The potential effects of Caper (Capparis spinosa L.) in the treatment of diabetic neuropathy
Hamid Reza Esmaeilpour, Reza Boostani, Ali Shoeibi, Mojtaba Mousavi Bazzaz, Roshanak salari, Mahdi Yousefi*
The potential effects of Caper (L.) in the treatment of diabetic neuropathy
Hamid Reza Esmaeilpour1, Reza Boostani2, Ali Shoeibi3, Mojtaba Mousavi Bazzaz4, Roshanak salari5, Mahdi Yousefi1*
1Department of Persian Medicine, School of Persian and Complementary Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.2Department of Neurology and HTLV-1 Foundation, Ghaem Hospital, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.3Department of Neurology, Ghaem Hospital, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.4Department of Community Medicine, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.5Department of Pharmaceutical Sciences in Persian Medicine, School of Persian and Complementary Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
Diabetic neuropathy (DN) is the most common form of neuropathy worldwide, with its prevalence rising alongside diabetes, and being characterized by sensory, motor or autonomic symptoms. DN is considered to be an incurable complication of diabetes, the management of which mainly consists of improving glycemic control, managing pain relief and ensuring continuous foot care. Although gabapentin, duloxetine and tricyclic antidepressants are commonly used to reduce patient symptoms, they do not affect the pathophysiology and progression of neuropathy. Furthermore, these drugs can have various side effects including insomnia, decreased appetite, arrhythmia, heart failure, and suicidal behavior.According totraditional Persian medicine, DN is recognized as a type of “or “(a sensory or motor disorder, respectively) that occurs due to the accumulation of sugars in the peripheral nerves.L., commonly known as the caper plant, has been recommended in authentic sources of traditional Persian medicine to treat such disorders.In this study, we reviewed the pharmacological properties ofusing the Web of Science, PubMed, Scopus and Google Scholar databases, and found thatL. could affect several pathways involved in DN pathogenesis, including aldose reductase activity, the secretion of inflammatory mediators (IL-17, TNF-α, IL-1β, IL-6), oxidative stress, hyperlipidemia, hyperglycemia and advanced glycation end product formation.Based on these findings, we hypothesize thatL., may prevent the progression and reduce the symptoms of diabetic neuropathy,and so canbe considered as a complementary treatment in this disorder. This hypothesis should be evaluated in well-designed in vitro and in vivo studies, and through clinical trials.
L.Diabetic neuropathy, Traditional Persian medicine, “
The authors hypothesize thatL., may prevent the progression of diabetic neuropathy, reduce its symptoms,and canbe considered as a complementary treatment for this disorder.
According to traditional Persian medicine, diabetic neuropathy is recognized as a type of “or “(a sensory or motor disorder, respectively) that occurs due to the accumulation of sugars in the peripheral nerves.L., commonly known as the caper plant,has been recommended as a treatment for such disorders by Avicenna (980 C.E.–1037 C.E.) and Razi (Rhazes) (864 C.E.–930 C.E.), two of the greatest scholars of traditional medicine.Different parts of the caper plant have been used in the treatment of rheumatism, gout, hypertension, diabetes, sciatica, as well as hepatic and splenic disorders.
Background
Neuropathy is a disorder of the sensory-motor or autonomic nerves and can result from various causes [1]. Currently, the most common cause of neuropathy worldwide, is diabetes [2, 3]. The prevalence of diabetes has increased in recent decades, mainly due to the changes in lifestyle [4]. Increased prevalence of diabetes will naturally be accompanied by an increase in diabetes related complications [5], with the most common complication of diabetes being neuropathy, generally affecting around 50% of diabetic patients [1, 6].Diabetic neuropathy (DN) progresses gradually over the years, and can have both physical and mental consequences, affecting patient quality of life [7]. A possible serious complication of DN is development of foot ulcers; the most common cause of non-traumatic amputation, and more often than other complications leads to the hospitalization of diabetic patients [8].
Several metabolic and immunologic pathways are involved in the pathogenesis of DN [9]. Oxidative stress, inflammation, glycation, ischemia and aldose reductase hyperactivity are mechanisms central to the cause of neural damage, with aldose reductase hyperactivity also leading to oxidative stress and the upregulation of some other pathogenic pathways[10, 11]. As such hyperglycemia plays a central role in the pathophysiology of this disorder [9]. Other conditions such as hypertension, hyperlipidemia and obesity may also increase the risk of developing DN. Currently DN is considered to be an incurable complication of diabetes, with its management mainly involving the improvement of glycemic control, managing pain relief and ensuring continuous foot care [3, 6]. Several drugs, including gabapentin, duloxetine and tricyclic antidepressants are currently used to reduce patient discomfort, however they do not affect the development of neuropathy and can also have various side effects, including decreased appetite, insomnia, suicidal behavior, dry mouth, arrhythmia and heart failure [12–14].
