Phosphorus removal by adsorbent based on poly-aluminum chloride sludge
Hui-fang Wu*,Jun-ping Wang,Er-gao Duan,Wen-hua Hu,Yi-bo Dong,Guo-qing Zhang
Department of Municipal Engineering,Nanjing Tech University,Nanjing 211800,China
Received 31 March 2020;accepted 4 June 2020
Available online 3 October 2020
Abstract Phosphorus adsorption tests were carried out using poly-aluminum chloride sludge(PACS),which was collected from a water treatment plant in Nanjing.The amount of phosphorus adsorbed by PACS increased quickly within the first hour and reached equilibrium after about 48 h.The adsorption behavior of PACS for phosphorus is consistent with the Langmuir adsorption isotherm equation(R2>0.99)and parallel first-order kinetic equation(R2>0.98).With the increase of the PACS concentration,the adsorption capacity of PACS for phosphorus decreased,and the removal rate increased.The results of batch tests showed that the adsorption capacities of PACS for phosphorus ranged from 1.64 to 1.13 mg/g when the pH value varied from 4 to 10.However,the adsorption capacity of PACS was not evidently influenced by temperature.In comparison with the ion exchange resin,the adsorption capacity of PACS was barely inhibited by competitive ions,such as,and Cl-.The PACS surface after adsorption became smooth,and the vibration peaks of Al-O and Al-OH shifted.Both HCl and NaOH have a strong desorption effect on PACS after adsorption saturation,and with higher concentrations of HCl and NaOH,the desorption effect was stronger.Results of column adsorption experiments showed that with lower phosphorus and hydraulic loads,the adsorption column took longer to reach saturation.This indicated that PACS could be used as an efficient material for removal of phosphorus from water.This study provides a new treatment method with PACS.
© 2020 Hohai University.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Keywords:Poly-aluminum chloride sludge(PACS);Phosphorus removal;Adsorption characteristics;Batch adsorption test;Column adsorption test
1.Introduction
Since the 1960s,research has been conducted on the recovery of aluminum from waterworks sludge.Acid-soluble,alkali-soluble,and ion-exchanged extraction methods have been used to extract aluminum from sludge.Although these methods have achieved high aluminum recovery rates,the purity of aluminum was low and the process was complicated and expensive(Dassanayake et al.,2015).Moreover,some researchers have directly used alum sludge as a coagulant to treat sewage and strengthen the removal of particulate and organic matter.The ability of alum sludge to eliminate a wide range of heavy metal pollutants,such as Hg,Pb,As,Cr,and Cu,from wastewater by adsorption and chemical precipitation has been verified(Bhatnagar and Sillanp¨a¨a,2010;Siswoyo et al.,2014).Alum sludge has a strong metal adsorption capacity due to the amorphous nature of hydroxyl-Al contained in the sludge,high surface area,and pore size.The application of alum sludge in removal of phosphorus from water has become a research hotspot(Yang et al.,2006a;Wang et al.,2016).Excessive phosphorus entering natural water bodies usually leads to eutrophication.Therefore,it is necessary to remove phosphorus in the tail water before it is discharged into water bodies(Alaba et al.,2018).Several extensive studies have been conducted on the possible utilization of alum sludge as an adsorbent and the effects of different factors on phosphorus adsorption.In particular,some research has been carried out on the decontamination performance of alum sludge for artificial wetland fillers(Zhao et al.,2011).
Yang et al.(2006b)used dehydration alum sludge(DAS)from a water supply plant with particle size less than or equal to 0.063 mm as an adsorbent,and studied its adsorption capacity and factors influencing orthophosphate removal at room temperature(20°C±2°C).The study revealed that pH plays a major role in the adsorption capacity of DAS and the Langmuir adsorption isotherm best fits experimental data.Huang and Chiswell(2000)used naturally dried alum sludge to adsorb phosphorus in a solution and found that the adsorption amount of phosphorus was approximately 0.30-0.33 mg/g.Kim et al.(2015)showed that the adsorption capacity of alum sludge for phosphorus could reach 25 mg/g.Galarneau and Gehr(1997)examined the adsorption properties of alum sludge for orthophosphate,polymeric phosphate,and organophosphate under theoretical and practical conditions,and found that when the molar ratio of aluminum to phosphorus was 3:1,the removal rates for orthophosphate,polymeric phosphate,and organophosphate were 70%,85%,and 15%,respectively.Babatunde et al.(2009)found that when the pH value increased from 4.0 to 9.0,the adsorption capacity of alum sludge for phosphorus decreased from 31.9 to 10.2 mg/g.Other researchers have used other types of alum sludge to study phosphorus removal(Guan et al.,2005;Park and Polprasert,2008;Razali et al.,2007).
