Evaluation of alum-based water treatment residuals used to adsorb reactive phosphorus
George Carleton,Teresa J.Cutright*
Department of Civil Engineering,The University of Akron,Akron,OH 44235-3905,USA
Received 18 November 2019;accepted 5 April 2020
Available online 1 October 2020
Abstract Excess reactive phosphorus(PO4)in waterways can lead to eutrophication.A low-cost approach to reducing PO4 levels in surface water was evaluated using the alum-based water treatment residual(Al-WTR)or Al-WTR augmented with powdered activated carbon(PAC-WTR).Batch adsorption-desorption and continuous flow column experiments were performed to assess the specific adsorption capacities under various concentration and flow conditions.Both Al-WTR and PAC-WTR exhibited the ability to adsorb PO4.The overall,cumulative sorbed amount after a 28-d desorption step for Al-WTR was 33.93 mg/kg,significantly greater than the PAC-WTR value of 24.95 mg/kg(p<0.05).The continuous flow column experiments showed a theoretical PO4 uptake of 9.00 mg/g for Al-WTR and 7.14 mg/g for PAC-WTR over 720 h.When surface water was used,the Al-WTR and PAC-WTR columns removed 67.4%and 62.1%of the PO4,respectively.These results indicated that Al-WTR was more effective for in-field evaluation.
© 2020 Hohai University.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Keywords:Phosphorous;Alum;Water treatment residuals;Adsorption;Desorption;Powered activated carbon
1.Introduction
Elevated bioavailable or reactive phosphorus(PO4)concentrations can lead to the deterioration of water quality in an ecosystem and formation of algal blooms(Agyin-Birikorang et al.,2007;Anderson et al.,2002).The proper operation of coagulation/flocculation,sedimentation,and filtration steps in conventional surface water treatment plants has been found effective in removing cyanobacteria cells(Cheung et al.,2013).It is still necessary to address the bioavailable or reactive phosphorous content entering a stream/tributary.
Previous studies suggested the use of drinking water treatment residuals(WTRs)to remove excess PO4from water(Babatunde and Zhao,2010;Dayton and Basta,2005).The sustainable and beneficial reuse of WTRs can reduce disposal costs and environmental impacts associated with WTRs.The sustainability of WTRs is evident from the fact of water treatment plants serving populations of 100 001 to 500 000,producing 20 000 t/year of WTRs(USEPA,2011).The most common coagulants used in drinking water plants are aluminum sulfate(i.e.,alum)and ferric chloride.During periods of warm weather,drinking water treatment plants add powdered activated carbon(PAC)upstream of the alum to further adsorb organic chemicals,which might cause taste and odor problems,and to adsorb cyanotoxins(Ho et al.,2011).PAC may also be added to address contaminants from seasonal runoff of agricultural chemicals(Sarkar et al.,2007).When PAC is added to alum,it is referred to as PAC-WTR.
Although studies have investigated the use of WTRs for phosphate adsorption,most of them processed WTRs before use.For instance,Dayton et al.(2003)crushed the WTRs to a particle size less than 0.2 mm before conducting experiments.Lee et al.(2015)showed that particle size can have an important impact on adsorption.However,no sample prepreparation was done to WTRs in this study.Responses from various water treatment personnel have indicated that water treatment facilities would be more likely to use WTRs as a potential sorption material if they could be used without additional processing.Personnel have also indicated that the most desirable sorbent would be the one that could be placed in a removable cartridge and added in the flow path of a tributary for one to four weeks to remove PO4prior to its entry into the reservoir/lake.Evaluating the cumulative desorption of PO4from WTRs would be essential to quantifying potential desorption of PO4that had been adsorbed during coagulation/sedimentation within the water treatment plant.Implementing changes to a traditional isotherm experiment will enable water treatment personnel to evaluate whether they can reuse WTRs to bind PO4.The objectives of this study were to(1)quantify the reactive PO4sorption uptake and desorption of both the alum-based water treatment residual(Al-WTR)and PACWTR to estimate potential adsorption capacity of the material;and(2)evaluate the potential differences in reactive PO4uptake of WTRs between surface water and distilled water.Distilled water was used to follow the traditional methodology,while the surface water was used to investigate the difference in PO4uptake when WTRs were simultaneously challenged with sorption and desorption,as well as competitive effects from other constituents present in the surface water.The hypothesis was that,for both WTRs,the uptake will be greater with distilled water.
2.Materials and methods
2.1.WTR source,collection,and characterization
The WTRs were obtained from separate drying lagoons located at the City of Akron's Water Treatment Plant(Kent,USA).WTRs were placed into separate clean buckets using clean shovels.Once filled,the buckets were sealed and transported to the laboratory.The potential amount of aluminum that would leach,pH,and moisture content of each WTR were measured to establish baseline properties.The particle size of the WTRs was determined according to U.S.standard sieves for WTRs taken directly from the field after drying.