In traditional Persian medicine, DN is recognized as a type of “or “(a sensory or motor disorder, respectively) which occurs due to the excessive accumulation of sugars in the peripheral nerves[15].L., commonly known as the caper plant, has been recommended as a treatment for such disorders by Avicenna (980 C.E.–1037 C.E.) and Razi (Rhazes) (864 C.E.–930 C.E.), two of the greatest scholars of traditional medicine[16, 17].Different parts of the caper plant have been used in the treatment of rheumatism, gout, hypertension, diabetes, sciatica, as well as hepatic and splenic disorders [18, 19]. This plant is found in many different parts of the world, including the Mediterranean basin, North Africa, Europe, Middle East and Tropical Asia [19, 20]. The presence of several biochemical components, such as flavonoids, alkaloids, phenolic compounds, terpenoids, saponins, tocopherols, and carotenoids, have been reported in this plant [19, 21].
Given the lack of effective treatments for DN, the adverse effects of conventional drugs, as well as the recommendation ofL. for the treatment of sensory and motor disorders in traditional Persian medicine, we evaluated the pharmacological properties ofL. and potential effects of this plant in the treatment of DN.
Hypothesis
Based on the findings of this study,L.may be useful in the treatment DN, due to several pharmacological properties, including hypoglycemic, hypolipidemic, hypotensive, antioxidant, anti-inflammatory, analgesic, antiglycation, anti-obesity and neuroprotective effects, as well as the inhibition of α-amylase, α-glucosidase and aldose reductase activity, all of which could affect the underlying mechanisms of DN[22–32]. However, further investigation of these mechanisms is required. We therefore recommend further studies on the effects ofL.on DNand, if effective, the use of this plant or its derivatives in the treatment of this disorder.
Evidence supporting the hypothesis
According to the available scientific evidence, the beneficial effects ofL. on diabetic neuropathy can be explained as follows(Figure 1):
1.L. has exhibited antioxidant properties in different in vivo and in vitro investigations, by increasing the activity of antioxidant enzymes and removing free radicals [25, 33–39].
2. Several in vivo and in vitro studies have revealed the anti-inflammatory properties ofL. and the biochemical mechanisms through which the inhibition of different inflammatory signaling pathways can occur [26, 27, 40–44].
3.L.has been shown to decrease blood glucose levels in animal studiesand inhibit α-glucosidase (having an acarbose like effect on postprandial hyperglycemia) as well as pancreatic α-amylase activity in vitro [22, 23, 30, 31, 45]. In a placebo-controlled clinical trial of 54 patients with type II diabetes,L.significantly decreased blood glucose and HbA1c levels [46].
4. Lipid-lowering effects ofL.have been demonstrated in experimental investigations. It has been shown to significantly reduce the blood levels of low-density lipoprotein, total cholesterol and triglyceride, and increase high density lipoprotein in the healthy and diabetic animals [45, 47, 48]. It also causes a significant decrease in blood triglyceride and a non-significantdecrease in blood cholesterol and low-density lipoprotein in patients with type II diabetes [46].
5.L.has the beneficial effect of reducing blood pressure and preventing neural ischemia. It has exhibited vasodilatory and diuretic activity in normal rats, a function of lowering systolic blood pressure in spontaneous hypertensive rats [24], and anti-atherogenic properties in human and animal subjects [47, 49].
6.L.[50], and some of the flavonoids found in this plant (naringin, rutin, and quercetin), have been shown to have inhibitory effects on aldose reductase activity [32, 51], and thereby may prevent its pathogenic consequences and the activation of other deleterious pathways in diabetes.
7. Obesity significantly increases the risk of neuropathy in diabetic patients and is also associated with higher intensity neuropathic pain[52, 53].L.exhibits weight-loss effects in diabetic rats and high fat diet mice[23, 47], and therefore, may reduce the risk of obesity-induced neuropathy and patient discomfort.
8. Pain relief is one of the main therapeutic targets in DN, and the analgesic effect ofL.has been revealed in a rat model of osteoarthritis and rheumatoid arthritis [27]. This plant has also traditionally been usedto relieve various pains such as headache, toothache and rheumatism [54], which may be unrelated to its anti-inflammatory properties.
