in the system(Khan and Khan,2015).This does not allow the buildup of ammonia(NH3),which is inhibitory to the cells if increased to 500-750 mg/L(Tam and Wong,1996).It eliminates the detrimental effect of ammonia and allows the microalgae cells to grow in an inhibition-free environment.During the stationary and death phase,dead cells of A.platensis are produced.These dead cells also exhibit comparable removal efficiencies due to binding of HMs through sorption.
Biosorption is the process of utilization or removal of HMs from a solution using biological materials.It can either be metabolically mediated or a physiochemical pathway of uptake(Fourest and Roux,1992),and it is classified into extracellular and intracellular accumulation(Kaplan,2013).Among most organisms,microalgae possess an immense metal binding capacity via extracellular accumulation(Schiewer and Volesky,2000).Usually,the net charge on the cell surface is negative due to the presence of phosphate and carboxyl residues whereas the HM in the solution is in a cationic form.Therefore,it is passively adsorbed on the cell surface.The intracellular accumulation takes place due to the presence of cytoplasmic ligands such as phytochelatin and metallothionein molecules(Mehta and Gaur,2005).Cell membrane-bound enzymes facilitate the transport of HM ions onto the surface of the microalgae cell.The metabolite glutamate is released when biomass is under HM stress and this,in turn,leads to proline formation.Proline and glutamate help in the sorption of HMs(Siripornadulsil et al.,2002;Kretsinger et al.,2013;Tsuji et al.,2003).Following the above-mentioned routes,A.platensis resulted in high removal efficiencies of Cu,Cd,Ni,Cr,Pb,and Co from the Yamuna River water of 52.8%-96.2%,71.7%-83.1%,36.7%-65.2%,72.1%-81.2%,68.9%-84.9%,and 35.1%-91.7%,respectively(Table 1).

Table 1 Effect of HM concentrations on microalgae growth and HM removal.
Batches of Cd fortified the Yamuna River water and exhibited the maximum YX/nitrateat 40 mg/L,which was 77.2%of that obtained in the control batch.However,the maximum removal efficiency of Cd was achieved at 60 mg/L and was comparable to that obtained at 40 mg/L.The specific removal capacity of Cd published earlier using Spirulina sp.was 0.436 mg/g in refinery waste effluent(Chojnacka et al.,2004),which was about 56.8% of that achieved in the present work.This clearly shows the high capacity of acclimatized A.platensis to remove Cd.
The maximum removal efficiency of Pb was obtained at the lowest concentration(20 mg/L)in the Yamuna River water,which remained almost constant with a further increase in the value of concentration up to 60 mg/L.The increased concentration of Pb at 60 mg/L resulted in 71.1% less YX/nitratethan that of the control batch.The maximum specific removal amount of Pb was observed as 0.8 mg/g,which was approximately 2.4 times that obtained from Porphyridium purpureum and Stigeoclonium tenue(Schmitt et al.,2001;Pawlik-Skowro′nska,2003).
Growth of microalgae under Cr stress exhibited both the maximum YX/nitrateand removal efficiency at 20 mg/L.Reduction in biomass yield was 70.9% of that in the control batch.Cu stress on microalgae from 40 to 80 mg/L maintained the YX/nitrateat approximately 0.1 g/g,which was 3.0 times that obtained at 20 mg/L.Previously published work on Spirulina platensis and Spirulina sp.reported specific removal amounts of Cu as 0.85 and 0.271 mg/g,respectively(Nalimova et al.,2005;Chojnacka et al.,2004),which were respectively 10.5% and 71.4% lower than the present result(0.95 mg/g).
Lower levels of Ni stress,i.e.,20-40 mg/L favored both YX/nitrateand removal efficiency of Ni in the Yamuna River water.The maximum specific removal amount of Ni was also approximately 3.0 times that obtained in previous work on Spirulina sp.(Chojnacka et al.,2004).