Poly-aluminum chloride sludge(PACS)is a byproduct of sedimentation tanks when water treatment plants use polyaluminum chloride as a coagulant.PACS is a type of alum sludge.Aluminum sulfate and ferric chloride with low molecular weights are gradually replaced by the new aluminum salt coagulant,poly-aluminum chloride(PAC),due to the lower concentration of PAC under the same water quality conditions,the better adaptability to the pH of raw water,and the larger amount of generated PACS(Gao et al.,2003).Although alum sludge has been widely used in phosphorus removal and its environmental and economic benefits have been verified,phosphorus adsorption of PACS has rarely been reported.As an aluminum-containing compound,PAC forms a polymeric cation after its addition to raw water,and its coagulation mechanism is similar to that of alum sludge.Therefore,assuming that PACS also has a strong removal effect on phosphorus is reasonable.
This study mainly investigated the characteristics of adsorption of phosphorus by PACS.Batch tests were conducted on PACS from a water treatment plant in Nanjing to study the adsorption performance and explore the influence of different factors.The effects of competitive ions were investigated through comparison with ion exchange resin adsorbents.Related characterization was performed to further investigate the influence of adsorption on the surface morphology and action mechanism between PACS and phosphorus.Column adsorption experiments were designed to study the penetrating performance of adsorption columns with different phosphorus loads and hydraulic retention times in order to apply PACS effectively in practice.
2.Materials and methods
2.1.Experimental materials
PACS with a concentration of 20-25 mg/L was collected from the inclined pipe sedimentation tank of a water treatment plant in Nanjing.After air drying to a moisture content of approximately 20%,PACS was dried in a vacuum oven at 60°C±1°C to a moisture content of 2.7%,ground into powder,and passed through a 100 mesh sieve.
The pure potassium dihydrogen phosphate(KH2PO4)was dried in a vacuum oven at a temperature of 110°C for 2 h and then naturally cooled.The phosphorus solution,which was artificially prepared by dissolving the pre-weighed KH2PO4in distilled water,was maintained airtight and incubated in the laboratory at 4°C.
Powdery PACS is difficult to settle,separate,and recycle.Therefore,PACS granular adsorbent should be pre-prepared so that it can be applied to an actual fixed bed and a fluidized bed.Using AlCl3with a mass ratio of 8% as the binder and starch with a 4% dosage ratio as the porogen,powdery PACS was balled and dried at 500°C for 2 h to transform it into a granular adsorbent.
D201 anion exchange resin was purchased from the Shanghai Jinkai Resin Factory.
2.2.Static batch tests
A series of batch tests were conducted in 250-mL flasks.100 mL of phosphorus solution was taken into the flask,the pH was adjusted,different amounts of PACS were added,the flasks were sealed and shaken in a constant temperature oscillator(SHA-C,Guohua Electric Co.,Ltd.,China)at 20°C and 150 rpm for 48 h,and the supernatant was filtered through 0.45-μm filter paper to investigate the residual phosphorus content in the solution.The phosphate and pH of the liquid were monitored using an ultraviolet-visible spectrophotometer(752s,Shanghai Lingguang Technology Co.,Ltd.,China)and a precision pH meter(PHS-3,Shanghai Precision Scientific Instrument Co.,Ltd.,China),respectively.
2.3.Dynamic column tests
Using PACS granular adsorbents as filler,a continuous penetration test with artificial water distribution was conducted to investigate the penetration performance of the adsorption column under different phosphorus and hydraulic loading conditions.The effluent samples were periodically collected,and phosphorus concentrations in the effluent were determined.A sketch of the dynamic column experimental device is shown in Fig.1.

Fig.1.Dynamic column experimental device.
3.Results and discussion
3.1.Adsorption characteristics
3.1.1.Specific surface area
The specific surface area and pore volume were calculated according to the Brunner-Emmett-Teller(BET)surface area test.The specific surface area of PACS was 23.124 m2/g,the pore volume was 0.076 345 cm3/g,and the average pore size was 13.206 nm.The pore size distribution curve is shown in Fig.2,where Vpis the pore volume(cm3/g),dpis the aperture(nm),and dVp/ddpis the hole area.The proportion of pores with a pore size of 10 nm is the largest.

Fig.2.Pore size distribution of PACS.
3.1.2.Adsorption isotherm
The adsorption isotherms of phosphorus by PACS are shown in Fig.3.As shown in Table 1,the adsorption of phosphorus by PACS under different pH conditions is well fitted by the Langmuir isotherm(Eq.(1)).The coefficients of determination(R2)were all greater than 0.99,indicating that the adsorption sites on the surface of PACS were evenly distributed and had the same affinity for phosphorus,and the adsorption was monolayer(Zuo et al.,2016).