9. Several studies have reported the neuroprotective effects ofL.in Alzheimer’s disease, as well as in lipopolysaccharide-and D-galactose-induced cognitive impairment in animals [29, 44, 55].
10.L. inhibits the non-enzymatic glycation of biological molecules and preventsadvanced glycation end product formation during a hyperglycemic state in vitro, indicating that it may protect peripheral nerves in diabetes [28].
Prospective
Based on our hypothesis,L. and caper-derived medications may be helpful in the treatment of DN. Current evidence supports this suggestion, and can be summarized as follows:
1. DN results from a combination of multiple pathogenic conditions, including hyperglycemia, hyperlipidemia, inflammation, oxidative stress, advanced glycation end product formation, aldose reductase hyperactivity, obesity, hypertension, and nerve ischemia.
2. Most currently used drugs have mainly a palliative effect in DN and do not affect the pathologic processes or progression of the disease.
3. Due to its high prevalence, progressive nature, disabling consequences, high economic burden, and the insufficiency and side effects of conventional drugs, DN requires new, more effective and safe treatments.
4.L.and caper-derived medicinesmay be more effective than currently used drugs, as, in addition topalliative properties and fewer side effects [31], it may influence the underlying mechanisms of neuropathy.
5.L. has been recommended by Avicenna, Rhazes and some other traditional medical scientists to treat peripheral nerve disorders and had also been used by Romans in the treatment of paralysis [54]. However, in recent centuries, due to the discovery of various chemical drugs, the use of natural remedies has declined.
Despite this positive evidence, challenges remain to implementing such treatments for DN. The inhibition of pathogenic pathways in DN has been evaluated in several in vivo and in vitro studies. Although, these investigations have been associated with positive results, evidence for the effectiveness of such a treatment approach in human is still insufficient. In addition, some limitations of this hypothesis can be identified. For example, the mechanisms involved in DN, as well asL.’s effect on these, are still not well understood. There are also insufficient studies to confirm the effective components ofL. in DN. Furthermore, studying the precise effects ofL. on DN requires invasive methods such as nerve biopsy. Considering these issues,practical solutions are suggested as follows:
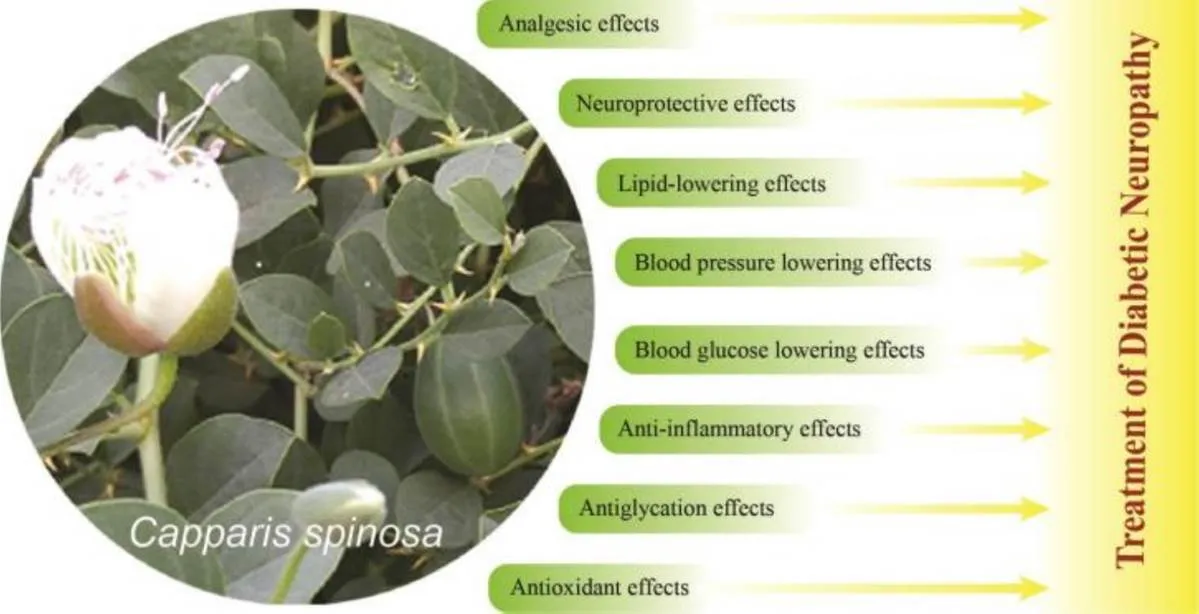
Figure 1 The beneficial effects ofL. on diabetic neuropathy.