The potential leaching of aluminum from the WTRs was investigated using 0.01 mol/L CaCl2with a contact time of 72 h,0.01 mol/L CaCl2with a contact time of 2 h,and distilled water with a contact time of 2 h(Maiz et al.,1997).All cases were performed with five replicates with 5 mg of WTRs added to 50 mL of the appropriate solution.Flasks were sealed and placed in a Lab-Line Orbital Environ-Shaker at 50 rpm for the specified contact time.Afterwards,the solutions in the flasks were decanted,filtered through Fisherbrand Q2 filter paper(particle retention>2μm),and analyzed following the Aluminum Method 8012(HACH,2009),using an HACH DR 890 Colorimeter.
The pH values of the WTR samples were determined using a ratio of WTRs to distilled water of 1:1(Ippolito,2015).Triplicate beakers containing 10 g of WTRs and 10 mL of distilled water were stirred for 15 min.The supernatant pH was measured using a Fisher Scientific Education AccuTupH.
The moisture content was determined using triplicate samples of each WTR.In brief,WTRs were added to an aluminum weigh dish and weighed.The samples were airdried at 25°C for one week and then reweighed.The difference between the initial and final weights was the moisture content of the WTR.
2.2.Surface water source
Surface water was collected at the intersection of Dawley Road and Eckert Ditch,approximately one mile upstream of Lake Rockwell.Water samples were taken on a weekly basis,weather permitting from October 2017 to November 2018.The reactive PO4concentration,aluminum concentration,and pH were tracked at each time.The reactive PO4was measured following the Reactive Phosphorus PhosVer 3 Method 8048(HACH,2009),using a HACH DR 890 Colorimeter.The aluminum concentration was measured following the Aluminum Method 8012(HACH,2009).pH values were measured using a Fisher Scientific Education AccuT pH probe.
2.3.Batch adsorption-desorption experiments and PO4 analysis
In batch adsorption-desorption experiments,1.6 g of WTRs were added to 40 mL of background solution in 40-mL glass vials.Depending on the specific experimental set,the background solution was either distilled water(control),distilled water spiked with PO4,or surface water spiked with PO4.The control was used to assess whether any of the PO4already present in the WTR would desorb.Distilled water spiked with PO4was used to provide the maximum adsorbed-desorbed amount without interference,while surface water spiked with PO4was used to mimic field conditions and assess competition in sorption when impurities were present,as well as simultaneous sorption-desorption(Lalley et al.,2016;Yu and Chen,2015).Spiked PO4was obtained by diluting a 1 mg/L of PO4standard purchased from HACH.The addition of the spiked PO4initiated the adsorption phase.After 24 h,the background solution was decanted,filtered through Fisherbrand Q2 filter paper(2μm pore size),and analyzed for reactive PO4following the Reactive Phosphorus PhosVer 3 Method 8048(HACH,2009).
Immediately after the background solution used in the sorption phase was decanted,fresh background solution(i.e.,with the same composition as the solution used in the sorption phase)was added to initiate the desorption phase measured at contact times of 1,2,3,7,14,21,and 28 d to provide insight into how long the WTR could be used in a tributary to lower PO4contents entering the reservoir.At each time interval the background solution was decanted for analysis,and new background solution was added.The first 24-h desorption step was used as the traditional equilibrium 24-h time step.Days 2,3,7,14,21,and 28 were used to assess the simultaneous adsorption-desorption that could occur when surface water was used(Hale et al.,2013).Fig.1(a)shows a simplified schematic diagram of the experimental procedure.
Although mixing would increase the contact between PO4and WTRs and enable direct comparison with results from other studies(Cucarella and Renman,2009),it would cause over-estimation of sorption potential of WTRs used in the field.As the appropriateness of laboratory procedures depends on the intended application(Klimeski et al.,2012),isotherm experiments were conducted at 20°C in a Lab-Line Orbital Shaker Bath under static conditions in the dark.
Each experimental set consisted of triplicate reactors.The initial spiked reactive PO4concentration used to initiate the experiment represented the only time that the samples were spiked.The spiked reactive PO4concentrations were 0.10,0.15,0.20,0.25,0.30,and 0.60 mg/L.The controls were not spiked with PO4.It is important to note that the 0.60-mg/L PO4sample with distilled water and Al-WTR was lost.
2.4.Batch adsorption-desorption data analysis
An adsorption isotherm describes the equilibrium between the concentration of adsorbate in the solution and its sorbed concentration(Sawyer et al.,2003).The sorbed amount qe(mg/kg)for the adsorption phase was determined as follows:

where Ciis the initial reactive PO4concentration(mg/L),Ceis the measured equilibrium solution concentration(mg/L),V is the volume of background solution(L),and m is the mass of sorbent(kg).For the desorption phase of the experiment,Eq.(1)was modified as follows:
where qe-1is the sorbed amount of the previous sorption step,CBis the reactive PO4concentration of the background solution(mg/L),Ce-1is the equilibrium concentration of the previous sorption step,and R is the fraction of supernatant replaced.All batch adsorption-desorption experiments had a V value of 0.04 L,an m value of 0.016 kg,and an R value of 0.95.Adsorption-desorption experiments had different PO4concentrations in the background solution.This led to different total masses of PO4that WTRs were challenged with during each trial.The total specific PO4adsorption percentage,N,was calculated with the following equation to determine the efficiency of each WTR:

where mtis the total mass of PO4(mg);and madsis the mass of adsorbed PO4(mg),which is calculated at each time step by converting the concentration to mass:

where Ci,nis the initial reactive PO4concentration of the time step n(mg/L).It is assumed that a small fraction of supernatant PO4is not replaced at each time step:

Data labels were used to identify the specific cumulative desorption time step for the qeand Cevalues.
2.5.Continuous flow column experiments
Reactive PO4with an influent concentration of 1.5 mg/L was used based on input from a water plant.This was two to five times higher than the typical concentration in the surface water and would be a worst case.It was made by diluting a 100-mg/L PO4stock solution(0.143 g KH2PO4,technical grade,Fisher Scientific)into 1 L distilled water.Addition of the PO4stock solution increased the pH of the distilled water from 5.79±0.09 to 5.90±0.11(p>0.05).When surface water was used,the average PO4concentration was determined and then raised to a final 1.5 mg/L PO4using the stock solution,which increased the pH from 7.45±0.15 to 7.59±0.05(p>0.05).The influent was pumped through 0.5-cm diameter tubing using a Masterflex L/S pump(Fisher Scientific)to the bottom of the 117.8 mm-long column with a 38.1-mm diameter and 7.5-min empty bed contact time.An upward flow of 18 mL/min was used throughout to avoid the wall channeling effect(Jafari and Jamali,2015).The water then exited the column at the top and discharged into a 113.46 L effluent tank(Fig.1(b)).A tee in the tubing just above the column allowed for sampling without interrupting the flow.

Fig.1.Schematic diagrams of experimental procedures for batch adsorption-desorption and continuous flow column experiments.
The first column experiment for each WTR used 1.5 mg/L PO4in distilled water to predict the uptake parameters that would be obtained when surface water was used.Due to sampling constraints,the run time was limited to 102.5 h for experiments with surface water in order to provide a homogeneous initial PO4concentration.The average PO4concentration in water from Eckert Ditch was 0.06 mg/L and was raised to the final 1.50 mg/L for PAC-WTR.The average PO4concentration in the surface water was 0.11 mg/L and was raised to 1.5 mg/L for Al-WTR.
Effluent concentrations were plotted against time,and a bestfit line was numerically integrated to obtain the area below the curve,Abelow((mg·h)/L).The total area,At((mg·h)/L),was the area bound by the influent PO4concentration and total time,t.The area above the curve,Aabove((mg·h)/L),was calculated as follows:

The PO4mass trapped in the bed,mads(mg),was then determined by Eq.(7):

where F is the volumetric flow rate(L/h)(Jafari and Jamali,2015).The PO4uptake of WTRs,Q(mg/g),could then be calculated using the following equation:

The total mass of PO4passed through the column,mt(mg),was calculated as follows:

2.6.Statistical analysis
The three-factor analysis of variance(ANOVA)was used to analyze the differences between the varying batch isotherm conditions using Minitab 18.1.The factors were WTRs(Al-WTR and PAC-WTR),isotherm condition,and spiked PO4concentration.The response variables for comparison of the factors were the cumulative 28-d desorption qevalues and change in qevalues from the 24-h adsorption to the cumulative 28-d desorption step.Depending on the differences obtained,a Tukey comparison test with 95%confidence interval was conducted.Responses with p<0.05 were statistically significant.
3.Results and discussion
3.1.General characteristics of WTR samples
The average moisture content was 48.42%±14.40% for the Al-WTR samples and 84.00%±12.90% for the PACWTR samples(p<0.05).PAC's higher specific surface area leads to more water being adsorbed by PAC-WTR(MWH Global et al.,2005).Al-WTR was composed of 29.11%gravel(4.75-75 mm),62.43% sand(0.075-4.75 mm),and 0.11%silt and clay(<0.075 mm).When dried,the particle size distribution of Al-WTR was 19.04%gravel,71.42%sand,and 8.66% silt and clay.For PAC-WTR,43.13% was gravel,58.70% was sand,and 0.09% was silt and clay.When dried,the distribution became 17.88% gravel,70.98% sand,and 10.25% silt and clay.The dominant particle size of both WTRs,whether wet or dried,was the sand(0.075-2 mm).
ThepotentialamountofAlthatcouldleachwasdeterminedfor each WTR.When distilled water was used,(0.019±0.025)mg/L and(0.029±0.030)mg/L of Al leached from Al-WTR and PACWTR,respectively.A 2-h contact with CaCl2yielded the same result of(0.018±0.028)mg/L for both WTRs.PAC-WTR leached slightly(p>0.05)more Al((0.030±0.034)mg/L)than Al-WTR((0.026±0.020)mg/L)when mixed with CaCl2for 72 h.Although PAC-WTR leached more Al,it was still within the acceptable range(0.63-3 200μg/L)according to the United States Environmental Protection Agency(USEPA,2018b)aquatic life criteria.
The pH values for Al-WTR and PAC-WTR were 6.99±0.21 and 6.81±0.12,respectively(p<0.05).This was similar to the results of Dayton and Basta(2001),who reported an average pH value of 7.1 for 17 Al-WTR samples.Neither WTR had a pH outside the optimum range of 6.5-8.5 for most aquatic organisms(USEPA,2018a),indicating that they would not pose detrimental effects associated with pH.
3.2.Characteristics of water samples collected from Eckert Ditch
The average reactive PO4concentration was(0.30±0.22)mg/L,the average aluminum concentration was(0.020±0.016)mg/L,and the average pH was 7.45±0.16.The majority of the low reactive PO4concentrations occurred during colder months(Fig.2).The highest PO4concentration values coincided with nutrient applications at nearby farms and strong storm events(3.35 mm of rain on June 26,2018 and 3.18 mm of rain on October 10,2018).

