Jadwar (Delphinium denudatum Wall.): a medicinal plant
Roghayeh Baghervand Navid, Mehrdad Karimi, Morteza Ghojazadeh, Seyed Mostafa Araj-Khodaei, Alireza Bagherzadeh karimi , Sanam Dolati, Mehri Bansans, Seyed Mohammad Bagher Fazljou
Jadwar (Wall.): a medicinal plant
Roghayeh Baghervand Navid1, Mehrdad Karimi2*, Morteza Ghojazadeh3, Seyed Mostafa Araj-Khodaei1, Alireza Bagherzadeh karimi1, Sanam Dolati4, Mehri Bansans5, Seyed Mohammad Bagher Fazljou1*
1Department of Persian Medicine, School of Traditional Medicine, Tabriz University of Medical Sciences, Tabriz5166616471, Iran;2Department of Iranian Traditional Medicine, School of Traditional Medicine, Tehran University of Medical Sciences, Tehran 1417613151, Iran;3Research Center for Evidence Based Medicine, Tabriz University of Medical Sciences, Tabriz 5166616471, Iran;4Physical Medicine & Rehabilitation Research Center, Aging Research Institute, Tabriz University of Medical Sciences, Tabriz 5166616471, Iran;5Department of Divinities and Islamic Sciences, Faculty of Theology, Tabriz University, Tabriz 5166616471, Iran.
Wall. is one of the important medicinal herbs of traditional Persian medicine and is known as Jadwar. Medicinal plants are the most widely used drugs in traditional Persian medicine and has been used for various diseases since earlier times. The medicinal uses ofWall. date back to over 1,000 years ago. Rhazes (845–925 C.E.) was the first Persian physician and scientist who reported the use ofWall. as a herbal remedy. During the following centuries, the usages ofWall. in the treatment of various diseases has been mentioned in the books and references of traditional Persian medicine for cures to various diseases such as neurologic and psychiatric disease, gastrointestinal disease, fever, pain, and poisoning. According to modern studies, the dried roots ofWall. have antipyretic, antimicrobial, anticonvulsant, hepatoprotective, antioxidant, and pain-relieving properties. Biomolecules from roots ofWall. were also identified as potential cures for central nervous system diseases as well as for the amelioration of morphine addiction.Wall., with its properties involving the prevention of mitochondrial dysfunction, reduction of oxidative stress, and inflammation and immune dysregulation, can be utilized in curing inflammatory disorders. The effective therapeutic influence of root extract ofWall. against several diseases needs to be confirmed through controlled clinical trials. This article reviews the different features ofWall. and focuses on the well-known therapeutic effects of this herbal drug on various human disorders and animal disease models.
Wall., Jadwar, root, Traditional Persian medicine, Neurologic and psychiatric effects, Anticonvulsant effects
This review summarizes the different features ofWall., popularly known as jadwar in the sub-continent, and focuses on the well-known therapeutic effects of this herbal drug on various human disorders and animal disease models, including anti-fatigue, anxiolytic and anti-depressive, anticonvulsant, analgesic activities, etc.
According to the historical written documents and books of traditional Persian medicine, medicinal ofWall. were recognized over 1,000 years ago. The first recorded report of theWall.medical utilization is credited to the great Persian chemist and physician, Rhazes, who lived between the ninth and tenth century (845–925 C.E.). He introducedWall. in his bookwhich was written in the tenth century C.E., as antidote to rat poison, and snake and scorpion venom. During the following centuries, the usage ofWall. in the treatment of various diseases has been mentioned in the books and references in traditional Persian medicine such as neurologic and psychiatric disease, gastrointestinal disease, fever, pain, and poisoning. Presently,Wall. are used in Iran, Pakistan, India, and some countries of Middle East for traditional medicine.
Background
Complementary and alternative medicines are commonly used as resources for both the prevention and treatment of various diseases [1]. During the past few decades, more attention has been focused on traditional medicines and their popularity has been rapidly increasing among adults [2, 3]. Plants as traditional medicines exert potent effects on inflammatory diseases via different cellular mechanisms, involving the down-regulation of pro-inflammatory cytokines, the suppression of oxidative stress, and the increase in antioxidant functions [4–6]. Although the people of different traditional societies and rural communities have been using plants for the treatment of different diseases for a long time, there are only a few documented studies validating their medicinal properties [7, 8].
Traditional Persian medicine is a holistic system of medicine that is an important branch of the complementary and alternative medicines, and has been used in Iran, India, Pakistan, and some countries of Middle East for thousands of years [9–12]. One of the most important medicinal plants that Traditional Persian medicine emphasizes for the treatment of many diseases is Jadwar (Wall.) [13].Wall. is a medicinally significant genus of the family Ranunculaceae, with several species found in the Himalayan region. TheWall. is one of the most important medicines used in traditional medicine in India, Pakistan, and Iran, particularly in the Unani system [14].
Dried roots ofWall. are a popular folk remedy for the treatment of epilepsy in the traditional Unani system of medicine (Figure 1).The first recorded report of theWall. medical utilization is related to the great Persian chemist and physician of ninth and tenth century, Rhazes (845–925 C.E.). He introducedWall. in his bookwhich was written in the tenth century C.E., as antidote for rat poison, and snake and scorpion venom [15, 16]. During the following centuries, the usage ofWall. in the treatment of various diseases has been mentioned in historical written documents and books of traditional Persian medicine. These diseases include neurologic and psychiatric disease, gastrointestinal disease, fever, pain, and poisoning, are summarized in Table 1[16–25]. Currently,Wall. is used in Iran, Pakistan, India, and some countries in the Middle East. The application ofbased on the record of ancient literature and Western medicine is summarized in Figure 2.
The present study aims to reviewWall. properties and it’s potentially beneficial role in the treatment of different disorders including nervous system disorders and other chronic diseases.

Figure 1 The whole plant and roots ofWall.
Botanical description and phytochemical study
The genusL. (Ranunculaceae) belongs to the tribe Delphinieae and includes about 385 species, mostly from temperate parts of the Northern hemisphere and mountainous regions of tropical Africa [26]. This genus includes 53 species in Flora Iranica area, 29 of which were reportedly from Iran.occurs in North East of Iran (Khorassan), Afghanistan and Turkmenistan [27]. A total of 27 species ofwere recorded from India [28]. The name “” is derived from the Latin for “dolphin”, referring to the shape of the nectary [29]. In June and July, the plant is topped with a raceme of many flowers, with different colors such as purple, blue, red, yellow, or white, with lengths varying between 40 to 80 cm [30, 31]. It is used in treating asthma and respiratory problems, skin disorders, and central nervous system (CNS)-related diseases. It has anticonvulsant and antimicrobial activities [32, 33].
