Green coffee bean hydroalcoholic extract accelerates wound healing in full-thickness wounds in rabbits
Reza Shahriari, Pari Tamri*, Amir Larki Harchegani, Alireza Nourian
Green coffee bean hydroalcoholic extract accelerates wound healing in full-thickness wounds in rabbits
Reza Shahriari1, Pari Tamri1*, Amir Larki Harchegani1, Alireza Nourian2
1Department of Pharmacology & Toxicology, School of Pharmacy, Medicinal Plants and Natural Products Research Center, Hamadan University of Medical Sciences, Hamadan 6517838678, Iran;2Department of Laboratory Sciences, Faculty of Para-Veterinary Sciences, Bu-Ali Sina University, Hamadan 6517838695, Iran.
Green coffee beans contain biochemical compounds including caffeine, chlorogenic acid, trigonelline, and diterpenoid alcohols. In traditional Iranian medicine, coffee bean powder is used for the treatment of wounds. Previous studies have shown that green coffee bean extract has a number of health benefits, including induction of weight loss, reduced blood pressure, and hepatoprotective and anti-Parkinson effects. The aim of this study was to investigate the wound-healing activity of green coffee bean extract on a full-thickness wound model.Full-thickness wounds of 20 × 20 mm were created on the back of New Zealand white rabbits. The animals were divided into 6 groups. Three concentrations of green coffee bean extract (5%, 10%, and 15% w/w) in a eucerin base were applied over the wounds in 3 test groups. One percent phenytoin cream was used in one group as a positive control. The rabbits of negative control and vehicle groups received no treatment and eucerin, respectively. For the evaluation of green coffee bean extract’s wound-healing effects, measures included: (1) wound-closure rate, by daily measuring of the wound surface area and calculating the reduction in area; (2) period of epithelialization, the number of days until the scab dropped from the wound; (3) hydroxyproline content, measured by a hydroxyproline assay kit; (4) lipid peroxidation, measured by a lipid peroxidation assay kit; and (5) histopathological state (hematoxylin-eosin stain) of wound-tissue samples on days 7 and 14 post-wounding.The results of this study showed significantly enhanced wound-closure rate, shorter period of epithelialization (< 0.01), increased hydroxyproline content (< 0.001) and suppressed lipid peroxidation (< 0.001 on day 14) of wounds on animals treated with the 10% green coffee bean extract compared to the negative control and eucerin-treated groups. Moreover, the therapeutic effects of 10% green coffee bean were significantly superior than those of phenytoin on enhancing wound-closure rate,decreasing period of epithelialization (< 0.05), increasing hydroxyproline content (< 0.001 on day 7 and< 0.0001 on day 14) and suppressing lipid peroxidation (< 0.0001 on day 14). In addition, histopathological study supported the wound healing activity of green coffee bean extract.Our results showed that green coffee bean extract has a potential for promoting wound healing, thus supporting its traditional use for this purpose.
Green coffee bean, Green coffee bean extract, Wound healing, Epithelialization,Hydroxyproline, Histopathology
A topical preparation of green coffee bean hydroalcoholic extract accelerated wound healing by enhancing wound-closure rate and hydroxyproline content, and by inhibiting the lipid peroxidation of wound-tissue samples in a full-thickness wound model in rabbits.
species are widespread and well known throughout the world. Traditional uses of coffee in human medicine have been reported in several publications. Moḥammad-Ḥossein Aghili Khorasani, a prominent Persian physician in the late 18th and early 19th centuries, reported that sprinkling coffee powder on wounds helped to heal them. In her book,(1980), L.M. Perry reported that coffee powder was used for the treatment of burn wounds.
Background
Wound healing is the body’s natural response to tissue injury [1]. Normal wound healing is a complex and dynamic process involving a cascade of molecular and cellular events correlating with the appearance of various cell types in the wound bed during distinct phases (hemostasis, inflammation, proliferation and maturation) of the healing process [2]. Many factors such as diabetes, vascular diseases, stress and malnutrition can affect the healing process by interfering with the phases of the process and leading to inappropriate healing [3]. Non-healing wounds result in significant burdens to both the individual and the health care system. Aside from the burden of non-healing or chronic wounds, minor wounds also require proper care and treatment to reduce scarring [3, 4]. It is no surprise then, the wound healing receives significant attention among researchers.