Fig.2.Reactive PO4 concentration and temperature in Eckert Ditch between October 2017 and December 2018.
3.3.Batch adsorption,desorption,and total specific adsorption with distilled water
Table 1 lists the total specific PO4adsorption(i.e.,the mass of PO4that was sorbed to WTR)for the six spiked concentrations to enable a direct comparison between PO4background concentrations,WTRs,and distilled vs.surface waters.The total specific PO4adsorption for Al-WTR generally increased as the total aqueous PO4concentration increased,a result that agreed with Bai et al.(2014).The set with spiked PO4concentration of 0.15 mg/L in distilled water had the lowest removal efficiency of 48.56%.Interestingly,the set spiked with 0.30 mg/L PO4in distilled water had a removal efficiency of 61.67%,lower than the set spiked with 0.25 mg/L PO4.This higher sorption may be explained by the particle distribution due to the heterogeneity of Al-WTR.Finer WTR particle sizes result in increased PO4sorption compared to coarse particles(Mortula and Gagnon,2007).
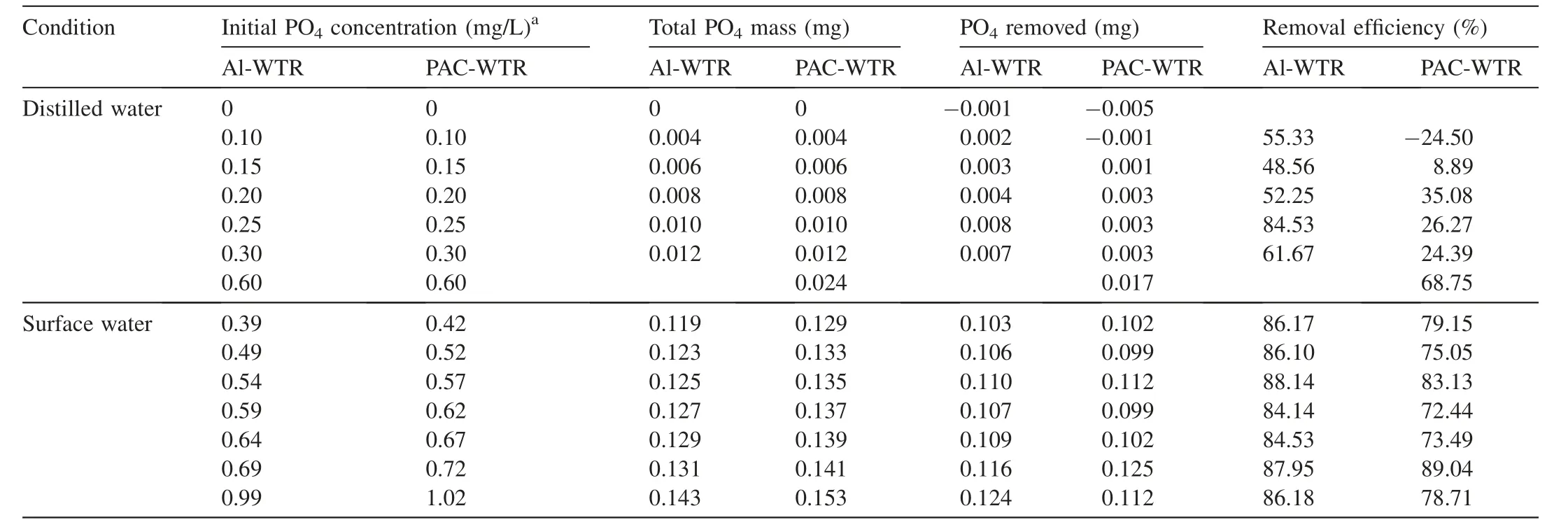
Table 1 Total specific PO4 adsorption calculations for batch isotherms.
As the initial spiked PO4concentration increased,the qevalues increased for Al-WTR and PAC-WTR(Fig.3).However,there was not a linear relationship between the initial spiked PO4concentration and the total specific PO4adsorption(Table 1).The adsorption results presented in Fig.3 give a preliminary indication that both WTRs may be effective at binding PO4.
Since distilled water was the background solution,and no PO4was added after the first day,desorption was easily quantified.The control set(0 mg/L PO4)shown in Fig.4(a)desorbed the previously bounded PO4.Only 0.001 mg of PO4was desorbed from the 1.6 g of Al-WTR over the experiment.The qecontinued to gradually decrease with each successive desorption step.For example,as shown in Fig.4(e),the Al-WTR qedecreased from 5.70 mg/kg after the first desorption step(day 1,referred to as d1 in Fig.4)to 5.63 mg/kg by day 7.This was expected,as washing of Al-WTR can lead to PO4desorption(Haustein et al.,2000).Dayton et al.(2003)investigated 15-h batch isotherms with PO4solutions with concentrations from 0 to 100 mg/L and found qevalues from 0.30 to 5.14 mg/kg.These slightly lower results were reasonable since they used a shorter equilibrium contact time.
Similar to Al-WTR,the negative qevalues depicted in Fig.5 correspond to the desorption of PO4that was bound when PAC-WTR was in use at the drinking water plant.For spiked PO4concentrations,PAC-WTR also depicted a general trend of increased cumulative 28-d qevalues as spiked PO4concentration increased(Fig.5(b)through(g)).A linear relationship was not present between the initial spiked PO4concentration and total specific PO4adsorption(Table 1).The set initially spiked with 0.60 mg/L PO4had the largest total specific PO4adsorption percentage,almost twice that of 0.30 mg/L(p>0.05).It also had the largest adsorption qevalue of 13.30 mg/kg,the largest cumulative 28-d qevalue of 9.61 mg/kg,and the largest removal efficiency of 68.75%.
Although the cumulative 28-d qevalues were larger for Al-WTR(Fig.4),this result was not significantly different from that with PAC-WTR(Fig.5,p>0.05).Nor was there a significant difference(p>0.05)between the change in qevalues from the 24-h adsorption to the cumulative 28-d desorption step.However,there was a significant difference between the change in Cevalues(p<0.05):0.007 mg/L for Al-WTR versus-0.057 mg/L for PAC-WTR.The negative value indicated that the amount of aqueous PO4decreased with greater PO4sorption.Comparison of all experimental data indicated that the PO4adsorption by PAC-WTR had a desorption ratio of 28%-142%,while Al-WTR performed better with a desorption ratio of 14%-53%.
3.4.Batch adsorption,desorption,and total specific adsorption with surface water
The surface water collected during the Al-WTR experiments had an average PO4concentration of 0.39 mg/L.The concentration in experimental sets was the spiked PO4concentration plus the average background surface water PO4concentration(combined PO4concentration).As expected,qeincreased as the combined PO4concentration increased(Fig.6(a)).For example,a combined PO4concentration of 0.39 mg/L yielded a 24-h adsorption qeof 6.49 mg/kg,while the qevalue was 14.57 mg/kg for a combined PO4concentration of 0.69 mg/L.