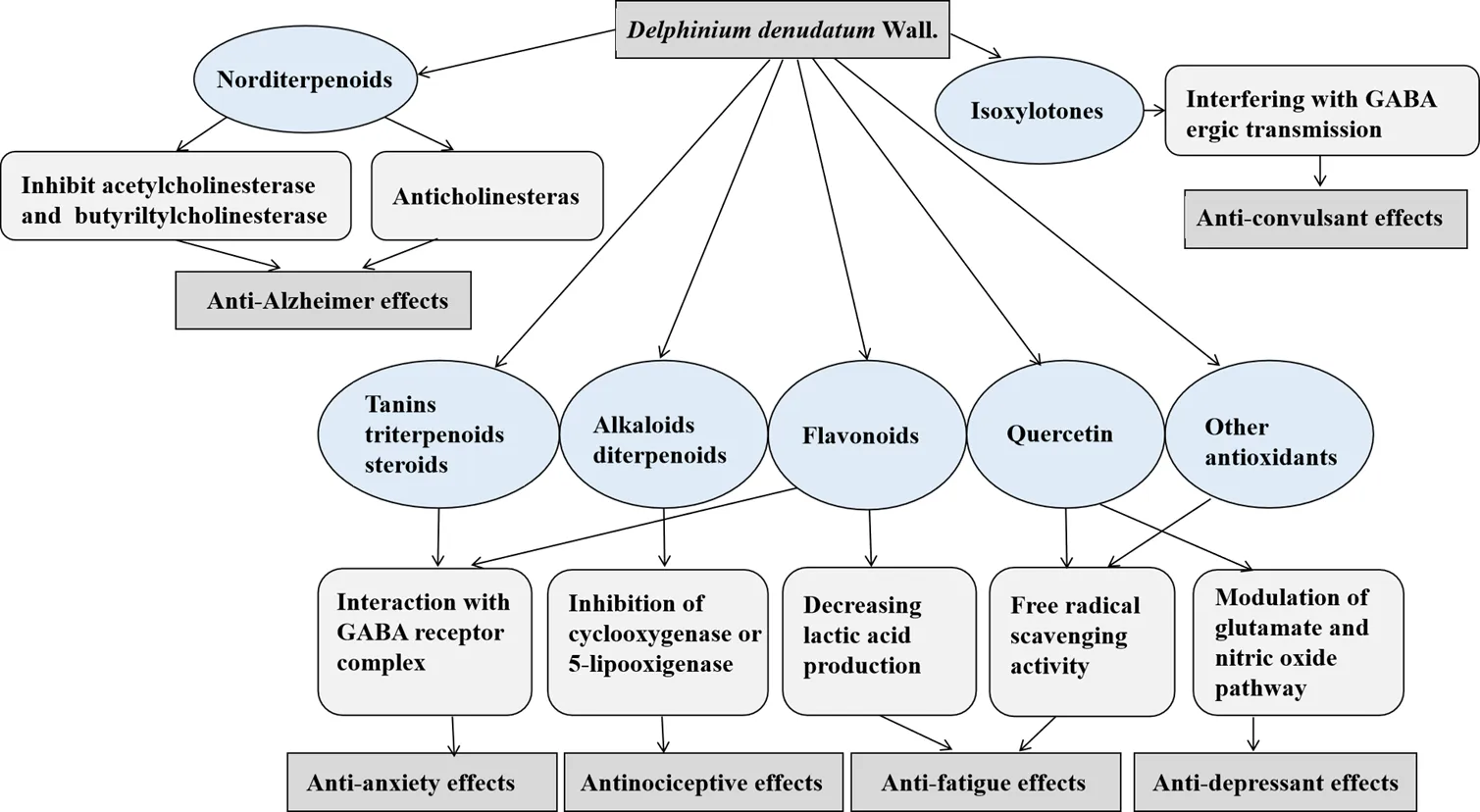
The mechanisms behind the pharmacological action ofWall. can significantly be assisted by the isolation of its active principle from the roots and determination of its structural and functional relationship. The chemical and phytochemical analysis of rootsofWall. has identified various active components, including different alkaloids, namely delphinine, stahisagrine, delphocurarine, condelphine, denudatin, and isotalatizidine, sterols, fatty acids, sugar, protein, starch, and flavonoids [34]. Furthermore, phenolic groups and tannins, carbohydrate, steroids, amino acids, glycosides, and terpenoids were also present in the extracts ofWall. [35]. The main probable mechanisms of the roots ofWall. pharmacological effects including anti-Alzheimer, anti-convulsant, anxiolytic, and antinociceptive effects are shown in Figure 3.
Wall. is well known as a rich source of diterpenoid and norditerpenoid alkaloids with sedative, cardiotonic and analgesic activities [36, 37]. The roots ofWall. have been reported to be beneficial for mitigating various diseases, including fungal infections, CNS-related diseases, toothaches, and mental and physical fatigue [38]. Its use has also been confirmed for ameliorating morphine addiction [39]. Quercetin is the main flavonoid content ofWall. which has antioxidant, anti-inflammatory, anti-aging, neuroprotective, anti-fatigue, and cardioprotective properties [40].
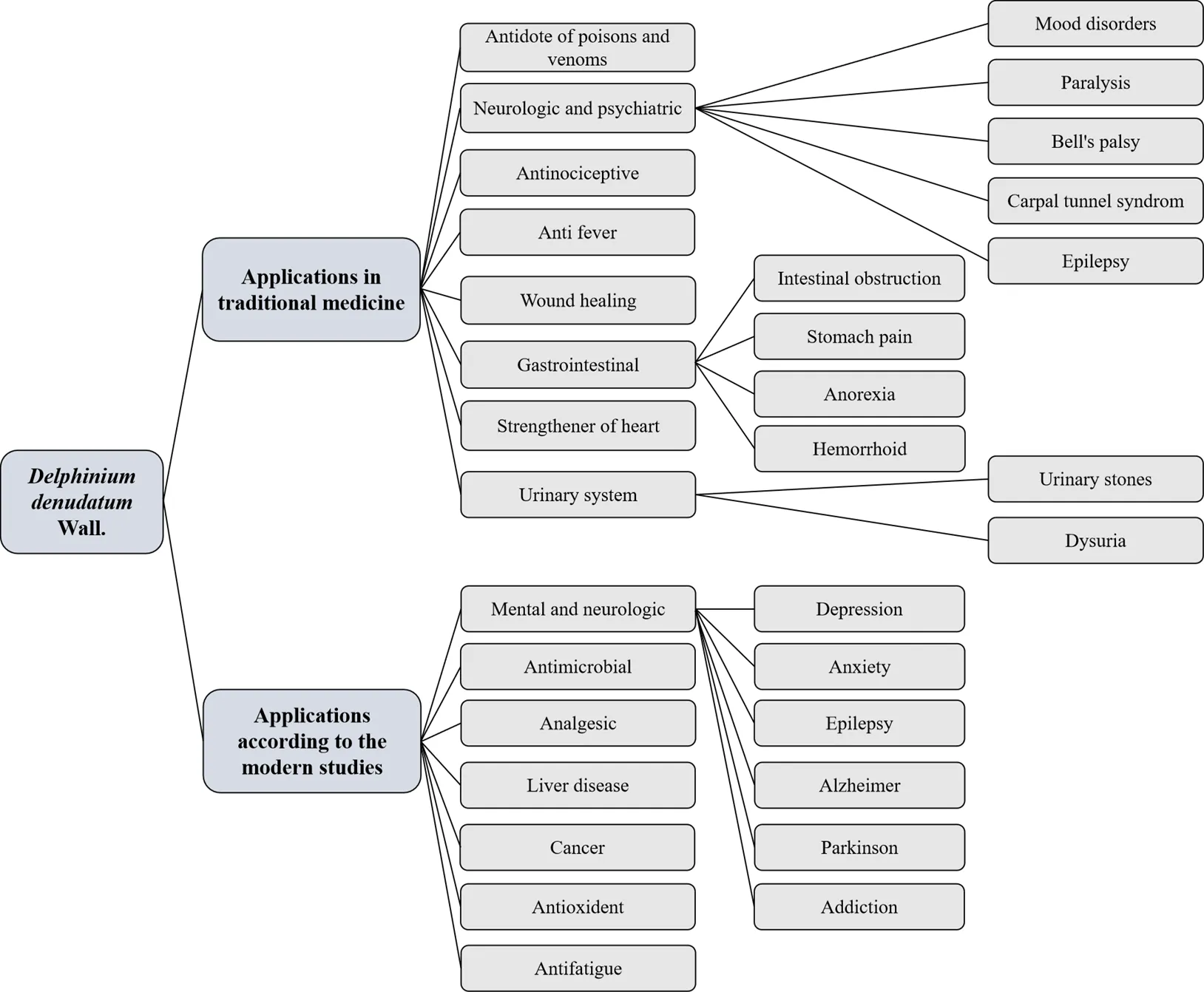
Figure 2 Summery of medicinal applications ofWall.