1. The effects of caper on DN could be evaluated in an experimental study in rats. After induction of DN, rats could be randomly divided into intervention (L. extract) and control (no treatment) groups.
2. The effects ofL. on the sciatic nerve can be evaluated using behavioral tests (such as hot plate, tail flick, Von Frey, and formalin assays), as well as electrophysiological and histopathological studies at appropriate intervals and compared with the control group using statistical tests.
3. To investigate the effects ofL. on the progression of DN and patient symptoms, a randomized placebo-controlled clinical trial could be designed. The intervention group can receive caper extract and the control group a placebo. The severity of diabetic neuropathy can be assessed by standard questionnaires and electrophysiological tests before and after the study and compared using appropriate statistical tests.
Conclusion
The positive effects ofL. on the pathogenic pathways involved in DN, and therapeutic properties discussed in reliable sources of traditional medicine, indicate that this plant can be useful in the treatment of DN. However, further studies are warranted to properly establish the beneficial effects ofL. in practice.
1. Singh R, Kishore L, Kaur N. Diabetic peripheral neuropathy: current perspective and future directions. Pharmacol Res 2014, 80: 21–35.
2. Said G. Diabetic neuropathy—a review. Nat Clin Pract Neurol 2007, 3: 331–340.
3. Iqbal Z, Azmi S, Yadav R, et al. Diabetic peripheral neuropathy: epidemiology, diagnosis, and pharmacotherapy. Clin Ther 2018, 40: 828–849.
4. Tuomilehto J, Lindström J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. New Engl J Med 2001, 344: 1343–1350.
5. Iqbal Z, Azmi S, Yadav R, et al. Diabetic peripheral neuropathy: epidemiology, diagnosis, and pharmacotherapy. Clin Ther 2018, 40: 828–849.
6. Figueroa-Romero C, Sadidi M, Feldman EL. Mechanisms of disease: the oxidative stress theory of diabetic neuropathy. Rev Endocr Metab Disord 2008, 9: 301–314.
7. Van Acker K, Bouhassira D, De Bacquer D, et al. Prevalence and impact on quality of life of peripheral neuropathy with or without neuropathic pain in type 1 and type 2 diabetic patients attending hospital outpatients clinics. Diabetes Metab 2009, 35: 206–213.
8. Vinik AI, Strotmeyer ES, Nakave AA, et al. Diabetic neuropathy in older adults. Clin Geriatr Med 2008, 24: 407–435.
9. Hosseini A, Abdollahi M. Diabetic neuropathy and oxidative stress: therapeutic perspectives. Oxid Med Cell Longev 2013.
10. Schreiber AK, Nones CF, Reis RC, et al. Diabetic neuropathic pain: physiopathology and treatment. World J Diabetes 2015, 6: 432.
11. Dewanjee S, Das S, Das AK, et al. Molecular mechanism of diabetic neuropathy and its pharmacotherapeutic targets. Eur J Pharmacol 2018, 833: 472–523..
12. Edwards JL, Vincent AM, Cheng HT, et al. Diabetic neuropathy: mechanisms to management. Pharmacol Ther 2008, 120: 1–34.
13. Quintero GC. Review about gabapentin misuse, interactions, contraindications and side effects. J Exp Pharmacol 2017, 9: 13.
14. Hossain SM, Hussain SM, Ekram AR. Duloxetine in painful diabetic neuropathy. Clin J Pain 2016, 32: 1005–1010.
15. Arzani MA. Teb-e-Akbari (Akbari’s Medicine). Qom: Jalaleddin press, 2008. (Persian)
16. Avicenna H. Al-Qanun fit-Tib (Canon of Medicine). Beirut: Ehyaol Toras al-Arabi Press, 2010. (Persian)
17. Rhazes Mz. Al-Hawi fi l-tibb (Comprehensive Book of Medicine). Beirut: Ehyaol Toras al-Arabi Press, 2001. (Persian)
18. Aghili-Shirazi MH. Makhzan-ol-Advieh (The Storehouse of Medicaments) 1 ed. Tehran: Bavardaran, 2001. (Persian)
19. Chedraoui S, Abi-Rizk A, El-Beyrouthy M, et al.L. in a systematic review: A xerophilous species of multi values and promising potentialities for agrosystems under the threat of global warming. Front Plant Sci 2017, 8: 1845.