Fig.3.Batch adsorption isotherm of PO4 for Al-WTR and PAC-WTR with distilled water stored statically at 20°C for different initial spiked PO4 concentrations.
For PAC-WTR,the surface water had an average PO4concentration of 0.42 mg/L.Similar to Al-WTR,the qevalues for all sets increased with time.For the 0.42,0.57,0.62,0.67,and 1.02 mg/L combined PO4sets,the Cevalue decreased with subsequent time steps in a linear fashion and then showed asymptotic behavior(Fig.6(b)).The cumulative Cevalue approached a limit of 0 as time went on,but it could not achieve this value,since previous PO4was not sorbed in past time steps.

Fig.4.Al-WTR batch desorption isotherms with distilled water for different initial spiked PO4 concentrations(data labels correspond to cumulative desorption day,e.g.,d21 represents cumulative desorption by day 21).

Fig.5.PAC-WTR batch desorption isotherms with distilled water and stored statically at 20°C for different initial spiked PO4 concentrations.
Covelo et al.(2007)investigated the simultaneous adsorption and desorption of various metals on soil and found that the isotherms were irregular due to competition for binding sites and the heterogeneity of the soils.Although WTRs were not soils,the sorption processes were similar.Therefore,irregular isotherms were expected due to the complex nature of the PO4sorption,variance in WTR composition,and fluctuating PO4concentrations in surface water.
In all cases,the qevalues increased significantly(p<0.05)during the desorption phase.This indicated continuous sorption of PO4,even with the simultaneous challenge of potential desorption.For Al-WTR(Fig.7),the values for days 1,2,and 3 in the desorption phase were greater than the adsorption Ce,indicating less PO4sorption.The values for days 7,14,21,and 28 were either similar to or less than the adsorption Cevalue,indicating greater PO4sorption(Fig.7(a)).For instance,the system with the 0.54 mg/L combined PO4concentration had a 24-h PO4adsorption Cevalue of 0.06 mg/L,while the Cevalues for the 7-d,14-d,21-d,and 28-d cumulative PO4desorption were 0.03,0.01,0.04,and 0.03 mg/L,respectively(Fig.7(c)).This suggested that the 24-h contact time was not long enough to sorb the PO4for a combined concentration higher than 0.5 mg/L.While there was less PO4sorption during the first few desorption steps,this was not concerning.At each desorption step,approximately 0.016 mg of PO4was introduced to Al-WTR,and at least 40% was removed.Additionally,at each desorption time step,the Cevalue was 50% lower than the average PO4concentration of 0.30 mg/L in Eckert Ditch.This further verified the fact that WTR successfully handled the higher PO4loads.

Fig.6.Batch adsorption isotherms for Al-WTR with surface water containing 0.39 mg/L PO4 and PAC-WTR with surface water containing 0.42 mg/L PO4 stored statically at 20°C for different initial combined PO4 concentrations.