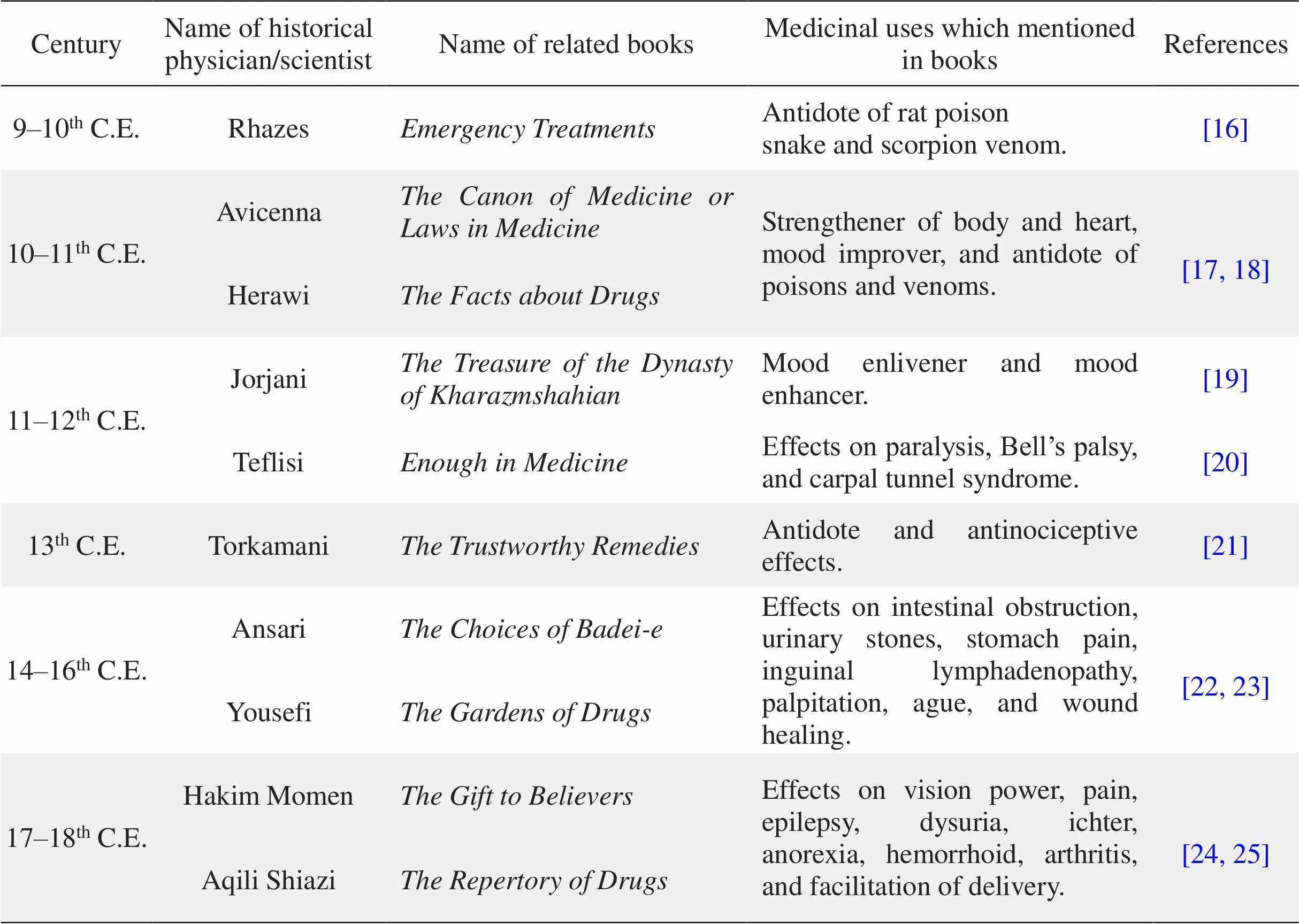
Table 1 The medicinal uses of Delphinium denudatum Wall. in historical written documents and books
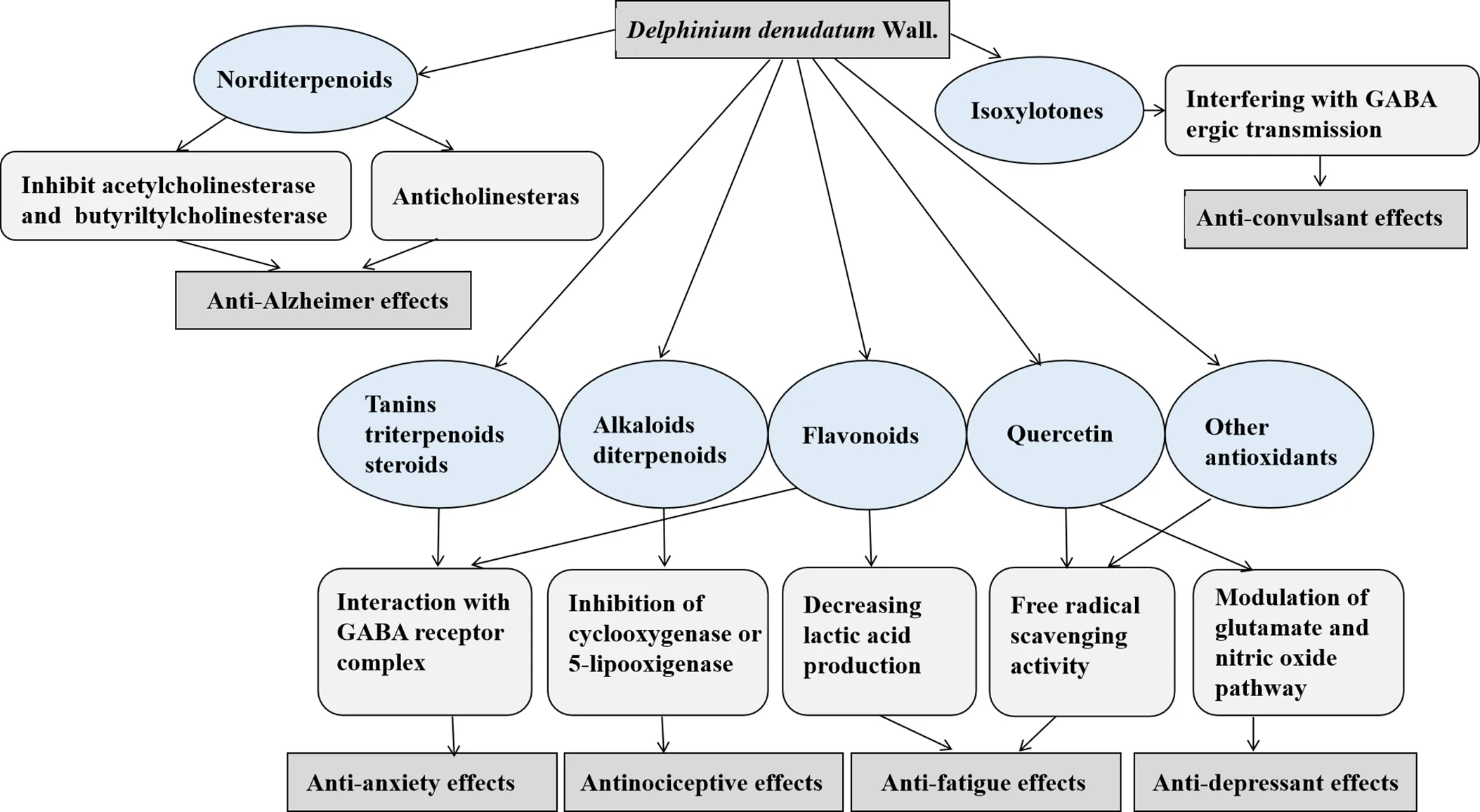
Figure 3 The main mechanisms behind theWall. pharmacological effects
Therapeutic effects
Anti-microbial effects
Wall. is used to treat intestinal worms and fluid retention [41]. The ethanol extracts of the roots ofWall. have shown antibacterial and antifungal activities. Most natural alkaloids and their derivatives are used as basic medicinal agents for their antibacterial properties [42]. Newly identified diterpenoid alkaloids of the roots ofWall., including 8-acetylheterophyllisine, alkaloids vilmorrianone, and panicutinehave have shown antifungal activity against a number of human pathogenic fungi [43]. The application of green synthesis of silver nanoparticles with aqueous root extract ofWall.has shown antibacterial effects againstaureus,coli,cereus, andaeruginosa in addition to anti-mosquito activity by effective larvicidal action against second instar larvae of dengue vector aedes aegypti [44]. For the biosynthesis of silver nanoparticles with aqueous root extract ofWall., 1.5 mL of plant extract was mixed with 30 mL of 1 mM aqueous silver nitrate solution and incubated for 2 h. The bio-reduction of the Ag+ions from silver nitrate solution was monitored periodically by measuring the UV-Vis spectroscopy. The silver nanoparticles acquired from the solution were purified by repeated centrifugation and finally, water-suspended nanoparticles were lyophilized [34].
Protective effects on liver
In recent times, there has been increasing interest in confirming the therapeutic potentials of plant derived molecules for liver disorders [45]. Co-administration of ethanolic root extract ofWall. (low, medium, and high doses (100, 200, 400 mg/kg)) leads to significant amelioration of alcohol-induced changes such as changes in aspartate transaminase, alanine transferase, and alkaline phosphatase, total bilirubin, and cholesterol in hepatotoxic rats [46]. Ethanolic root extract ofWall. prevents alcohol-mediated lipid peroxidation. In the above research, a 400 mg/kgWall. dose gave optimal results, similar to that of silymarin.Wall. treatment restored the changed parameters in a dose-dependent manner (36–100%). These results suggest thatWall. has potential as a source of treatment for alcohol-induced liver dysfunction [46].
Anti-fatigue effects
Traditional medicinal therapies can be used for treating symptoms of fatigue [47]. Different active contents from plants, including alkaloids, some proteins, anthraquinones, terpenes, saponin, polyphenols, and unsaturated fatty acid monomer compounds, have anti-fatigue properties [48]. Flavonoids and fatty acids are important components ofWall. that have anti-fatigue properties. However, other kinds of alkaloids have been found inWall., and it is important to examine their probable