20. Gull T, Anwar F, Sultana B, et al. Capparis species: a potential source of bioactives and high-value components: a review. Ind Crops Prod 2015, 67: 81–96.
21. Vahid H, Rakhshandeh H, Ghorbani A. Antidiabetic properties ofL. and its components. Biomedi Pharmacother 2017, 92: 293–302.
22. Kazemian M, Abad M, Haeri MR, et al. Anti-diabetic effect ofL. root extract in diabetic rats. Avicenna J Phytomed 2015, 5: 325.
23. Lemhadri A, Eddouks M, Sulpice T, et al. Anti-hyperglycaemic and anti-obesity effects ofand Chamaemelum nobile aqueous extracts in HFD mice. Am J Pharmacol Toxicol 2007, 2:106–110.
24. Naoufel Ali Z, Eddouks M, Baptiste Michel J, et al. Cardiovascular effect ofaqueous extract. Part III: Antihypertensive effect in spontaneously hypertensive rats. Am J Pharmacol Toxicol 2007, 2: 111–115.
25. Allaith AAA. Assessment of the antioxidant properties of the caper fruit (L.) from Bahrain. J Assoc Arab Univ Basic Appl Sci 2016, 19: 1–7.
26. Moutia M, El Azhary K, Elouaddari A, et al.L. promotes anti-inflammatory response in vitro through the control of cytokine gene expression in human peripheral blood mononuclear cells. BMC Immunol 2016, 17: 26.
27. Maresca M, Micheli L, Di Cesare Mannelli L, et al. Acute effect ofroot extracts on rat articular pain. J Ethnopharmacol 2016, 193: 456–465.
28. Yarizade A, Niazi A, Kumleh H. Investigation of antiglycation and antioxidant potential of some antidiabetic medicinal plants. J Pharma Sci Res 2017, 9: 2382–2387.
29. Goel A, Garg A, Kumar A. Effect ofLinn. extract on lipopolysaccharide-induced cognitive impairment in rats. Indian J Exp Biol 2016, 54: 126–132.
30. Selfayan M, Namjooyan F. Inhibitory effect ofextract on pancreatic alpha-amylase activity. Zahedan J Res Med Sci 2016.
31. Mollica A, Zengin G, Locatelli M, et al. Anti-diabetic and anti-hyperlipidemic properties ofL.: in vivo and in vitro evaluation of its nutraceutical potential. J Funct Foods 2017, 35: 32–42.
32. Reddy G, Puppala M, Akileshwari C, et al. Inhibition of aldose reductase and sorbitol accumulation by dietary rutin. Cur Sci 2011, 101: 1191–1197.
33. Stefanucci A, Zengin G, Locatelli M, et al. Impact of different geographical locations on varying profile of bioactives and associated functionalities of caper (L.). Food Chem Toxicol 2018, 118: 181–189.
34. Germanò MP, De Pasquale R, D'Angelo V, et al. Evaluation of extracts and isolated fraction fromL. buds as an antioxidant source. J Agric Food Chem 2020, 50:1168–1171.
35. Bhoyar MS, Mishra GP, Naik PK. Estimation of antioxidant activity and total phenolics among natural populations of Caper () leaves collected from cold arid desert of trans-Himalayas. Aust J Crop Sci 2011, 5: 912–919.
36. Mousavi SH, Hosseini A, Bakhtiari E, et al.reduces doxorubicin-induced cardio-toxicity in cardiomyoblast cells. Avicenna J Phytomed 2016, 6: 488–494.
37. Mansour RB, Jilani IBH, Bouaziz M, et al. Phenolic contents and antioxidant activity of ethanolic extract of. Cytotechnology 2016, 68: 135–142.
38. Cao YL, Li X, Zheng M.protects against oxidative stress in systemic sclerosis dermal fibroblasts. Arch Dermatol Res 2010, 302: 349–355.
39. Kalantari H, Foruozandeh H, Khodayar MJ, et al. Antioxidant and hepatoprotective effects ofL. fractions and quercetin on tert-butyl hydroperoxide-induced acute liver damage in mice. J Tradit Complem Med 2018, 8: 120–127.
40. Feng X, Lu J, Xin H, et al. Anti-arthritic active fraction ofL. fruits and its chemical constituents. Yakugaku Zasshi 2011, 131: 423–429.