Fig.7.Al-WTR batch desorption isotherms with surface water containing 0.39 mg/L PO4 and stored statically at 20°C for different initial combined PO4 concentrations.
A direct comparison cannot be made between Al-WTR and PAC-WTR that were stored statically at 20°C because the surface water background PO4concentrations were different.The surface water for PAC-WTR had a PO4concentration that was 7% greater than Al-WTR,equating to PAC-WTR being challenged with 0.010 mg of PO4more than Al-WTR.The Al-WTR cumulative 28-d desorption step qevalues were significantly greater(Fig.7,p<0.05)than those of PAC-WTR(Fig.8).There was no significant difference between the qevalues of WTRs from the 24-h adsorption step to the cumulative 28-d desorption step(p>0.05)of individual experiments,indicating that both WTRs sorbed similar amounts of PO4.Comparing the overall 28-d cumulative desorption across experiments,qewas 33.93 mg/kg for Al-WTR and 24.95 mg/kg for PAC-WTR(p<0.05).
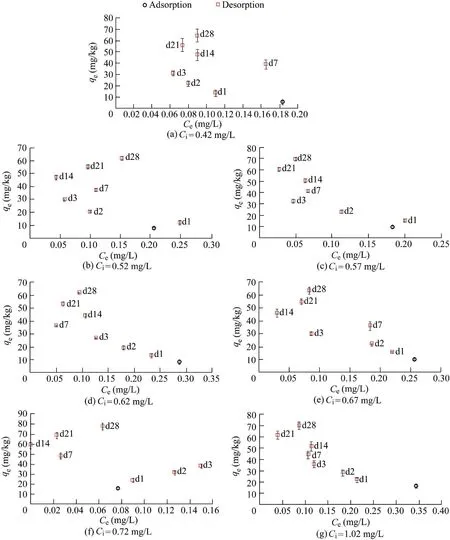
Fig.8.PAC-WTR batch desorption isotherms with surface water containing 0.42 mg/L PO4 and stored statically at 20°C for different initial combined PO4 concentrations.
All experiments with Al-WTR had a total specific PO4adsorption of 84.14% or greater(Table 1).The results indicated that Al-WTR was still capable of removing PO4when the PO4loading increased.Since the PO4concentration of 0.39 mg/L in the background surface water was higher,the impact of the initial spiked PO4concentration was minimal compared to what was sorbed from the surface water.In the set with an initial PO4concentration of 0.99 mg/L,approximately 0.024 mg,or just 20%,of the total PO4was due to the spiked concentration of 0.60 mg/L.Overall,the average total specific PO4adsorption was 78.71%±5.86% for the PAC-WTR trial with surface water.
3.5.Column experiments with distilled water
Al-WTR was subjected to 1.5 mg/L PO4in distilled water over 720 h(Fig.9).The PO4uptake was 9.00 mg/g with additional parameters listed in Table 2.The effluent PO4concentration dropped to 0.74 mg/L at 319 h,rebounded back to 1.10 mg/L over the next 50 h,and remained about 1.1 mg/L from 400 to 720 h.Al-WTR continuously sorbed PO4from the water,even after receiving 1.5 mg/L PO4for several weeks,indicating that Al-WTR can successfully handle the much lower PO4loading of 0.30 mg/L in Eckert Ditch.The Al-WTR uptake was comparable to those found in literature.Razali et al.(2007)found that the PO4uptake decreased as the pH increased.Babatunde et al.(2009)investigated removal efficiencies over time using influent PO4concentrations that began with 10 mg/L and increased up to 316 mg/L over a 25-week period.
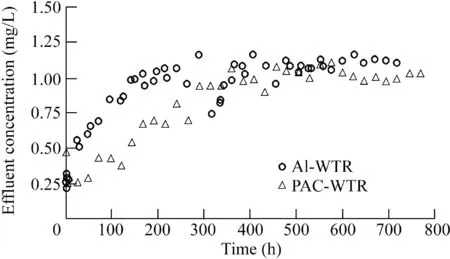
Fig.9.Variation of effluent concentrations with time for continuous flow column experiments with Al-WTR and PAC-WTR.
Fig.9 also contains the results for PAC-WTR subjected to 1.5 mg/L PO4in distilled water over 768 h,which yielded a PO4uptake of 7.14 mg/g(Table 2).The first effluent reading,0.49 mg/L taken at 0.75 h,was twice the amount of the second reading,0.27 mg/L taken at 3 h.This indicated that PO4uptake increased during the first few hours of the experiment.Jafari and Jamali(2015)also determined that the metal concentration in the effluent of consecutive adsorption-desorption column experiments initially had a high uptake,before it gradually decreased as the column approached breakthrough.O'Neill and Davis(2012)reported a similar trend,where removal was much higher at the beginning and gradually decreased with time.A column not reaching breakthrough is typically attributed to the specific concentration of PO4and flow rate used.Although increasing the PO4concentration would decrease the time needed to reach saturation,it would not match field conditions(Klimeski et al.,2012).