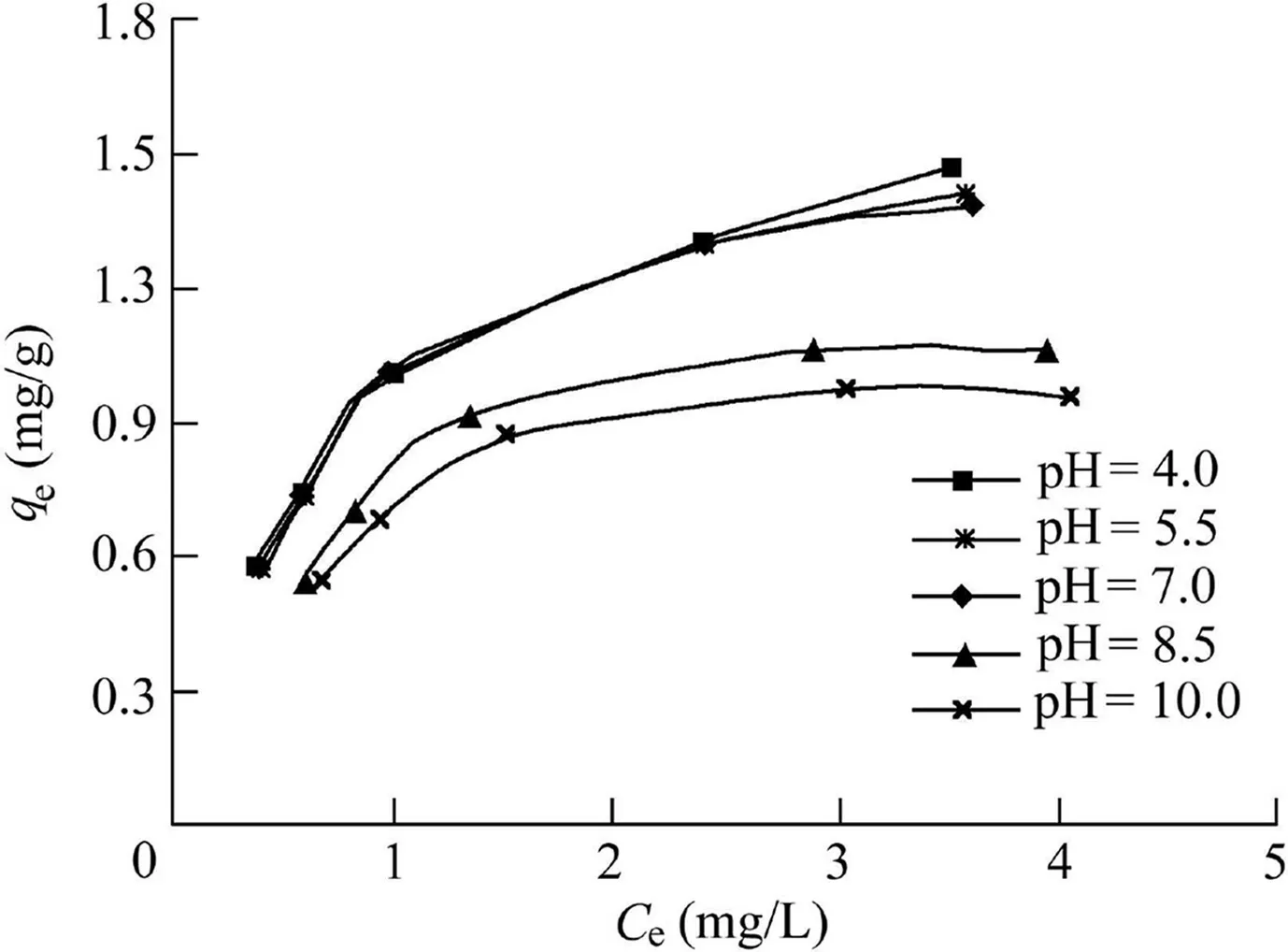
Fig.3.Adsorption isotherms of phosphorus by PACS with difference pH values.

where qeis the adsorption capacity at equilibrium(mg/g),Ceis the equilibrium phosphorus concentration(mg/L),q0is the theoretical maximum adsorption capacity(mg/g),and b is the constant related to phosphorus binding energy(L/mg).
3.1.3.Adsorption kinetics
In the study of solid-liquid adsorption systems,multiple adsorption kinetic models,such as the pseudo first-order kinetic equation(Eq.(2)),pseudo second-order kinetic equation(Eq.(3)),and parallel first-order kinetic equation(Eq.(4)),were used to describe the solid-liquid interface:

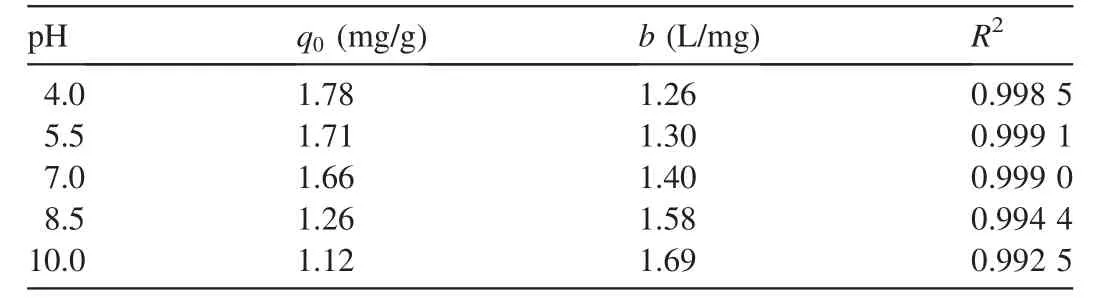
Table 1 Langmuir adsorption isotherm.
where qtis the adsorption of phosphorus by unit mass PACS(mg/g)at time t(min);k1is the pseudo first-order equilibrium adsorption rate constant(min-1);k2is the pseudo second-order equilibrium adsorption rate constant(g/(mg·min));qe1and qe2are the equilibrium adsorption amount(mg/g)of pseudo first-order reaction and pseudo secondary-order reactions,respectively;and k1aand k2aare the equilibrium adsorption rate constants(min-1)of pseudo first-order reaction and pseudo second-order reaction,respectively.