41. Zhou HF, Xie C, Jian R, et al. Biflavonoids from Caper (L.) fruits and their effects in inhibiting NF-kappa B activation. J Agric Food Chem 2011, 59: 3060–3065.
42. Hamuti A, Li J, Zhou F, et al.fruit ethanol extracts exert different effects on the maturation of dendritic cells. Molecules 2017, 22.
43. El Azhary K, Tahiri Jouti N, El Khachibi M, et al. Anti-inflammatory potential ofL. in vivo in mice through inhibition of cell infiltration and cytokine gene expression. BMC complem altern med 2017, 17: 1–12.
44. Mohebali N, Shahzadeh Fazeli SA, Ghafoori H, et al. Effect of flavonoids rich extract ofon inflammatory involved genes in amyloid-beta peptide injected rat model of Alzheimer's disease. Nutrit Neurosci 2018, 21:143–150.
45. Jalali MT, Mohammadtaghvaei N, Larky DA. Investigating the effects ofon hepatic gluconeogenesis and lipid content in streptozotocin-induced diabetic rats. Biomed Pharmacother 2016, 84:1243–1248.
46. Huseini HF, Hasani-Rnjbar S, Nayebi N, et al.L. (Caper) fruit extract in treatment of type 2 diabetic patients: a randomized double-blind placebo-controlled clinical trial. Complement Ther Med 2013, 21: 447–452.
47. Eddouks M, Lemhadri A, Michel JB. Hypolipidemic activity of aqueous extract ofL. in normal and diabetic rats. J Ethnopharmacol 2005, 98: 345–350.
48. Negahdarizadeh M, Mokhtari M, Malekzadeh J, et al. The effects ofhydroalcoholic extract on blood glucose and lipids serum in diabetic and normal male rats. Armaghane Danesh 2011, 16: 181–190.
49. Khavasi N, Somi M, Khadem E, et al. Daily consumption of thereduces some atherogenic indices in patients with non-alcoholic fatty liver disease: a randomized, double-blind, clinical trial. Iran Red Crescent Med J 2018, 20.
50. Kheirollah A, Aberumand M, Ramezani Z, et al. Inhibition of aldose reductase and red blood cell sorbitol accumulation by extract of. Jundishapur J Nat Pharm Prod 2015, 10: e24331.
51. Goodarzi M, Zal F, Malakooti M, et al. Inhibitory activity of flavonoids on the lens aldose reductase of healthy and diabetic rats. Acta medica Iranica 2006, 44.
52. Smith AG, Singleton JR. Obesity and hyperlipidemia are risk factors for early diabetic neuropathy. J Diabetes Complications 2013, 27: 436–442.
53. Hozumi J, Sumitani M, Matsubayashi Y, et al. Relationship between neuropathic pain and obesity. Pain Res Manag 2016: 2487924.
54. Zhang H, Ma ZF. Phytochemical and pharmacological properties ofas a medicinal plant. Nutrients 2018, 10: 116.
55. Turgut NH, Kara H, Arslanbas E, et al. Effect ofL. on cognitive impairment induced by D-galactose in mice via inhibition of oxidative stress. Turk J Med Sci 2015, 45: 1127–1136.
:
This study was supported by Mashhad University of Medical Sciences Research Council, Mashhad, Iran.
:
DN, diabetic neuropathy.
:
There are no conflicts of interest.
:
Hamid Reza Esmaeilpour, Reza Boostani, Ali Shoeibi, et al. The potential effects of Caper (L.) in the treatment of diabetic neuropathy. Traditional Medicine Research 2020, 5 (6): 442–448.
: Nuo-Xi Pi.
: 11 December 2019,
5 January 2020,
:26 February 2020.
Mahdi Yousefi. Department of Persian Medicine, School of Persian and Complementary Medicine, Mashhad University of Medical Sciences,Vakilabad Blvd, Mashhad, Iran. E-mail: Yousefim@mums.ac.ir.
10.12032/TMR20200223167
 Traditional Medicine Research2020年6期
Traditional Medicine Research2020年6期
- Traditional Medicine Research的其它文章
- Is “Pangolin (Manis Squama) is not used in medicine" an improvement in the protection of precious and rare species or an improvement in the safety of using medicine?
- The gap between clinical practice and limited evidence of traditional Chinese medicine for COVID-19
- Effects of herbal medicine in gastroesophageal reflux disease symptoms: a systematic review and meta-analysis
