The gap between clinical practice and limited evidence of traditional Chinese medicine for COVID-19
Hui Luo
The gap between clinical practice and limited evidence of traditional Chinese medicine for COVID-19
Hui Luo1*
1Institute for Tibetan Medicine of China Tibetology Research Center, Beijing 100101, China.
Background
Traditional Chinese medicine (TCM) is the set of knowledge and practices concerning life, health, illness prevention, and treatment that originated in China thousands of years ago. Presently, TCM is still one of the mainstream medical systems, and has been given an equal legal status and place as Western medicine in the healthcare system of China [1]. Whether it was the plague in ancient times or the SARS and influenza A in the past decades, TCM has always played a major role in the prevention and treatment of diseases [2]. As early as the outbreak of COVID-19 in January 2020, the Chinese government established the treatment principle of integrated Chinese and Western medicine, and since then TCM has been fully and deeply involved in the management of the epidemic in China. Till August 18, 2020, the Chinese health authority had issued eight versions of guidelines for the diagnosis and treatment of COVID-19, and starting from the third version, a TCM approach including herbal formulae and drugs has been included. According to the White Paper issued by the Chinese government in June 2020, 92% of all confirmed cases received TCM-based treatment that proved to be effective [3].
Along with the extensive clinical application, a large number of clinical trials have been registered and carried out to evaluate the effectiveness and safety of TCM interventions. According to the data provided by Chinese Clinical Trial Registry, as of August 27, 2020, a total of 165 TCM clinical trials had been registered, accounting for 22.3% (165/741) of all trials on COVID-19 in Chinese Clinical Trial Registry. However, despite the widespread clinical use and so many clinical trial registrations, current high-quality evidence on TCM in the treatment of COVID-19 is still rare. In this comment, TCM clinical trials on COVID-19 were searched in Google Scholar, Web of Science, and PubMed on August 27, 2020. Studies published in journals indexed by Science Citation Index would be identified as influential research and selected for review.
Published clinical trials of TCM in treating COVID-19
Ten studies were obtained through searching and screening [4–13]. Only five studies were randomized controlled trials (RCTs), and one of them was a multicenter study; the rest were either retrospective studies or case reports [4–13]. The sample sizes of the ten studies ranged from 4 to 721, with a median of 51. These studies were heterogeneous in terms of TCM interventions, which included various Chinese patent medicines, decoctions, and comprehensive treatment based on guidelines. Only the Lianhua Qingwen capsule (approval number of CFDA: Z20040063) was evaluated in two RCTs [7, 12]. Guideline-based routine treatment of Western medicine was applied as the basic treatment in both the TCM treatment groups and the control groups of all studies. Placebo control and blinding of participants and personnel were not implemented in any of the studies. One RCT adopted blinding of outcome assessment [10]. In the five RCTs, the details of random sequence generation were reported, while allocation concealment was neither reported nor adopted. The endpoints for outcome measures included symptoms, virus removal, fatality rate, chest CT examinations, length of hospitalization, rate of progression to severe disease, usage of antibiotics and antiviral drugs, etc. All retrospective studies reported positive results, while two out of five RCTs reported negative results on the primary endpoints including the fatality rate of severe cases and symptom improvement of suspected and non-severe cases [8, 12]. The detailed characteristics of clinical trials of TCM in treating COVID-19 are shown in Table 1.
In general, several specific Chinese patent medicines or herbal formulae have been shown to improve symptoms, reduce progression to severe disease in mild and moderate COVID-19 patients. However, the majority of these drugs or formulae were evaluated in a single clinical trial, with a small sample size and moderate to high risk of bias (Figure 1). Therefore, the results should be interpreted with caution.
The gap between clinical practice and limited evidence
Despite the widespread application in clinical practice and recommendation from the national guideline, high-quality clinical research evidence for the treatment of COVID-19 with TCM is still lacking so far. There is limited quality and quantity of evidence from the review of existing studies. The gap between clinical practice and limited evidence could be mainly explained by the following three aspects:
Firstly, there was a paucity of newly-confirmed cases. The majority of trials were simultaneously registered and carried out during the outbreak, resulting in limited sample sizes. The outbreak of COVID-19 in China was brought under control in a short time, which implied that most trials could not attain the predetermined sample size [14]. Secondly, methodological drawbacks weakened the strength of evidence. Due to the small sample size, absence of placebo, absence of blinding, inappropriate outcome endpoints, retrospective design, and other methodological drawbacks, the quality of the current evidence is still limited and not so satisfactory [15]. Lastly, the particularity of TCM interventions inhibited the publication of the study in high-impact journals. Since the preparation of placebos of Chinese medicine, especially decoctions were difficult [16], and due to the urgency of the epidemic, all studies adopted an open-label design instead of placebo-control and blinding. Besides, the composition of TCM formulae was complex, and there was a lack of basic data on chemical composition and pharmacological research, making it difficult to be accepted outside China.