The kinetic data of PACS adsorption for phosphorus with different initial phosphorus concentrations and the fitting curves of the three kinetic equations are shown in Fig.4.It can be seen that the PACS adsorbs phosphorus at a high rate at the beginning,but that the adsorption gradually decreases with time.This is because in the early stage of adsorption,the number of vacant adsorption sites on PACS is relatively large.The phosphate concentration gradient formed at the solidliquid interface of the adsorption system is also large,leading to a large mass transfer driving force,which is conducive to ion exchange between phosphate and active functional groups.With the occurrence of the reaction,the number of active sites on PACS decreases continuously and the concentration gradient diminishes,finally reaching equilibrium.At the same time,the time for the adsorption reaction to reach a basic equilibrium extends as the concentration of phosphorus increases.
Compared with pseudo first-order and pseudo second-order kinetic equations,the parallel first-order kinetic equation can better fit the adsorption kinetic test data.The fitting results are shown in Table 2.The R2values are greater than 0.98 in the tests examined.The adsorption reaction consisted of two subreactions(Rodrigues and da Silva,2010).
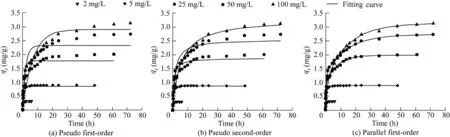
Fig.4.Kinetics of phosphorus adsorption on PACS with different initial phosphorus concentrations and fitting curves of pseudo first-order,pseudo second-order,and parallel first-order kinetic equations.
3.2.Batch adsorption test
3.2.1.Effect of PACS concentration
With the initial phosphorus concentration of 5 mg/L,pH value of 6.8-7.2,and temperature of 25°C,the adsorption behavior was monitored by measuring the residual phosphorus until the equilibrium concentration was reached.As a result,the PACS of the water plant had a strong removal effect on the phosphorus in the aqueous solution,especially in cases with a large amount of adsorbent.The relationship between the unit amount of phosphorus adsorbed by PACS with different concentrations and time is shown in Fig.5.The adsorption amount reached 70%-90% of the equilibrium adsorption amount in the first hour,and the adsorption rate decreased slowly with the increase of adsorption time after 1 h.The number of active adsorption sites on the PACS surface per unit mass remained constant.Thus,the adsorption reaction reached equilibrium after 48 h,in accordance with the conclusion of Yang et al.(2006b).With the increase of PACS concentration,the removal rate at equilibrium increased while the adsorption capacity of phosphorus by unit mass of PACS decreased from 1.43 mg/g to 0.52 mg/g,a finding in line with the general adsorption rule.
3.2.2.Effect of pH
Under the condition of PACS concentration at 1 g/L and temperature at 25°C,the effect of pH on adsorption capacity was examined through a series of adsorption tests with fixed amounts of sludge addition and with different pH values.The results are shown in Fig.6.The amount of phosphorus adsorbed by PACS decreased with the increase of the initial value of pH.The main reasons for this phenomenon are the following:(1)The pH directly affects the form of phosphorus in water.When the pH value in the solution is less than 2.16,the phosphorus mainly exists in the form of H3PO4and is not easily adsorbed.With the increase of the pH value,phosphorus mainly exists in the form ofand,which is favorable for adsorption.(2)A change of pH will change the charge on the PACS surface.PACS is positively charged when the pH value is lower than the isoelectric point of the PACS surface,which is beneficial for adsorption.The PACS surface is negatively charged when the pH value rises above the isoelectric point of the PACS surface,which is not conducive to the adsorption of phosphorus in the form ofand(Yang et al.,2006b).(3)The process of adsorbing phosphate by PACS is accompanied by the release of OH-(Eq.(5)).When pH rises,the concentration of OH-increases,and the reaction of phosphate adsorption is suppressed.


Table 2 Parameters for kinetic models of phosphorus adsorption with different initial phosphorus concentrations.
Although PACS had a higher phosphate adsorption capacity in an acidic pH region than in an alkaline pH region,the effect of pH on the phosphate adsorption capacity of PACS was not evident.When the pH value increased from 4.0 to 10.0,the adsorption amount only decreased from 1.64 to 1.13 mg/g,in contrast to the research results of Yang et al.(2006b),which showed that pH significantly affects the adsorption capacity of PACS for phosphorus,with maximum adsorption capacities ranging from 0.7 to 3.5 mg/g when the pH value varied from 9.0 to 4.3.Large differences were observed in the adsorption capacity of different forms of alum sludge for phosphorus(0.30-23.9 mg/g)(Yang et al.,2006b).This was possibly related to different types and surface physicochemical properties of alum sludge produced by different types of coagulants,concentrations,and raw water used in water treatment plants.In short,the data obtained in this experiment fell between the 0.30-0.33 mg/g reported by Huang et al.(2008)and the 0.7-3.5 mg/g reported by Yang et al.(2006b).