anti-fatigue properties as well [49].Wall. root powder can significantly ameliorate the physical, mental, and emotional perception of individuals, thereby relieving both mental and physical fatigue [50]. The saturated (CH3(CH2)nCOOH) and also ω-3 and ω-6 polyunsaturated fatty acids (CH3(CH2)nCH=CH(CH2)nCOOH), which have been identified in roots ofWall., may possibly be involved in its anti-fatigue properties [50, 51]. It has been demonstrated that using quercetin supplementation for seven days was associated with a modest increase in VO2maxand endurance capacity [52]. These advantages of quercetin can have significant implications for the improvement of athletic and military performance.
Antioxidant effects
The ethanolic extract ofWall. contains the free radical α-diphenyl-β-picrylhydrazyl (DPPH) and superoxide-scavenging inhibitors acting as primary antioxidants, whereas ethyl acetate had hydroxyl radical scavenger as a primary antioxidants [53]. The β-sitosterol from the roots ofWall. is one of the phytosterols with a chemical structure identical to that of cholesterol and has the ability to scavenge the radicals generated by DPPH method [54]. This suggests that β-sitosterol has potential antioxidant properties. Furthermore, other reports have shown the significant free radical scavenging capacity of β-sitosterol [55]. Moreover, the chloroform extract of the root part ofWall. showed the highest DPPH scavenging activity (86.56%), which was higher than the standard ascorbic acid (82.77%) at a similar dose [56]. Also, flavonoids act as antioxidants and free radical scavengers, which inhibit oxidative cell damage [53]. Therefore, various extracts fromWall. are prevent oxidation, have anti-carcinogenic and anti-atherogenic properties, and can have the potential for the inhibition and treatment of human cancer [57].
Anti-cancer effects
Cancer is the most important public health problem in both developed and developing countries [58]. Forty-four extracts from 16 plants used traditionally as anticancer agents were assessed in vitro for their antiproliferative activity against human epithelial type 2, MCF-7, and vero cell lines [59].Wall., with different extracts that have antioxidants effects, could act as an anti-tumor agent. Currently, the number of patients leaning towards the use of such herbal medicines is increasing in cancer patients. The chloroform extract of the root part ofWall. and chloroform extract of the aerial part as well as the methanolic extracts of the root part ofWall. have shown selective cytotoxicity against leukemia, ovarian cancer cell line, and breast cancer cell line, with an inhibition percentage of 50–100% [56]. Moreover, the novel sulfated derivative of quercetin (as a flavonoid content ofWall.) has strong antitumor activity exerted through a reactive oxygen species-dependent apoptosis pathway, and can potentially be developed into an antitumor precursor compound [60].