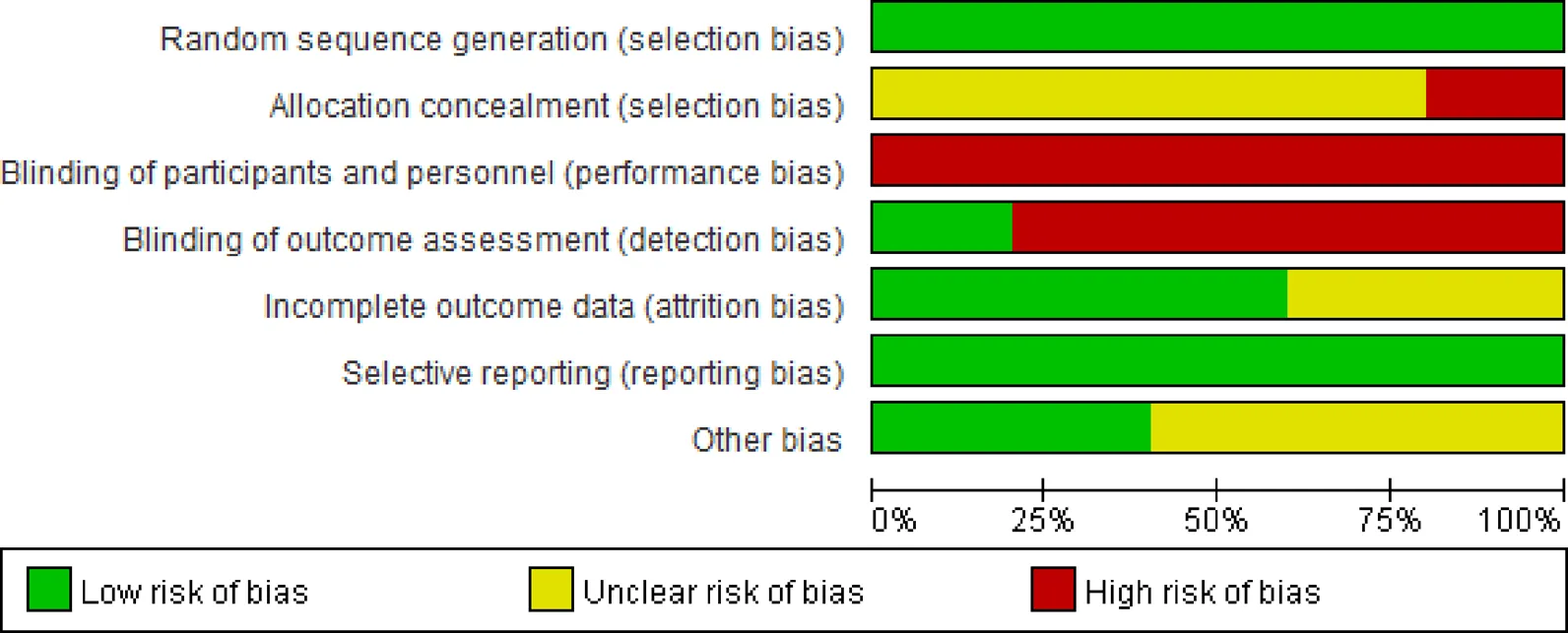
Figure 1 Graphical representation of the risk of bias of randomized controlled trials of traditional Chinese medicine in treating COVID-19
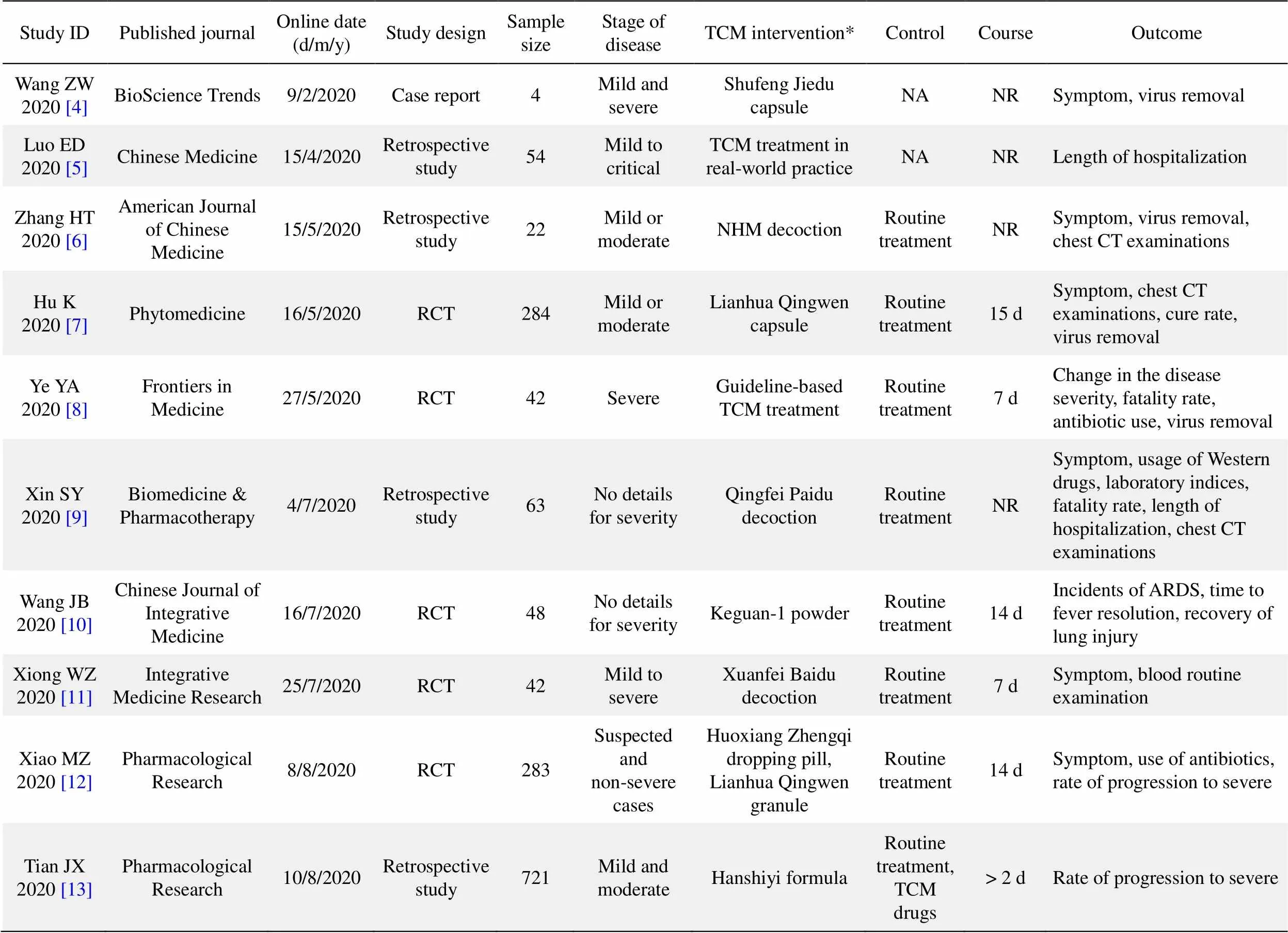
Table 1 The characteristics of clinical trials of traditional Chinese medicine in treating COVID-19
RCT, randomized controlled trial; TCM, traditional Chinese medicine; NA, not applicable; NR, not report; ARDS, acute respiratory distress syndrome.
*Details of TCM interventions:
Shufeng Jiedu capsule (Chinese patent medicine), approval number of China Food and Drug Administration (CFDA): Z20090047, ingredients:(Huzhang),(Lianqiao),(Banlangen),(Chaihu),(Baijiangcao),(Mabiancao),(Lugen),(Gancao).
TCM treatment in real-world practice (Experience formula), most frequently used herbs:(Fuling),(Yiyiren),(Zexie),(Xingren),(Baizhu),(Huangqin).
NHM decoction (Experience formula), ingredients:(Jinyinhua),(Biandou),(Fuling),(Shengdi),(Huangqin),(Shuiniujiao),(Pipaye),(Xuanshen),(Lianqiao).