Fig.5.Relationship between phosphorus adsorption capacity and time for different PACS concentrations.
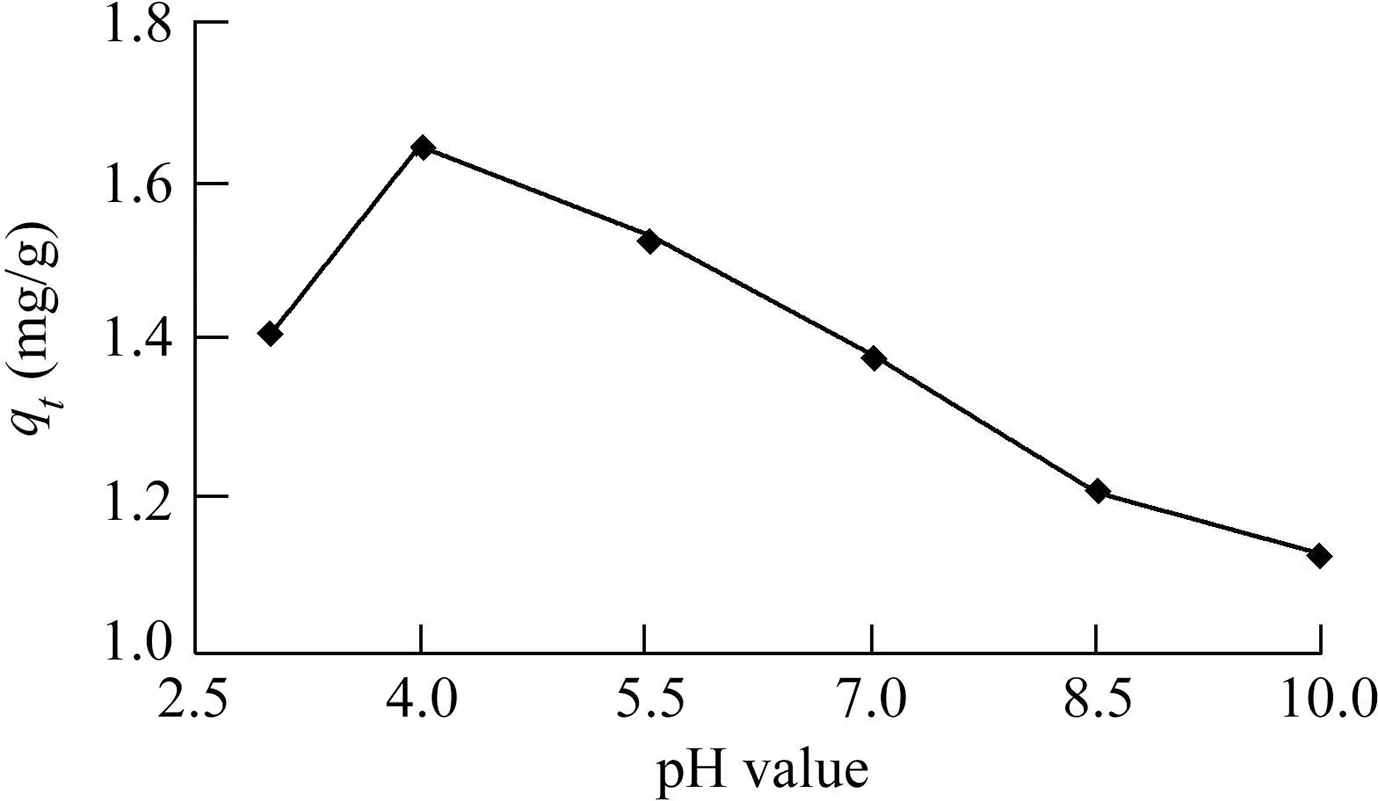
Fig.6.Effect of pH on phosphorus adsorption.
3.2.3.Effect of temperature
Adsorption tests were also conducted at various temperatures under conditions of sludge concentration of 1 g/L and pH values of 6.8-7.2.Fig.7 shows that the adsorption reaction of PACS was mildly sensitive to temperature,and the adsorption amount slightly increased from 1.34 to 1.41 mg/g when temperature varied from 10°C to 40°C.The adsorption reaction of PACS to phosphorus was spontaneous.The irregular molecular thermal motion in the solution was pronounced at high temperatures,which promoted the exchange of molecules on the adsorbent surface.With a higher temperature,there was a greater degree of spontaneity and a shorter time required to reach equilibrium.