The maximum YX/nitrateand removal efficiency under Co stress showed similar patterns in varying levels of 20-100 mg/L,which declined to 34.28%and 59.6%,respectively,when the Co stressincreasedfrom20to40mg/L.Resultsofmaximumspecific removal also highlighted the potential of acclimatized A.platensis by providing the maximum value as approximately 78.0 times that obtained using Spirulina sp.(Chojnacka et al.,2004).
When HMs enter the cell,they bind to the extracellular ligand of the microalgae cell and enter with the help of transporter proteins.Glutathione,which is already synthesized in the cell byγ-glutamylcysteine,binds the HM ions to form a metallothionein-HM complex in the presence of phytochelatin synthase(PCS).On exposure to sulfide ions(S2-),low-and high-molecular weight complexes of metallothionein-HM are formed,of which the latter are sequestered into organelles such as vacuoles,chloroplast,and mitochondria.Apart from the toxic effects of HMs,some constructive applications in metabolic reactions also occur when Cd,Ni,Cu,Co,Pb,and Cr combine with enzymes as cofactors.Examples include the following:binding of Ni to urease and hydrogenase;Cu to cytochrome c oxidase,laccase,and nitrite reductase;Co to nitrile hydratase and methionyl aminopeptidase;and Cr to affect the PS I and PS II activity resulting in alteration of pigments production and the sustainability of microalgae(Stohs and Bagchi,1993;Hultberg et al.,2001;Heiss et al.,2003;Cracan and Banerjee,2013;Sydor and Zamble,2013;Freisinger and Vasak,2013;Putri et al.,2017).
In summary,the decreasing order of the complexation rate for bioaccumulation and biosorption in A.platensis was found to be Cu>Co>Pb>Cd>Cr>Ni.The maximum specific removal amounts of Cu,Cd,Ni,Cr,Pb,and Co were 54.0,58.0,39.0,62.8,58.9,and 45.3 mg/g of biomass,respectively,which indicates the strong potential of acclimatized live cells to sequester HMs.The removal of HMs using biomass by activated carbon as an adsorbent has been reported in different studies.The maximum specific removal amounts of extracted Cu,Cd,Ni,Cr,and Pb were 248.0 mg/g(surface modified Strychnos potatorum seeds),54.6 mg/g(porous activated carbon),74.3 mg/g(porous activated carbon),83.0 mg/g(acidtreated lantana camara fruit),and 408.0 mg/g(treated cashew nut shell),respectively(Murugesan et al.,2018;Prabu et al.,2017;Anitha et al.,2015;Nithya et al.,2016;Suganya et al.,2017;Kumar et al.,2011,2012;Kumar,2013).These studies on dead biomass show higher HM sequestration but the adsorbents used are not as environmentally friendly as live microalgae cells.When dried and rehydrated Spirulina platensis were used as biosorbents for the removal of Cr(III)and Cu from water,it resulted in a maximum adsorption of 30.6 mg/g for Cr and 96.8 mg/g for Cu(Lodi et al.,2008;Solisio et al.,2006).However,the present study on active live cells exhibited 105.0% higher and 44.2% lower removal efficiencies of Cr and Cu,respectively,than that of Spirulina platensis.This clearly indicates a better absorption capacity of A.platensis for Cr and Spirulina platensis for Cu.Suspension of live microalgae cells in water clearly exhibited better performance under osmotic stress.
3.2.Growth inhibition model
The growth of A.platensis was inhibited under HM stress following the model described in Eqs.(3)and(4).However,a positiveμmax,obswas found even at a high HM stress.As shown in Table 2,the values of model parameters n and Pmalso indicate the robust nature of acclimatized A.platensis,which can withstand Pmlevels of 392.9,210.7,424.5,386.4,and 358.9 mg/L of Cd,Ni,Cu,Co,and Cr,respectively.The highest values of n obtained for Co and Cr signify fast growth inhibition in the presence of these HMs.