Throughout the world, plants and their secondary products have been used for the treatment of diseases since ancient times [5].(Rubiaceae) is considered as the most useful of all coffee plants, providing 70% to 80% of the world’s coffee production [6]. Seeds and other parts of the coffee plant have been used in traditional medicine throughout the world for conditions such as flu, backache, measles, asthma, migraine, stomatitis, pharyngitis and purulent wounds Figure 1[6–10]. Coffee bean extract has been used traditionally for wound healing in certain parts of the world. Moḥammad-Ḥossein Aghili, a prominent Persian physician of the late 18th and early 19th centuries reported that sprinkling of coffee powder on wounds helped to heal them [11]. The traditional use of coffee bean powder for the treatment of burn wounds was reported in the book(1980) by L.M. Perry [12]. According to the’(2000), in folk medicine the coffee coal (charcoal) has been used for the treatment of purulent wounds [10]. Green coffee beans are obtained from coffee cherries (the raw fruit of the coffee plant) by removing both the pulp and hull using either a wet or dry method. Caffeine is the most commonly known component of coffee beans, but the bean has several other components, including cellulose, minerals, sugars, lipids, tannin, and polyphenols such as quercetin, kaemferol, and chlorogenic and nicotinic acids [6, 13–16]. Additionally, coffee beans contain several amino acids and vitamins [17, 18]. Previous studies have shown that coffee and its components such as chlorogenic acid and caffeine have antioxidant, anti-inflammatory and antibacterial effects [13, 19–23]. The results of one study suggested that ingestion of coffee polyphenols extracted from coffee beans can improve the hydration, permeability barrier function and microcirculatory function of the skin [24]. In this study we aimed to investigate the healing effects of green coffee bean hydroalcoholic extract (GCBE) on skin wounds in animals because of its antioxidant, antibacterial and nutritional properties.
Figure 1whole plant and green coffee beans
Materials and methods
Drugs and reagents
Phenytoin cream was obtained from the Darou Pakhsh pharmaceutical manufacturing company (Iran), 400% eucerin was obtained from Emad Darman Pars Co., Ltd. (Iran), and hydroxyproline and lipid peroxidation assay kits were purchased from KiaZist life sciences (Iran). Ethanol was obtained from Merck company (Germany).
Extract preparation
Green coffee beans were purchased from a local market (Isfahan, Iran) and identified at the Department of Pharmacognosy, School of Pharmacy, Hamadan University of Medical Sciences. The coffee beans were ground to a powder. Hydroalcoholic extract of the coffee beans was obtained using a maceration method. Two thousand grams of dry powder was added to 6 liters of 70% (v/v) ethanol and kept at room temperature for 72 h. It was recovered by filtration on cellulose support under reduced pressure, followed by concentration by a rotary evaporator (Yamato RE300, Japan) at a temperature of 40 °C. The concentrated extract was freeze dried to yield a dry powder. The extraction procedure was repeated 3 times. The yield of GCBE was about 20% (w/w). The prepared extract was stored in a refrigerator at 4 °C.
Preparation of ointments containing GCBE
Ointments containing 5%, 10% and 15% (w/w) of GCBE were prepared using a levigation method. The dried powder of GCBE was dissolved in glycerin then incorporated in eucerin as the ointment base.
Animals
Thirty-six New Zealand white rabbits (weighing 1.8–2.2 kg) of both sexes, obtained from Department of Animal Sciences, Pasteur Institute of Iran, were used in this study. The animals were housed in individual cages under standard conditions (temperature 24–26 °C, humidity 55 ± 10 %, 12/12 hours cycle of light and darkness) with access to food and drinking water ad libitum. The animal protocol was conducted according to National Guidelines for the Care and Use of Laboratory Animals published by National Academies Press in 2011 ((800) 624-6242). and approved by the ethics committee of Hamadan University of Medical Sciences. The ethical approval code is IR.UMSHA.REC.1395.167, date: June 17, 2017. The rabbits were randomly divided into 6 equal groups as a standard control group, negative control group, vehicle group and three test groups.