Neurologic and psychiatric effects
In traditional Persian medicine, a number of herbal medicines have been used for different neurological disorders due to their neuroprotective potentials.
Antinociceptive effects. Many traditional medicines have been used for pain relief. Herbal medicines have anti-inflammatory and also analgesic effects on patients with arthritis [61]. The acute oral toxicity of the aqueous root extract ofWall. was determined in Swiss albino mice. Doses of up to 14,000 mg/kg did not induce death but caused abnormal physical behavior for 6 h along with CNS depression [62]. At the four dose levels used (200–1,600 mg/kg),Wall. extract confirmed its dose-dependent antinociceptive effect which was measured by tail-flick latency and acetic acid induced writhing in the thermal and chemical models of analgesia. There is no scientific report available from the toxicological and antinociceptive perspective about this observation [62]. Administration of ethanolic extract ofWall. in low dose (300 mg/kg) and high dose (600 mg/kg), and the low dose (200 mg/kg) and high dose (400 mg/kg) of methanol fraction exhibited statistically significant enhancements in reaction time and pain threshold in both eddy’s hot plate and the tail flick test in Wistar albino rats [63]. Alkaloids have analgesic properties. It was shown that diterpenoid alkaloids selectively interact with nicotinic acetylcholine receptors in the CNS [64].
Anticonvulsant effects. Bioassay-guided isolation studies onWall. were conducted in several studies to isolate its anticonvulsant components [65]. The non-alkaloidal aqueous extract of the plant was further exposed to vacuum liquid chromatography, which provided partially purified oily subfraction by elution with acetone [65]. The ethanolic extract (EE) and aqueous fraction (AF) were used to evaluate the effectiveness ofWall., with EE doses of 200, 400, 600 mg/kg and the AF doses of 400, 600, 800 mg/kg in male CF-1 mice [66]. The aqueous fraction greatly reduced the duration of the hind limb tonic extension of the maximal electroshock test in a dose-dependent manner (at the doses of 600 and 800 mg/kg) and had stronger anticonvulsant activity against seizures. However, EE had weak dose-dependent anticonvulsant effects on seizures (at a dose of 600 mg/kg) [66]. The results of one study showed that the partially purified oily subfraction ofWall. (0.06 mg/mL) inhibits sustained repetitive firing (SRF) in hippocampal neurons in a use-dependent and voltage-dependent manner in Sprague Dawley rat pups [67]. At 0.6 mg/mL, the AF exhibited anticonvulsant activity similar to that of phenytoin at the cellular level in blocking SRF in Sprague Dawley rat pups [68].
There is a new class of anti-seizure compounds isolated inWall. that prevent sodium channel function and inhibits the development of epileptic seizures. Isoxylitone, a molecule discovered inWall., is a strong inhibitor of voltage-gated sodium channels, which was shown to have anticonvulsant properties, stopped stage five seizures, and reduced the brain-derived neurotrophic factor mRNA expression in experimental epilepsy using the kindling model [69, 70]. Based on the above research, it may be implied that the root extract ofWall. shows significant antiepileptic activity.
In recent times, nanotechnology in drug formulation is being increasingly used and its advantages, such as enhancing compound solubility, decreasing medicinal doses, and increasing the absorbency of herbal medicines, have been appreciated [71]. Water-soluble phytoconstituent molecules, such as flavonoids, are poorly absorbed because of their poor miscibility with oils and other lipids, or due to their multiple-ring large-sized molecules [22]. The binding constituents ofWall. AF towards phosphatidylcholine, followed by nanosizing, results in the preparation ofWall. nanophytosome (DNP) [72]. This particle size of the complex (around 500 nm) increase compound solubility and improves the absorbency of herbal medicines [73]. DNP also exhibits anxiolytic and antidepressant effects in a dose-dependent manner [72]. DNP (400 and 800 mg/kg) has better bioavailability and has better access to the CNS because it can easily cross the blood brain barrier. It significantly altered the seizure latency time in comparison with the free aqueous fraction in Swiss albino mice [74].
Effects on Alzheimer and Parkinson diseases. In one study, norditerpenoids alkaloids including 1β-hydroxy, 14β-acetyl condelphine, jadwarine-A, and jadwarine-B together with alkaloids including isotalatizidine hydrate and dihydropentagynine were isolated fromWall. [75]. Jadwarine-A, alkaloids isotalatizidine hydrate and dihydropentagynine displayed competitive inhibitory effects through inhibiting acetylcholinesterase and butyrylcholinesterase, respectively [75, 76]. Isotalatizidine hydrate has anticholinesterase properties in comparison with standard drugs being used, such as allanzanthane A and galanthamine, and can be used as a target drug for treating Alzheimer’s disease [77].
Parkinson’s disease is a relatively common age-dependent neurodegenerative disease [78]. Herbal medicine could possibly improve the symptoms of Parkinson’s disease and they are considered to be safe and well-tolerated [79]. The ethanolic extract treatment ofWall. for three weeks in a rat model of Parkinsonism considerably weakened the activities of superoxide dismutase and catalase in striatum, which were significantly decreased by lesioning [80]. Additionally, an increase in the expression of tyrosine hydroxylase in the ipsilateral striatum following the ethanolic extract treatment withWall. was exhibited [80].
Effects on opium addiction. Morphine addiction is the worst socio-economic global problem [81]. Oral administration of the aqueous root extract ofWall. (200–1,600 mg/kg) exhibited a significant dose-dependent inhibition of naloxone-induced (1 mg/kg) withdrawal on morphine-induced tolerance in mice [82].Wall. methanolic extract (especially 700 mg/kg) reduced symptoms resulting from morphine withdrawal in rats [83]. The use of ethanolic root extract ofWall. from a single dose (600 mg/kg) and methanolic root fraction ofWall. from a single dose (400 mg/kg) significantly decreased the mean scores of several “counted signs” such as chewing, headshakes, and yawning, and “checked signs” such as screaming after touching, which are symptoms of morphine withdrawal syndrome in Wistar albino rats. Therefore, it may be an alternative medicine in for treating symptoms of withdrawal in recovering morphine addicts [81]. The α7 nicotinic acetylcholine receptor blockade byWall. may explain the reductions of scores in different counted and checked signs, which were demonstrated during morphine withdrawal [84, 85].
Antidepressant effects.Wall. has beneficial properties against several neurological disorders. Quercetin ofWall. has promising effects on anxiety and depression [50]. The aqueous extract (200–1,600 mg/kg, orally) ofWall. has shown significant antidepressant effects depressive paradigms in mice [86]. The antidepressant effect of quercetin could potentially be influenced by the inhibition of the N-Methyl-d-aspartic acid receptors and/or the synthesis of nitric oxide through the modulation of the glutamate and nitric oxide pathway. It has also been confirmed that the administration of quercetin decreases the levels of cyclic guanosine monophosphate. Furthermore, the antioxidant effects of quercetin are also involved in its anti-depressive properties, with regard to the decrease of lipid hydroperoxide levels in the hippocampus [87].