3.2.4.Effect of competitive ions
The experimental results showed that D201 a nion exchange resin has a high adsorption capacity for phosphorus.When the concentration was 1 g/L,the phosphorus removal rate reached 86.06% in the simulated phosphorus solution.The amount of PACS should be 5 g/L in order to achieve the same removal rate.Therefore,in the absence of competitive ions,the adsorption capacity of resin for phosphoric acid is considerably stronger than that of PACS.,,and Cl-,which are common in natural water bodies,were selected as competitive ions to investigate their effects on phosphorus adsorption.Competitive tests were performed under the condition of 1 g/L D201 resin concentration,5 g/L PACS concentration,and 5 mg/L initial phosphorus concentration.

Fig.7.Effect of temperature on phosphorus adsorption.
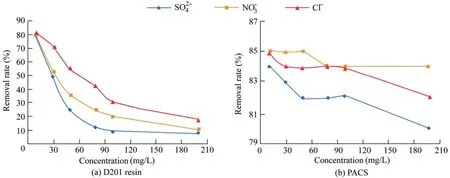
Fig.8.Effects of ,,and Cl-concentrations on phosphorus removal rate of D201 resin and PACS.
3.2.5.Surface morphology
The surface morphology of PACS before and after adsorption was observed via cold field emission scanning electron microscopy(SEM;S-4800,Hitachi,Japan)to investigate the effect of adsorption on the surface morphology of PACS,as shown in Fig.9(a)and(b).Phosphorus was adsorbed by a precipitation reaction occurring on the PACS surface.Therefore,the PACS surface after adsorption was relatively smooth,not as complicated as that before adsorption,and the pores were lessened.This confirms the conclusion of Yang et al.(2006a)to some extent,indicating that phosphate replaced the functional groups from the surface of alum sludge,which implies that ligand exchange is the dominating mechanism for phosphate removal(Yang et al.,2006a).
At a pH value of 7,granular PACS before and after phosphorus adsorption was analyzed with Fourier transform infrared spectroscopy(FTIR),and the results are shown in Fig.10.In Fig.10(a),the absorption peaks in the vicinity of 3 447 cm-1were caused by the stretching vibration of the hydroxyl group,and the absorption peak appearing at 1 636 cm-1was caused by the bending vibration of the adsorbed water.The stretching vibration-induced peak of the structural hydroxyl group of powdery PACS at 3 620 cm-1disappeared after molding.This is because some mineral components in the PACS were changed at high temperature(Xu et al.,2015).The non-stretching vibration of the powdery PACS at 1 385 cm-1was caused by C=O,which became quite small after molding,indicating that carbon in the PACS was burned out at high temperature during molding.A strong characteristic peak was also observed at 477 cm-1,which was caused by the vibration of the hydroxyl group connected to Al.It can be seen from Fig.10 that the peak of PACS at 1 031 cm-1became narrow after phosphorus adsorption,which may be due to the coincidence of P-O peak in this range.The peak of PACS after adsorption at 477 cm-1moved toward 472 cm-1.Peaks of PACS after adsorption at 600-778 cm-1also shifted to varying degrees due to phosphate adsorption by Al-O or Al-OH.Therefore,Al compounds are crucial to phosphorus adsorption by PACS adsorbent(Yang et al.,2006a).
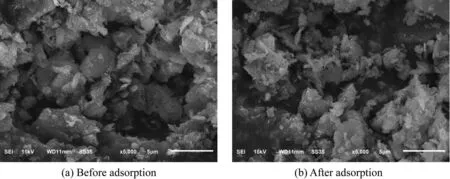
Fig.9.SEM of PACS before and after adsorption.

Fig.10.FTIR spectra of PACS.
3.2.6.Desorption and regeneration
The PACS can be regenerated by desorption.The desorption efficiencies of the acid and alkali desorption agents at different concentrations for the adsorption of phosphorussaturated PACS are shown in Fig.11.The test results show that both HCl and NaOH have a certain effect on the desorption of phosphorus.The desorption reaction is basically stable after about 1 h.After 1 h,even if the time increases,the efficiency cannot be greatly improved.The desorption rate of 0.10 mol/L HCl to phosphorus can reach 92%.The desorption efficiencies of acid and alkali desorption agents from phosphorus-saturated PACS are greatly affected by their concentrations.That is to say,the amount of hydrogen ions or hydroxide ions in the desorption system greatly affects the desorption efficiency.