Lianhua Qingwen capsule (Chinese patent medicine), approval number of CFDA: Z20040063, ingredients:(Lianqiao),(Jinyinhua),(Mahuang),(Kuxingren),(Shigao),(Banlangen),(Mianma guanzhong),(Yuxingcao),(Guanghuoxiang),(Dahuang),(Hongjingtian),(Bohenao),(Gancao).
Guideline-basedTCMtreatment,availableat:http://www.nhc.gov.cn/xcs/zhengcwj/202001/4294563ed35b43209b31739bd0785e67.shtml.
Qingfei Paidu decoction (Experience formula), ingredients:(Mahuang),(Zhigancao),(Xingren),sm(Shengshigao),(Guizhi),(Zexie),(Zhuling),(Baizhu),(Fuling),(Chaihu),(Huangqin),(Jiangbanxia),(Shengjiang),(Ziwan), Fararae(Kuandonghua),(Shegan),(Xixin),(Shanyao),(Zhishi),(Chenpi),(Huoxiang).
Keguan-1 powder (Experience formula), ingredients:(Jinyinhua),(Lianqiao),(Sangye),(Juhua),(Yiyiren),(Zhebeimu),(Kuxingren).
Xuanfei Baidu decoction (Experience formula), ingredients:(Mahuang),(Xingren),(Shengshigao),(Cangzhu),(Yiyiren),(Huoxiang),(Huzhang),(Tinglizi),(Mabiancao),(Lugen),(Qinghao),(Juhong),(Shenggancao).
Huoxiang Zhengqi dropping pill (Chinese patent medicine), approval number of CFDA: Z20000048, ingredients:(Cangzhu),(Chenpi),(Houpo),(Baizhi),(Fuling),(Dafupi),(Banxia),(Shenggancao),(Huoxiang),(Zisu).
Hanshiyi formula (Experience formula), ingredients:(Mahuang),(Shigao),(Xingren),(Qianghuo),(Tinglizi),(Guanzhong),(Dilong),(Xuchangqing),(Huoxiang),(Peilan),(Cangzhu),(Fuling),(Shengbaizhu),(Shanzha),(Shenqu),(Maiya),(Houpo),(Binglang),(Caoguo),(Shengjiang).
Bridging the gap between clinical practice and limited evidence
Presently, the public’s knowledge of the efficacy of TCM in the treatment of COVID-19 is mainly based on the introduction of official press conferences and media publicity, supplemented by published clinical trials. However, we should realize that in the long run, only high-quality published clinical trials will be the most convincing and lasting pieces of evidence to prove the effectiveness of TCM and support its use worldwide.
Strategies could be adopted to bridge the gap between clinical practice and limited evidence of TCM for COVID-19 and other infectious diseases in the future. For the TCM authorities at all levels of China, the management and coordination of registered clinical trials should be strengthened during the outbreak. The limited medical, scientific, and case resources should be prioritized for evaluating TCM interventions that have potential advantages and/or historical evidence of use in similar diseases. For TCM researchers and physicians, the study design should be given high priority before the start of clinical trials. Only a trial with a rigorous design and standardized procedure can generate high-quality evidence.
As for clinical trials on TCM in COVID-19, multicenter RCTs with large samples are warranted. On the basis of providing all participants with guideline-based Western medicine treatment, the use of placebo in the control group is in line with medical ethics and can be accepted by patients. Blinding should be adopted to evaluate symptoms and other subjective outcomes. Core outcome of COVID-19 including the rate of recovery and time taken for recovery/improvement/progression/death to occur, SARS-CoV-2 nucleic-acid tests, rate of prevention of the progression mild-to-moderate cases to severe disease, and symptoms should be adopted for the assessment of efficacy [17]. Currently, the global epidemic is still far from being controlled; imported and indigenous cases in China occur from time to time. Therefore, there are still opportunities to perform or continue those promising TCM trials in China or through international cooperation, so as to generate more high-quality evidence of the efficacy of TCM in the treatment of COVID-19.
1. Hesketh T, Zhu WX. Health in China. Traditional Chinese medicine: one country, two systems. BMJ 1997, 315: 115–117.
2. Luo H, Tang QL, Shang YX, et al. Can Chinese medicine be used for prevention of corona virus disease 2019 (COVID-19)? A review of historical classics, research evidence and current prevention programs. Chin J Integr Med 2020, 26: 243–250.