Anxiolytic effects. Anxiety is an ambiguous, nervous feeling of discomfort or dread that is accompanied by an autonomic (self-controlling) response [88]. Psychotropic medicines are usually prescribed for anxiety; however, they have major side effects.Wall. can be used in the management of anxiety because it can mitigate the effects of psychiatric disorders. TheWall. has antispasmodic effects on rats and has wide-ranging anti-stress properties exerted on different stressors [89]. Results of a new study showed that the hydroalcoholic root extract ofWall. provided anxiolytic effects in Wistar albino rats at doses of 200 and 400 mg/kg [89]. The root extract ofWall. significantly reduced the number of rearing and number of steps climbed. It also decreased spontaneous locomotor activity in Wistar rats [90]. These properties may be due to the interaction of flavonoids, alkaloids, tannins, triterpenoids, and steroids of the plant with the gamma-aminobutyric acid/benzodiazepine receptor complex in the brain. Nevertheless, flavonoids have proven anxiolytic activities [90].
Conclusion
Traditional herbal medicines used by local populations play a significant role in treating different illnesses. It appears that determining the efficacy of traditional medicine therapies concerning for a variety of diseases needs to be further studied and researched.Wall., found on the outer ranges of Western Himalayas, is one of the most important drugs used in Indian traditional medicine. It was shown that different root extracts ofWall. possess antioxidant, anticonvulsant, anti-fatigue, anti-microbial, and anxiolytic activities and could have a promising role in the treatment of various diseases, including mental disorders such as depression, anxiety, insomnia, and stress, neurological disorders, and inflammatory diseases. Our recommendations for prospective of studies onWall. are to consider investigating the mechanisms underlying the medical efficacy ofWall. and to assess the efficacy ofWall. for treating various illnesses.
1. World Health Organization (WHO). WHO traditional medicine strategy: 2014–2023. Geneva Switzerland Who 2013: 76.
2. De Luigi AJ. Complementary and alternative medicine in osteoarthritis. PM&R 2012, 4: S122–S133.
3. Govindu S, Adikay S. Evaluation of antiepileptic activity of chloroform extract ofin mice. Pharmacognosy Res 2014, 6: 108–112.
4. Farzaei MH, Farzaei F, Abdollahi M, et al. A mechanistic review on medicinal plants used for rheumatoid arthritis in traditional Persian medicine. J Pharm Pharmacol 2016, 68: 1233–1248.
5. Bahramsoltani R, Farzaei MH, Farahani MS, et al. Phytochemical constituents as future antidepressants: a comprehensive review. Rev Neurosci 2015, 26: 699–719.
6. Kumar S, Kumar R, Khan A. Medicinal plant resources: manifestation and prospects of life-sustaining healthcare system. Cont J Biol Sci 2011, 4: 19–29.
7. Ghafouri RR, Araj-Khodaei M, Targhi ST, et al, First report of a disease by Rhazes 10 centuries ago. Int J Prev Med 2019, 10: 6.
8. Kumar S, Chand G, Sankhyan P. Herbal folk remedies for curing various ailments in Lug Valley of district Kullu, Himachal Pradesh (NW Himalaya). Int J Ayurvedic Herb Med 2013, 3: 1308–1314.
9. Karimi AB, Elmi A, Zargaran A, et al. Clinical effects of date palm (L.): a systematic review on clinical trials. Complement Ther Med 2020, 51: 102429.
10. Jazani AM, Hamdi K, Tansaz M, et al. Herbal medicine for oligomenorrhea and amenorrhea: a systematic review of ancient and conventional medicine. Biomed Res Int 2018, 2018: 3052768.
11. Khodaei MA, Noorbala AA, Parsian Z, et al. Avicenna (980–1032 C.E.): The pioneer in treatment of depression. Transylvanian Rev 2017.
12. Karimi AB, Elmi A, Mirghafourvand M, et al. Effects of date fruit (L.) on labor and delivery outcomes: a systematic review and meta-analysis. BMC Pregnancy Childbirth 2020, 20: 210.
13. Azgomi RND, Zomorrodi A, Nazemyieh H, et al. Effects ofon reproductive system: a systematic review of the available evidence. Biomed Res Int 2018, 2018: 4076430.
14. Rahman S, Khan RA, Kumar A. Experimental study of the morphine de-addiction properties ofWall. BMC Complement Altern Med 2002, 2: 6.
15. Briggs JP, Longo DL, Fauci AS, et al. Complementary, alternative, and integrative health practices. Harrison’s Princ InternMed, 2015.
16. Razi M. Emergency Treatments. Iran: Tehran University of Medical Sciences, 2005: 61–71.
17. Ibn-e-Sina AAH. The canon of medicine. Beirut: Dare Ehyae al-Torathe al-Arabi, 2005: 395.
18. Harawi M. The facts about drugs. Iran: Tehran University, 1967: 178.
19. Jorjani E. The treasure of the Dynasty of Kharazmshahian. Iran: Ehya Teb E-tabiei Institute, 2012: 198.
20. Teflisi A. Enough in medicine. Iran: Iran University of Medical Sciences, 2008: 293.
21. Torkamani MM. The trustworthy remedies. Lebanon: Dar al-Ketab al-Elmiye, 2000: 52.
22. Ansari A. The choices of Badei-e. Iran: Sherkate Daru Pakhsh Razi, 1992: 93.
23. Yousefi HY. The Gardens of drugs. Iran: Almaei Pulications, 2012: 66.
24. Hakim Momen M. The gift to believers. Iran: Noor-e-Wahi Publication, 2011: 261.
25. Aghili Shirazi MH. The repertory of drugs. Iran: Tehran University of Medical Sciences, 2011: 284.
26. Jabbour F, Renner SS. A phylogeny of(Ranunculaceae) shows thatis nested withinand that Late Miocene transitions to long life cycles in the Himalayas and Southwest China coincide with bursts in diversification. Mol Phylogenet Evol 2012, 62: 928–942.
27. Sharifnia F, Hasanbarani M, Assadi M. Notes on some species of the genus(Ranunculaceae) in Iran. Iranian J Bot 2013, 19: 202–210.
28. Agnihotri P, Jena SN, Husain D, et al. Perspective of the genusLinnaeus (Ranunculaceae) in India. Pleione 2014, 8, 344–352.
29. Nayab M, Ansari AN, Anwar M. An overview of cardio protective drugs in the light of Advia-e-Qalbia. Int J Unani Integr Med 2017, 1: 12–16.
30. Shaheen F, Ahmad M, Rizvi TS, et al. Norditerpenoid alkaloids fromkohatense Munz. Rec Nat Prod 2015, 9: 76–80.
31. Nizami, Qudsia, Jafri MA. Unani drug, Jadwar (Wall.): a review. Indian J Tradit Knowl 2006, 5: 463-467.
32. Alam MA, Ahmed Z, Ansari AH, et al. Iksir-e-Badan (Elixir): unique influence from Unani medicine: a review. Int J Herb Med 2014, 1: 37–42.