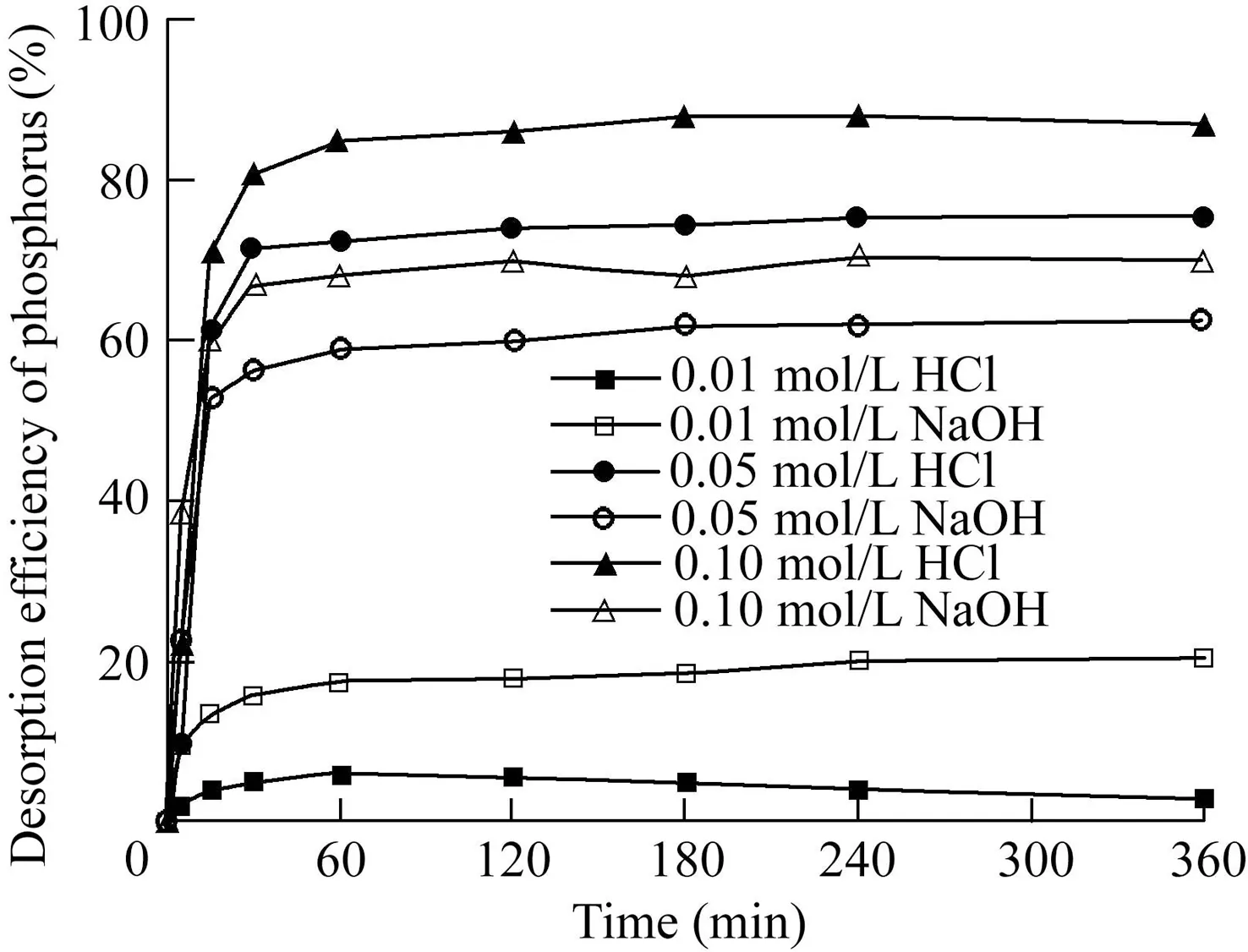
Fig.11.Phosphorus desorption by HCl and NaOH.
3.3.Column adsorption test
The effects of phosphorus and hydraulic loads on phosphorus removal by adsorption columns are shown in Fig.12(a)and(b),respectively.As shown in Fig.12(a),the effluent phosphorus concentration was nearly zero within the first 10 h.With a higher influent phosphorus concentration,the time required for the adsorption column to reach saturation is shorter.Intraparticle diffusion was the main process that affected the adsorption of the dynamic column,which resulted in a steep adsorption curve for a high influent phosphorus concentration.As shown in Fig.12(b),the time needed to reach the adsorption saturation was different under varying hydraulic loads.Under the condition of the same column height and phosphorus load,the phosphorus in the solution had a long contact time with PACS when the hydraulic load was low.When the hydraulic load was extremely high,the mass transfer resistance of the PACS surface was lowered.Thus,the PACS adsorbent filler could not be fully utilized,thereby resulting in a decrease in dynamic adsorption performance.The lower the influent flow rate,the better the treatment effect.Therefore,the hydraulic retention time was the main factor affecting the effluent phosphorus concentration in the dynamic experiment.The concentration of phosphorus in the effluent was very low before leakage.From adsorption leakage to breakthrough,PACS still had a large adsorption capacity.A thick adsorption layer or multi-stage adsorption can more effectively improve the utilization rate of PACS.
In practical applications,suitable phosphorus and hydraulic loads contribute to the durability of PACS to ensure the adsorption rate of phosphorus and the quality of the effluent.Therefore,determining the appropriate phosphorus and hydraulic loads in practical applications through research is necessary to ensure the suitability of the saturation time,the volume of treated water,the adsorption rate of phosphorus,and the quality of effluent.