The growth inhibition model was introduced for studying theμmax,obsof microalgae.This model was tested with linear regression analysis using the curve fitting tool in MATLAB and validated by comparing experimental with predicted input parameters(Yexpand Ypre,respectively).Different data sets of HMs used for validation were derived from different experimental studies.The values of n and Pmwere obtained with the model for each HM level in the media and fitted with the experimental data given in Table 2.The highest value of Pmimplies inhibited growth at a very high input concentration of HM.Cu showed the highest value and Ni showed the lowest amongst all batches.The model parameter Yprewas plotted against the observed Yexpfor validation as shown in Fig.2.The maximum number of points lies on and around the line y=x(where y is the Yprevalue and x is the Yexpvalue)in the graph,which proves the validity of the model.

Table 2 Model parameters n and Pm obtained by curve fitting(MATLAB).
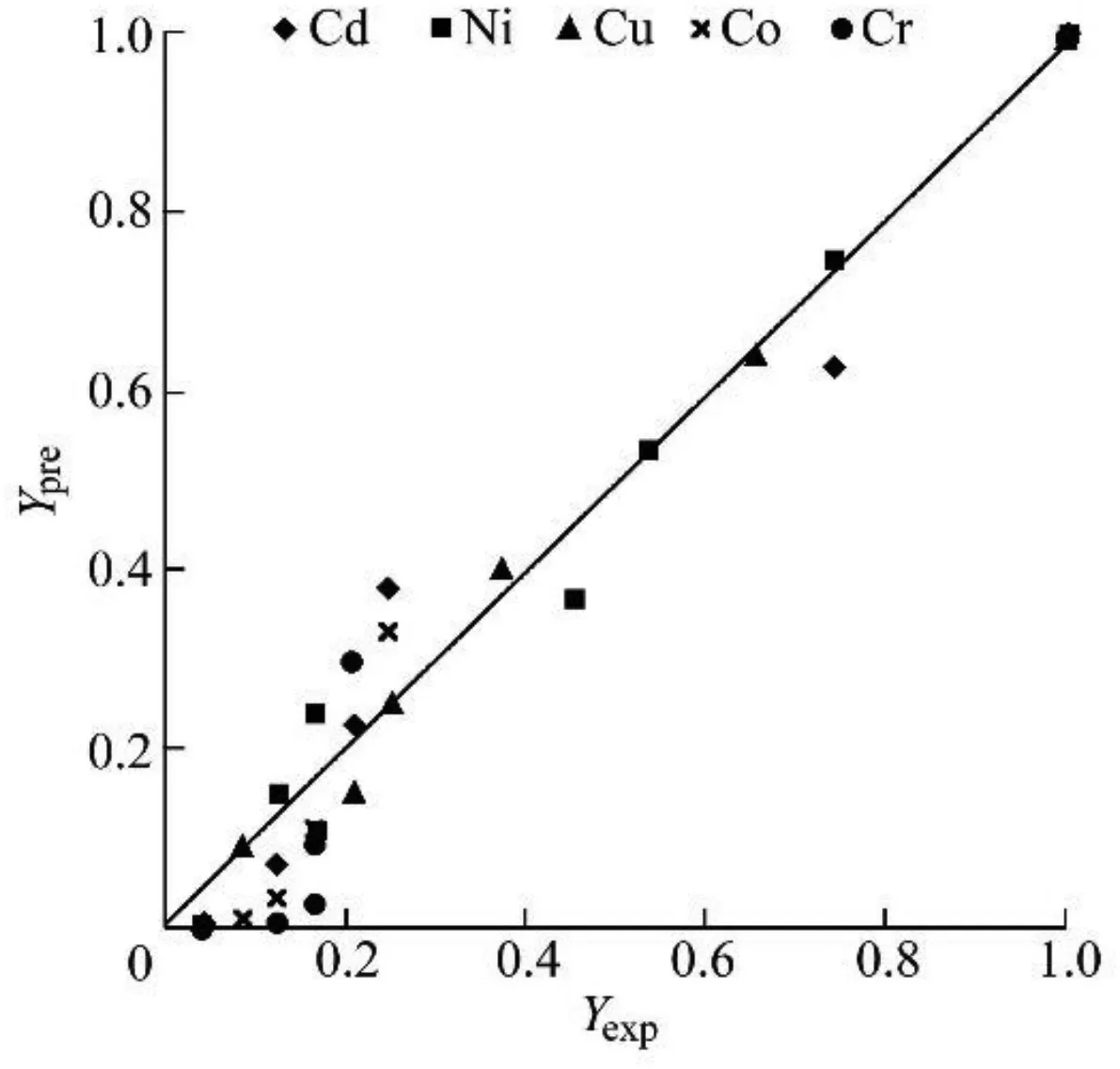
Fig.2.Validation of growth inhibition model parameters.