33. Gupta AK, Khan M, Khan D. Physicochemical study ofWall (Ranunculales: Ranunculaceae) and their antioxidant activity. Brazilian J Biol Sci 2019, 6: 161–169.
34. Siddique NA, Mujeeb M. Determination of heavy metal in medicinal plants by atomic absorption spectroscopy (AAS). Inter J Phytother Res 2013, 4, 21–26.
35. Sathish Kumar T, Rajakararan P, Shanmugam S, et al. Phytochemical constituents and antibacterial activities ofRoxb. andLinn. leaves. Adv Biotechnol 2007: 23–25.
36. Amal B, Najlae M, Ouarda A, et al. Immunomodulating properties of protein fractions isolated fromstaphysagria seeds. J Med Plants Res 2016, 10: 29–34.
37. Borcsa B, Widowitz U, Csupor D, et al. Semisynthesis and pharmacological investigation of lipo-alkaloids prepared from aconitine. Fitoterapia 2011, 82: 365–368.
38. Song L, Liu XY, Chen QH, et al. New C(19)-and C(18)-Diterpenoid Alkaloids fromvar. savatieri. Chem Pharm Bull 2009, 57: 158–161.
39. Rawat VS, Jalal JS. Sustainable utilization of medicinal plants by local community of Uttarkashi district of Garhwal, Himalaya, India. Eur J Med Plants 2011, 1: 18–25.
40. Wang WY, Sunn CX, Mao LK, et al. The biological activities, chemical stability, metabolism and delivery systems of quercetin: a review. Trends Food Sci Technol 2016, 56: 21–38.
41. Rasool S, Khan MH, Hamid S, et al. An overview and economical importance of few selected endangered medicinal plants grown in Jammu and Kashmir region of India. Phytomed 2016, 5: 27–37.
42. Kumar U, Kumar B, Bhandari A, et al. Phytochemical investigation and comparison of antimicrobial screening of clove and cardamom. Int J Pharm Sci Res 2010, 1: 138–147.
43. Atta-ur-Rahman, Nasreen A, Akhtar F, et al. Antifungal diterpenoid alkaloids from. J Nat Prod 1997, 60: 472–474.
44. Suresh G, Gunasekar PH, Kokila D, et al. Green synthesis of silver nanoparticles usingroot extract exhibits antibacterial and mosquito larvicidal activities. Spectrochim Acta A Mol Biomol Spectrosc 2014, 127: 61–66.
45. Vankadari RMG, Hussain MA, Venkat RN, et al. Protective effect of SR-101, a poly herbal formulation against alcohol induced hepatotoxic rats. Adv Pharmacol Toxicol 2011, 12: 89.
46. Hussain MA, Suresh DK, Gupta RMV, et al. Hepatoprotective activity of ethanolic extract delphinium root, against alcohol induced hepatotoxic rats. Adv Pharmacol Toxicol 2012, 13: 75.
47. Wang YY, Li XX, Liu JP, et al. Traditional Chinese medicine for chronic fatigue syndrome: a systematic review of randomized clinical trials. Complement Ther Med 2014, 22: 826–833.
48. Zhou SS, Jiang JG. Anti-fatigue effects of active ingredients from traditional Chinese medicine: a review. Curr Med Chem 2019, 26: 1833–1848.
49. D'Andrea G. Quercetin: a flavonol with multifaceted therapeutic applications? Fitoterapia 2015, 106: 256–271.
50. Daneshfard B, Yekta NH, Khoshdel A, et al. The effect of(Jadwar) on fatigue: a randomized double blind placebo-controlled clinical trial. Complement Ther Med 2019, 46: 29–35.
51. Alfano CM, Imayama I, Neuhouser ML, et al. Fatigue, inflammation, and ω-3 and ω-6 fatty acid intake among breast cancer survivors. J Clin Oncol 2012, 30: 1280–1287.
52. Davis JM, Carlstedt CJ, Chen S, et al. The dietary flavonoid quercetin increases VO(2max)and endurance capacity. Int J Sport Nutr Exerc Metab 2010, 20: 56–62.
53. Mohanapriya S, Siva GV. Phytochemical analysis and antioxidant potential ofwall. J Modern Biotechnol 2013, 2: 53–58.
54. Kamboj A, Saluja AK. Isolation of stigmasterol and β-sitosterol from petroleum ether extract of aerial parts of(Asteraceae). Int J Pharm Pharm Sci 2011, 3: 94–96.
55. Patra A, Jha S, Murthy PN, et al. Isolation and characterization of stigmast-5-en-3β-ol (β-sitosterol) from the leaves ofT. Anders. Int J Pharma Sci Res 2010, 1: 544–549.
56. Bhat HMU. Chemoprofiling of delphinium denudatum Jadwar and achillea millefolium Biranjasif and their unani formulations. Jiwaji University, 2014.
57. Yoshida Y, Niki E. Antioxidant effects of phytosterol and its components. J Nutr Sci Vitaminol 2003, 49: 277–280.
58. Ghazizadeh J, Hamedeyazdan S, Torbati M, et al.L. hydro-alcoholic extract inhibits anxiety and depression through prevention of central oxidative stress and apoptosis. Exp Physiol 2020, 105: 707–720.
59. Talib WH, Mahasneh AM. Antiproliferative activity of plant extracts used against cancer in traditional medicine. Sci Pharm 2010, 78: 33–45.
60. Zhang HS, Zhang M, Yu LH, et al. Antitumor activities of quercetin and quercetin-5′, 8-disulfonate in human colon and breast cancer cell lines. Food Chem Toxicol 2012, 50: 1589–1599.
61. Fotouhi A, Maleki A, Dolati S, et al. Platelet rich plasma, stromal vascular fraction and autologous conditioned serum in treatment of knee osteoarthritis. Biomed Pharmacother 2018, 104: 652–660.
62. Zafar S, Ahmad MA, Siddiqui TA. Acute toxicity and antinociceptive properties of. Pharm Biol 2003, 41: 542–545.
63. Zaheer I, Rahman SZ, Khan RA. Evaluation of analgesic activity of extracts ofin animal models: a dose dependent pre-clinical trial. J Clin Diagn Res 2018, 12.
64. Alves de Almeida AC, Meira de-Faria F, Dunder RJ, et al. Recent trends in pharmacological activity of alkaloids in animal colitis: potential use for inflammatory bowel disease. Evid Based Complement Alternat Med 2017, 2017: 8528210.
65. Rahman, A., Choudhary MI, Shaheen F, et al. Anticonvulsant compounds. US: US7399888 B2 2008.
66. Raza M, Shaheen F, Choudhary MI, et al. Anticonvulsant activities of ethanolic extract and aqueous fraction isolated from. J Ethnopharmacol 2001, 78: 73–78.
67. Raza M, Shaheen F, Choudhary MI, et al. Anticonvulsant effect of FS-1 subfraction isolated from roots ofon hippocampal pyramidal neurons. Phytother Res 2003, 17: 38–43.
68. Raza M, Shaheen F, Choudhary MI, et al. Inhibition of sustained repetitive firing in cultured hippocampal neurons by an aqueous fraction isolated from. J Ethnopharmacol 2004, 90: 367–374.