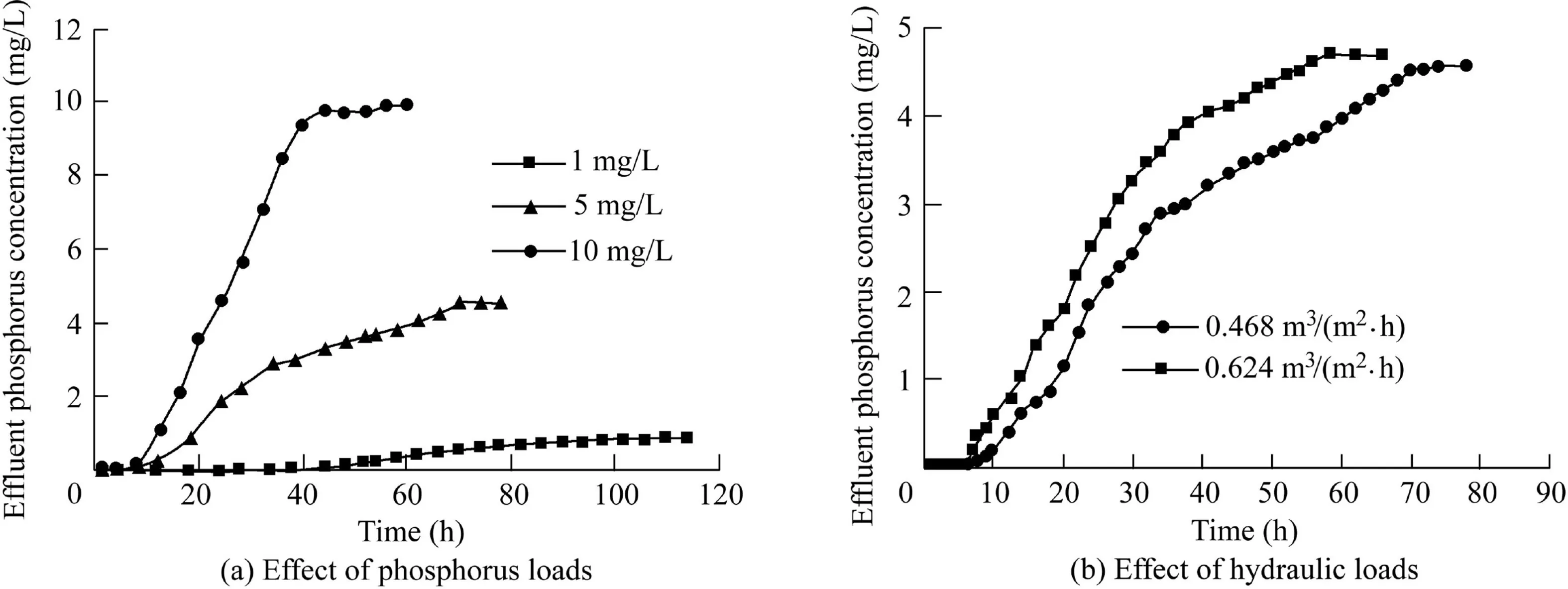
Fig.12.Effects of phosphorus and hydraulic loads on effluent phosphorus concentration.
4.Conclusions
(1)The adsorption of phosphorus by PACS progressed quickly within the first hour and reached equilibrium after about 48 h.The behavior of phosphorus adsorbed by PACS conforms to the Langmuir adsorption isotherm equation(R2>0.99),and is monolayer adsorption.PACS adsorption of phosphorus at different initial phosphorus concentrations(2-100 mg/L)conforms to the parallel first-order kinetic equation(R2>0.98).
(2)With the increase of the PACS concentration(from 1 to 8 g/L),the amount of phosphorus adsorbed by PACS decreased,but the removal rate of phosphorus gradually increased.The adsorption capacity of PACS ranged from 1.64 to 1.13 mg/g when the pH value of the phosphorus solution varied from 4 to 10.The adsorption amount slightly increased with temperature,thereby showing mild sensitivity to temperature.The PACS surface after adsorption became smooth,and the vibration-induced adsorption peaks of Al-O and Al-OH shifted.
(3)In comparison with D201 ion exchange resin,the phosphorus adsorption capacity of PACS was hardly inhibited by competitive ions,such as,and Cl-.This characteristic is beneficial for its use in practical applications.With higher concentrations of HCl and NaOH,the effect on phosphorus desorption from PACS after adsorption saturation is better.The desorption reaction basically reached equilibrium in 1 h.
(4)PACS had effectively removed phosphorus in batch and column tests.The column adsorption experiments show that the saturation of the adsorption column takes a long time when the phosphorus and hydraulic loads are low.
Declaration of competing interest
The authors declare no conflicts of interest.
 Water Science and Engineering2020年3期
Water Science and Engineering2020年3期
- Water Science and Engineering的其它文章
- Impacts of topographic factors on regional snow cover characteristics
- Evaluation of alum-based water treatment residuals used to adsorb reactive phosphorus
- Characterization of cobalt ferrite-supported activated carbon for removal of chromium and lead ions from tannery wastewater via adsorption equilibrium
- Acclimatization of microalgae Arthrospira platensis for treatment of heavy metals in Yamuna River
- Three-dimensional modelling of shear keys in concrete gravity dams using an advanced grillage method
- Influences of flow rate and baffle spacing on hydraulic characteristics of a novel baffle dropshaft