3. The State Council Information Office of the People's Republic of China [Internet]. Fighting COVID-19: China in action [cited 2020 August 27]. Available from: http://english.scio.gov.cn/ node_8018767.html (Chinese)
4. Wang Z, Chen X, Lu Y, et al. Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and western medicine treatment. Biosci Trends 2020, 14: 64–68.
5. Luo E, Zhang D, Luo H, et al. Treatment efficacy analysis of traditional Chinese medicine for novel coronavirus pneumonia (COVID-19): an empirical study from Wuhan, Hubei Province, China. Chin Med 2020, 15: 34.
6. Zhang HT, Huang MX, Liu X, et al. Evaluation of the adjuvant efficacy of natural herbal medicine on COVID-19: a retrospective matched case-control study. Am J Chin Med 2020, 48: 779–792.
7. Hu K, Guan WJ, Bi Y, et al. Efficacy and safety of Lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: a multicenter, prospective, randomized controlled trial. Phytomedicine 2020: 153242.
8. Ye YA, G-CHAMPS Collaborative Group. Guideline-based Chinese herbal medicine treatment plus standard care for severe coronavirus disease 2019 (G-CHAMPS): evidence from China. Front Med (Lausanne) 2020, 7: 256.
9. Xin SY, Cheng XQ, Zhu B, et al. Clinical retrospective study on the efficacy of Qingfei Paidu decoction combined with western medicine for COVID-19 treatment. Biomed Pharmacother 2020, 129: 110500.
10. Wang JB, Wang ZX, Jing J, et al. Exploring an integrative therapy for treating COVID-19: a randomized controlled trial. Chin J Integr Med 2020, 26: 648–655.
11. Xiong WZ, Wang G, Du J, et al. Efficacy of herbal medicine (Xuanfei Baidu decoction) combined with conventional drug in treating COVID-19: a pilot randomized clinical trial. Integr Med Res 2020, 9: 100489.
12. Xiao MZ, Tian JX, Zhou YN, et al. Efficacy of Huoxiang Zhengqi dropping pills and Lianhua Qingwen granules in treatment of COVID-19: a randomized controlled trial. Pharmacol Res 2020, 161: 105126.
13. Tian JX, Yan SY, Wang H, et al. Hanshiyi formula, a medicine for Sars-CoV2 infection in China, reduced the proportion of mild and moderate COVID-19 patients turning to severe status: a cohort study. Pharmacol Res 2020, 161: 105127.
14. Yang M, Shang YX, Tian ZY, et al. Characteristics of registered studies for coronavirus disease 2019 (COVID-19): a systematic review. Integr Med Res 2020, 9: 100426.
15. Wei XX, Zhao MZ, Zhao C, et al. The global registry of COVID-19 clinical trials: indicating the design of traditional Chinese medicine clinical trials. TMR Mod Herb Med 2020, 3: 140–146.
16. Bian LQ, Li BS, Li ZH, et al. A preparation model of Chinese medicine decoction placebo. Chin J Integr Med 2017, 23: 631–634.
17. Qiu R, Zhao C, Liang T, et al. Core outcome set for clinical trials of COVID-19 based on traditional Chinese and western medicine. Front Pharmacol 2020, 11: 781.
:
TCM, traditional Chinese medicine; RCT, randomized controlled trial; CFDA, China Food and Drug Administration.
:
The authors declare no conflicts of interest.
:
Hui Luo. The gap between clinical practice and limited evidence of traditional Chinese medicine for COVID-19. Traditional Medicine Research 2020, 5 (6): 428–432.
: Nuo-Xi Pi, Jing-Na Zhou.
: 29 August 2020,
04 September 2020,
:09 September 2020.
Institute for Tibetan Medicine of China Tibetology Research Center, No.131 North Fourth Ring Road, Chaoyang District, Beijing 100101, China. E-mail: luohui09@hotmail.com.
10.12032/TMR20200906197
 Traditional Medicine Research2020年6期
Traditional Medicine Research2020年6期
- Traditional Medicine Research的其它文章
- Is “Pangolin (Manis Squama) is not used in medicine" an improvement in the protection of precious and rare species or an improvement in the safety of using medicine?
- The potential effects of Caper (Capparis spinosa L.) in the treatment of diabetic neuropathy
- Effects of herbal medicine in gastroesophageal reflux disease symptoms: a systematic review and meta-analysis