Table 2 PO4 uptake and removal efficiencies for column experiments.
3.6.Column experiments with surface water
Prior to initiating column experiments with surface water from Eckert Ditch,the effluent concentration at 102.5 h was determined from Fig.9 to be 0.84 mg/L.If the constituents in the water did not impact sorption,the effluent concentration at 102.5 h with Al-WTR and surface water would also be 0.84 mg/L.As shown in Fig.10(a),the actual Al-WTR reading was 0.86 mg/L,or 2.3% greater.The distilled water data for the first 102.5 h from Fig.9 were added to Fig.10 for visual comparison.The reactive PO4uptake during the 102.5 h was 1.23 mg/g(Table 2).The effluent concentrations were closer for the Al-WTR surface and distilled water columns than those for the PAC-WTR columns(Fig.10).The final removal efficiency for the Al-WTR surface water column was 67.44%,almost twice that of the 34.89%for the distilled water column due to the significant difference in operating times.When adjusting both times to 120 h,the distilled water column removal efficiency was 57.25%.

Fig.10.Effluent concentrations during continuous column experiments with Al-WTR and PAC-WTR.
For PAC-WTR,the distilled water run had an effluent concentration of 0.55 mg/L at 102.5 h,while the effluent with surface water was 0.76 mg/L,a 38.2% underestimation.The distilled water experiment had lower effluent concentrations than the surface water between 6 and 102 h.WTR sorption was non-site specific,meaning that it would sorb more PO4,not just that in the water(Ippolito et al.,2011).In distilled water,PO4was the only loading present,whereas the surface water had other constituents,such as total suspended solids and nitrogen.Reactive PO4sorption onto the WTR was dependent upon the availability of positively charged adsorption sites(Babatunde et al.,2009).The PO4uptake for PACWTR with surface water was 1.27 mg/g(Table 2).PACWTR exhibited the ability to sorb PO4over time from both distilled water and surface water.Using a run time of 120 h,the distilled water column removed 75.16% of PO4,slightly higher than the 62.13% removal efficiency for the surface water column(Fig.10(b)).
In the distilled water continuous flow column experiments,Al-WTR performed better than PAC-WTR.In the 102.5-h surface water continuous flow column experiments,the PO4uptake for PAC-WTR was 1.27 mg/g,3.3% greater than the 1.23 mg/g PO4uptake of Al-WTR.One possible explanation for this was the greater surface area of PAC,leading to more adsorption sites for PO4(MWH Global et al.,2005).Even with these differences,the theoretical prediction of Al-WTR PO4uptake was much closer to the actual value than that of PACWTR PO4uptake.
Although evaluation of the impact of mass transfer kinetic limitations on sorption was outside the scope of this research,it is important to note that the limitations can be magnified in column studies.Slow adsorption-desorption can occur in batch isotherms due to intraparticle diffusion(Lalley et al.,2016).However,this can often be overcome when the sorbate concentration is higher than that of the sorbents and mixing occurs.In a continuous flow system,high mass transfer limitations can be present due to a low-concentration gradient of solute from solution to the solid surface,little-to-no mixing,and a lower contact time(Kalaruban et al.,2018;Sanford and Crawford,2000).
4.Conclusions
This study quantified the PO4uptake values of Al-WTR and PAC-WTR and determined the potential differences between the WTRs.Although both Al-WTR and PAC-WTR were suitable sorbent materials,Al-WTR had greater PO4uptake values than PAC-WTR.The average cumulative 28-d desorption qeduring the batch isotherms of Al-WTR was higher than that for PAC-WTR(p<0.05).Similarly,the cumulative 28-d Cevalues for Al-WTR were lower than those for PAC-WTR(p<0.05).Al-WTR sorbed more PO4in the surface water experiments than in the distilled water experiments.Therefore,Al-WTR was selected for the full-scale passive PO4system to be installed in Eckert Ditch.
Declaration of competing interest
The authors declare no conflict of interest.
 Water Science and Engineering2020年3期
Water Science and Engineering2020年3期
- Water Science and Engineering的其它文章
- Impacts of topographic factors on regional snow cover characteristics
- Phosphorus removal by adsorbent based on poly-aluminum chloride sludge
- Characterization of cobalt ferrite-supported activated carbon for removal of chromium and lead ions from tannery wastewater via adsorption equilibrium
- Acclimatization of microalgae Arthrospira platensis for treatment of heavy metals in Yamuna River
- Three-dimensional modelling of shear keys in concrete gravity dams using an advanced grillage method
- Influences of flow rate and baffle spacing on hydraulic characteristics of a novel baffle dropshaft