Wound procedure
Two identical full-thickness excisional wounds (20 × 20 mm) were created in the back of all rabbits. Briefly the hairs of the animals’ lower backs were shaved and disinfected with 70% ethanol, then the animals were anesthetized with subcutaneous injection of 2% lidocaine. The full-thickness wounds were made with bistouries, forceps and scissors [25]. The wounds were washed with a normal saline solution. Topical treatments were started 2 h after wound creation. The standard control group received 1% phenytoin cream, the negative control group received no treatment, the vehicle group received eucerin and the test groups received 3 different concentrations of GCBE: 5%, 10%, and 15% (w/w). The treatments were applied twice daily.
Calculation of wound contraction
The wounds’ closure rates were evaluated on days 1, 4, 7, 10, 13, 16, 19 and 21 by tracing the outlines of the wounds onto sterile transparent paper with a permanent marker. The wounds’ areas were measured using squared paper. The measured area was used to calculate the percentage of wound contraction as shown below.

Epithelialization period measurement
Epithelialization is the formation of a new epithelium over the denuded skin following dermal injury. Epithelialization is a key component of the wound-healing process and is used as an important measure of successful wound healing [26]. The number of days required for the dropping of the scab from the wound was considered as the epithelialization period [27].
Preparation of samples for biochemical analysis
Wound-tissue samples from all experimental groups were collected on days 7 and 14 post-wounding. Skin wound tissues (100 mg of tissue) were excised with a sterile scalpel blade. The samples were stored at −80 °C until the biochemical analysis was performed. (Note: there were 12 wounds in each group, 6 wounds were used for wound-contraction evaluation, 3 wounds for sampling on day 7 and 3 wounds for sampling on day 14).
Hydroxyproline assay
The hydroxyproline content of the wound-tissue samples was determined using a hydroxyproline assay kit (KiaZist life sciences, Iran) according to the manufacturer’s instructions. Briefly, the tissue samples were weighed, homogenized and incubated with 12 M HCL at 120 °C for 3 h in sealed tubes to be hydrolyzed. Twenty milliliters of each sample and standard were added to each well. The hydrolysates were neutralized with 100 µL of oxidizing agent (Chloramine T) and subjected to chromogen solution at 60 °C for 30 minutes. Finally, the samples and standard absorbance was read at 540 nm using a microplate reader (BioTEK, USA). Hydroxyproline concentrations (µg/mg tissue) in samples were determined from the standard curve.
Lipid peroxidation assay
Lipid peroxidation is a process in which free radicals take electrons from cell membrane lipids, resulting in cell damage. The lipid peroxidation of samples was evaluated with a lipid peroxidation assay kit (KiaZist life sciences, Iran). Briefly, a thiobarbituric acid solution was added to samples, incubated at 95 °C for 60 minutes, then cooled in an ice bath for 10 minutes. The samples were precipitated with n-butanol, centrifuged and dried. The resuspended pellet was transferred to the wells and analyzed with a microplate reader (BioTEK, USA). The absorbance was read at 532 nm.
Histopathological studies
The cross sectional full-thickness skin specimens from all experimental groups were taken on days 7 and 14 post-wounding. Samples were fixed in 10% formalin. After processing and blocking in paraffin, the specimens were sectioned to 5µm on a microtome (SCILAB Co., Ltd., UK) and stained with hematoxylin and eosin. In the histological study the basic components of the wound-healing process including angiogenesis (identification of microvessels from hematoxylin-eosin-stained images by a pathologist), inflammation (the number of inflammatory cells present in tissue samples), granulation tissue formation (considered complete when a layer of granulation tissue fills the entire wound gap) and re-epithelialization (the resurfacing of the wound area with a new epithelium) were evaluated.
Statistical analysis
All the data are expressed as mean ± standard deviation. The data analysis was carried out using a two-way analysis of variance method followed by the Bonferroni multiple comparison test. The analysis was performed with GraphPad, InStat 8 statistical software (GraphPad software limited liability company, USA).
Results
Wound contraction
The results of the wound-contraction assessment showed that GCBE has a significant effect on the wound-closure rate in rabbits. The percentage of wound closure on days 1, 4, 7, 10, 13, 16, 19 and 21 are shown in Figure 2. The GCBE accelerated wound contraction significantly at 3 applied doses from days 10 to 21 compared with the negative control and eucerin-treated groups. The wound-closure rate in 10% GCBE-treated animals was significantly higher compared to that of the 5% GCBE-treated group from day 10 onward. Significant wound contraction was observed for the phenytoin-treated group from day 10 onward compared to the negative control and eucerin-treated groups. The rate of wound closure in animals treated with 10% GCBE was significantly higher compared to that of the phenytoin-treated animals from day 19 onward. Complete wound closure occurred on day 21 for the 10% GBCE group, day 24 for 5% GCBE, day 25 for phenytoin, day 26 for 15% GCBE and day 29 for the no-treatment and eucerin-treated animals. Hence the 10% ointment of GCBE was the most effective dose for wound healing in this study (Figure 3).