3.3.Pigment production
A.platensis produces the pigments,chlorophyll,and phycocyanin in the presence of HMs in the Yamuna River water.Chlorophyll acts as an antioxidant and anti-mutagen,exhibits fluorescence,and is also known to have stimulating effects(Hosikian et al.,2010).Phycocyanin has strong antiinflammatory,neuroprotective,immunomodulating,antitumor,and hepatoprotective properties(Wu et al.,2016).These high value-added products were produced in the cells regardless of HM stress.The yields and specific productivities of chlorophyll and phycocyanin obtained are given in Table 3.However,the lower concentrations of HMs found induced higher production of chlorophyll and phycocyanin(Hemlata and Fatma,2009).Interestingly,Cu triggered the phycocyanin concentration at lower HM levels of 20 and 40 mg/L,as discussed earlier.
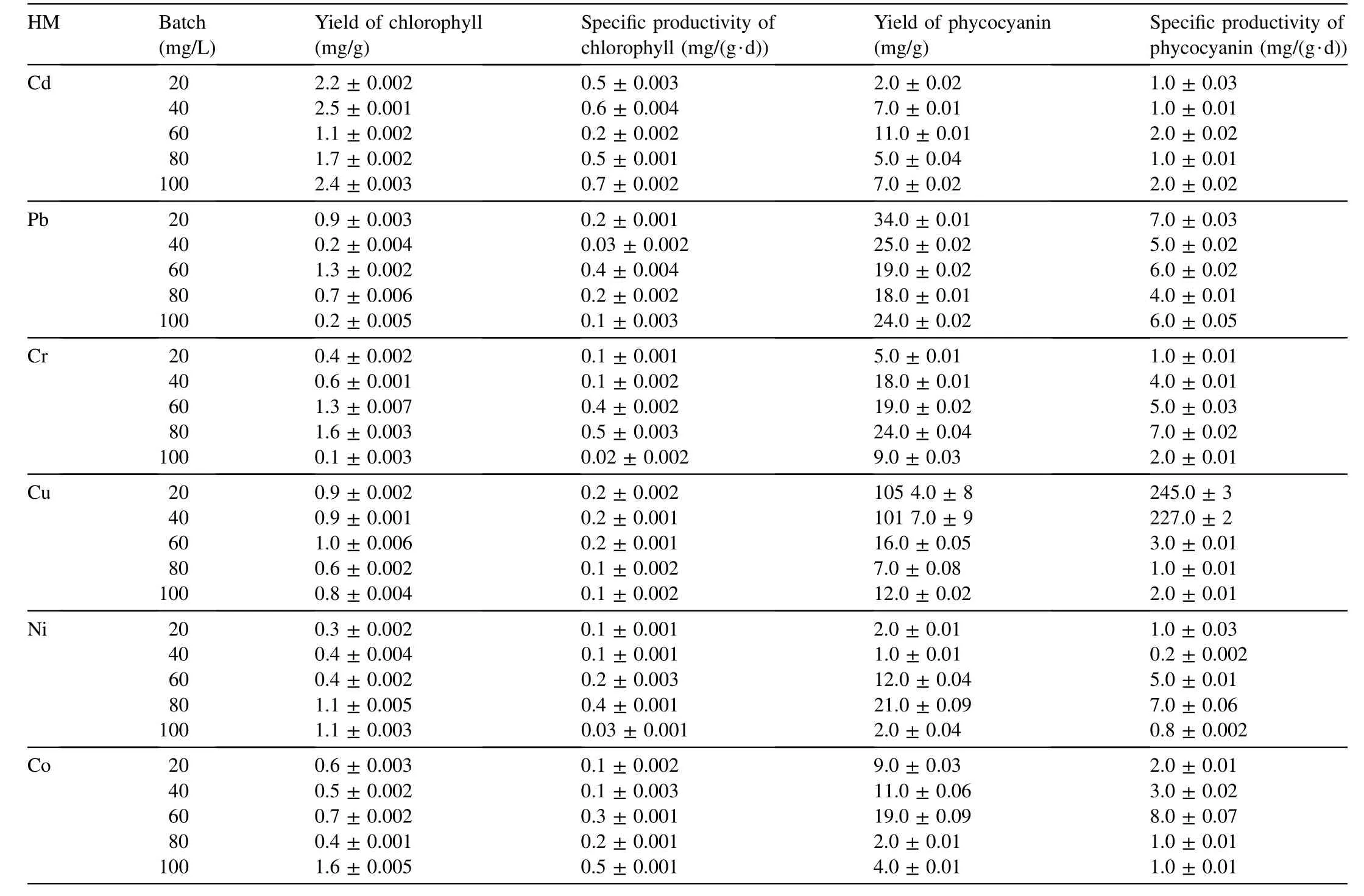
Table 3 Pigment production in microalgae under stress of HMs.
3.4.Comparison of live acclimatized microalgae with other adsorbents
Acclimatization produced enhanced HM removal efficiency in A.platensis.In comparison to the published adsorbents,live and acclimatized microalgae in the present work exhibited maximum adsorption of all the HMs used(Table 4).

Table 4 Comparison of HM removal efficiency from this study with published literature.
4.Conclusions