69. Ashraf MN, Gavrilovici C, Ali Shah SU, et al. A novel anticonvulsant modulates voltage-gated sodium channel inactivation and prevents kindling-induced seizures. J Neurochem 2013, 126: 651–661.
70. Simjee SU, Shaheen F, Choudhary MI, et al. Suppression of c-Fos protein and mRNA expression in pentylenetetrazole-induced kindled mouse brain by isoxylitones. J Mol Neurosci 2012, 47: 559–570.
71. Zha RT, He XT, Du T, et al. Synthesis and characterization of chitosan nanoparticles modified by glycyrrhetinic acid as a liver targeting drug carrier. Chem J Chin Univ 2007: 28: 1098–1100.
72. Abbas Zaidi S. Enhanced neurobehavioral effects of Jadwar () aqueous fraction by implying nanotechnology based approach. Archiv Neurol Neurosurg 2016, 101: 2–6.
73. Li HF, Liu MX, Liu QF, et al. Preparation of pharmacokinetics of breviscapine-loaded poly (D, L-lactic acid) nanoparticles. Chin J New Drugs 2007, 16: 614. (Chinese)
74. Musthaba SM, Ahmad S, Ahuja A, et al. Nano approaches to enhance pharmacokinetic and pharmacodynamic activity of plant origin drugs. Curr Nanosci 2010, 5: 344–352.
75. Ahmad H, Ahmad S, Ali M, et al. Norditerpenoid alkaloids ofas cholinesterase inhibitors. Bioorg Chem 2018, 78: 427–435.
76. Taheri-Targhi S, Gjedde A, Araj-Khodaei M, et al. Avicenna (980–1037 C.E.) and his early description and classification of dementia. J Alzheimers Dis 2019, 71: 1093–1098.
77. Ahmad H, Ahmad S, Khan E, et al. Isolation, crystal structure determination and cholinesterase inhibitory potential of isotalatizidine hydrate from. Pharm Biol 2017, 55: 680–686.
78. Cookson MR. Parkinson’s disease, in disease-modifying targets in neurodegenerative disorders. Elsevier 2017: 157–174.
79. Wang Y, Xie CL, Lu L, et al. Chinese herbal medicine paratherapy for Parkinson’s disease: a meta-analysis of 19 randomized controlled trials. Evid Based Complement Alternat Med 2012, 2012: 534861.
80. Ahmad M, Yousuf S, Khan MB, et al. Protective effects of ethanolic extract ofin a rat model of Parkinson’s disease. Hum Exp Toxicol 2006, 25: 361–368.
81. Zaheer I, Rahman SZ, Khan RA, et al. An experimental study of ethanolic extract and methanolic fraction ofWall in morphine withdrawal syndrome. J Med Res 2016, 2: 71–76.
82. Zafar S, Ahmad MA, Siddiqui TA. Effect of roots aqueous extract ofon morphine-induced tolerance in mice. Fitoterapia 2002, 73: 553–556.
83. Ebrahimie M, Bahmani M, Shirzad H, et al. A review study on the effect of Iranian herbal medicines on opioid withdrawal syndrome. J Evid Based Complement Altern Med 2015, 20: 302–309.
84. Feng B, Xing JH, Jia D, et al. Blocking α4β2 and α7 nicotinic acetylcholine receptors inhibits the reinstatement of morphine-induced CPP by drug priming in mice. Behav Brain Res 2011, 220: 100–105.
85. Rahman SZ, Khan RA, Kumar A. Preclinical study ofwall. in morphine de-addiction. J Pharmacol Toxicol Methods 2007, 56: e4.
86. Zafar S, Ahmad MA, Siddiqui TA. Evaluation of the central depressant activity of Jadwar (Wall) in mice. Indian J Tradit Knowl 2002, 1: 59–64.
87. Holzmann I, Mota da Silva L, Corrêa da Silva JA, et al. Antidepressant-like effect of quercetin in bulbectomized mice and involvement of the antioxidant defenses, and the glutamatergic and oxidonitrergic pathways. Pharmacol Biochem Behav 2015, 136: 55–63.
88. Vanin JR. Overview of anxiety and the anxiety disorders. Humana Press 2008, 1–18.
89. Yasmeen S, Sadia N, Arun M, et al. Role of Unani neuroprotective herbal drugs in the management of autism. Inter J Res Rev 2019.
90. Abid M, Gosh AK, Khan NA. In vivo psychopharmacological investigation ofandspinosus extracts on Wistar rats. Basic Clin Neurosci 2017, 8: 503–512.
:
Roghayeh Baghervand Navid and Alireza Bagherzadeh karimi wrote the manuscript and edited the final version of the manuscript; Morteza Ghojazadeh and Seyed Mostafa Araj-Khodaeidesigned and wrote some parts of manuscript; Sanam Dolati and Mehri Bansans drew figures and submitted the paper; Mehrdad Karimi and Seyed Mohammad Bagher Fazljou supervised the study and correspondence during the paper submission.
:
Authors would like to acknowledge Department of Persian Medicine, School of Traditional Medicine, Tabriz University of Medical Sciences, Tabriz, Iran for their great help.
:
CNS, central nervous system; DPPH, α-diphenyl-β-picrylhydrazyl; EE, ethanolic extract; AF, aqueous fraction; SRF, sustained repetitive firing; DNP,nanophytosome.
The authors declare that there is no conflict of interest.
:
Roghayeh Baghervand Navid, Mehrdad Karimi, Morteza Ghojazadeh, et al. Jadwar (Wall): a medicinal plant. Traditional Medicine Research 2020, 5 (6): 487–497.
:Jing-Na Zhou.
: 10 July 2020,
30 August 2020,
:10 October 2020
Mehrdad Karimi. Department of Iranian Traditional Medicine, School of Traditional Medicine, Tehran University of Medical Sciences, Building of the Ahmadiye, No. 27, Corner Alley Tabriz, Sarparast Shomali Street, Taleghani Ave, Tehran 1416663361, Iran. E-mail: mehrdadkarimi@yahoo.com; Seyed Mohammad Bagher Fazljou. Department of Traditional Medicine, Tabriz University of Medical Sciences, Daneshgah Street, Golgasht Ave, Tabriz 5165665931, Iran. E-mail: fazljou.mohammadbagher@gmail.com.
10.12032/TMR20200910198
 Traditional Medicine Research2020年6期
Traditional Medicine Research2020年6期
- Traditional Medicine Research的其它文章
- Green coffee bean hydroalcoholic extract accelerates wound healing in full-thickness wounds in rabbits
- Gastrointestinal effects of Artemisia absinthium Linn. based on traditional Persian medicine and new studies
- Evaluation of scientific evidence for abortifacient medicinal plants mentioned in traditional Persian medicine
- Effects of chicory (Cichorium intybus L.) on nonalcoholic fatty liver disease
- Is “Pangolin (Manis Squama) is not used in medicine" an improvement in the protection of precious and rare species or an improvement in the safety of using medicine?
- The gap between clinical practice and limited evidence of traditional Chinese medicine for COVID-19