A.platensis was successfully acclimatized to heavy doses of HMs,as confirmed by the model concentrations of 210.7-424.5 mg/L for different HMs,and produced a positive observed maximum specific growth rateμmax,obs.This acclimatized culture was proven to be an excellent scavenger of HMs.The developed empirical model describing its growth in the presence of HMs showed high Pmand positive values of n,further ensuring it is a highly robust type of microalgae.Acclimatized microalgae also showed a high capacity to sequestrate HMs from waste water collected opposite the thermal power plant.Maximum chlorophyll and phycocyanin yields were 2.5 and 1 054.0 mg/g of biomass,respectively,due to well-developed chloroplast during autotrophic growth.This study proves that the treatment of HMs present in waste water is possible with acclimatized A.platensis.
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgements
The authors acknowledge the support and facilities provided by University School of Chemical Technology,at Guru Gobind Singh Indraprastha University.
 Water Science and Engineering2020年3期
Water Science and Engineering2020年3期
- Water Science and Engineering的其它文章
- Impacts of topographic factors on regional snow cover characteristics
- Evaluation of alum-based water treatment residuals used to adsorb reactive phosphorus
- Phosphorus removal by adsorbent based on poly-aluminum chloride sludge
- Characterization of cobalt ferrite-supported activated carbon for removal of chromium and lead ions from tannery wastewater via adsorption equilibrium
- Three-dimensional modelling of shear keys in concrete gravity dams using an advanced grillage method
- Influences of flow rate and baffle spacing on hydraulic characteristics of a novel baffle dropshaft