Epithelialization period
Complete epithelialization occurred in a significantly shorter period in the 10% GCBE, 5% GCBE and phenytoin-treated animals comparing to the negative control and eucerin-treated groups (< 0.05). The 10% GCBE ointment-treated group showed a faster rate of epithelialization (< 0.01) compared to the negative control and eucerin-treated groups. In addition, the 10% GCBE group showed a significant difference of epithelialization period compared to the 5% GBCE, 15% GCBE and phenytoin-treated groups (< 0.05). There were no significant differences among the 5% GCBE, 15% GCBE and phenytoin-treated groups in the rate of epithelialization. The period of epithelialization in the negative control and eucerin-treated groups was not significantly different (Table 1).

Figure 2 Effect of GCBE on wound-closure rate in full-thickness wound model in rabbits. Values are shown as mean ± standard deviation.GCBE, green coffee bean extract.
Figure 3 Comparison of the wounds of different experimental groups on days 1 and 21. GCBE, green coffee bean extract.
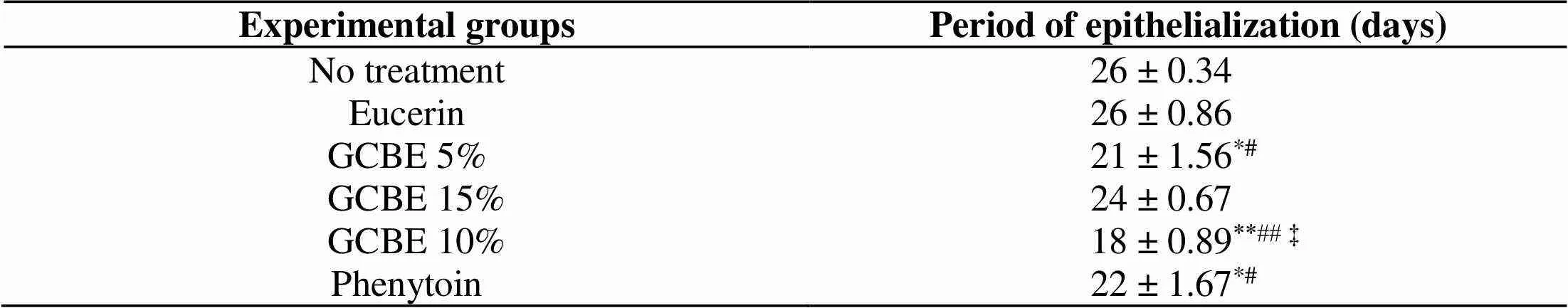
Table 1 Effect of GCBE on the period of epithelialization compared to control groups in rabbit full-thickness wound model
Data are expressed as mean ± standard error of the mean. *< 0.05 and **< 0.01 indicate the significant differences from the no-treatment group.#< 0.05 and##< 0.01 indicate the significant differences from the eucerin-treated group.‡< 0.05 indicates the significant differences from the GCBE at 5%, 15% and phenytoin-treated groups. GCBE, green coffee bean extract.
Hydroxyproline content estimation
The results of the hydroxyproline content assay of samples obtained from the experimental groups on day 7 after wounding showed significantly higher levels of hydroxyproline in animals treated with 10% GCBE compared to those in the no-treatment, eucerin treated (< 0.0001) and phenytoin (< 0.001) treated groups. There were no significant differences among other groups in the hydroxyproline content of tissue samples at day 7 post-injury. The hydroxyproline content of tissue samples obtained from the 10% GCBE (< 0.0001), 5% GCBE, 15% GCBE and phenytoin-treated groups (< 0.05) was significantly higher than that of the negative control and eucerin-treated groups on day 14 post-wounding. The hydrxyproline content of 10% GCBE was significantly higher (< 0.0001) than that of phenytoin-treated group on day 14 post wounding (Figure 4).
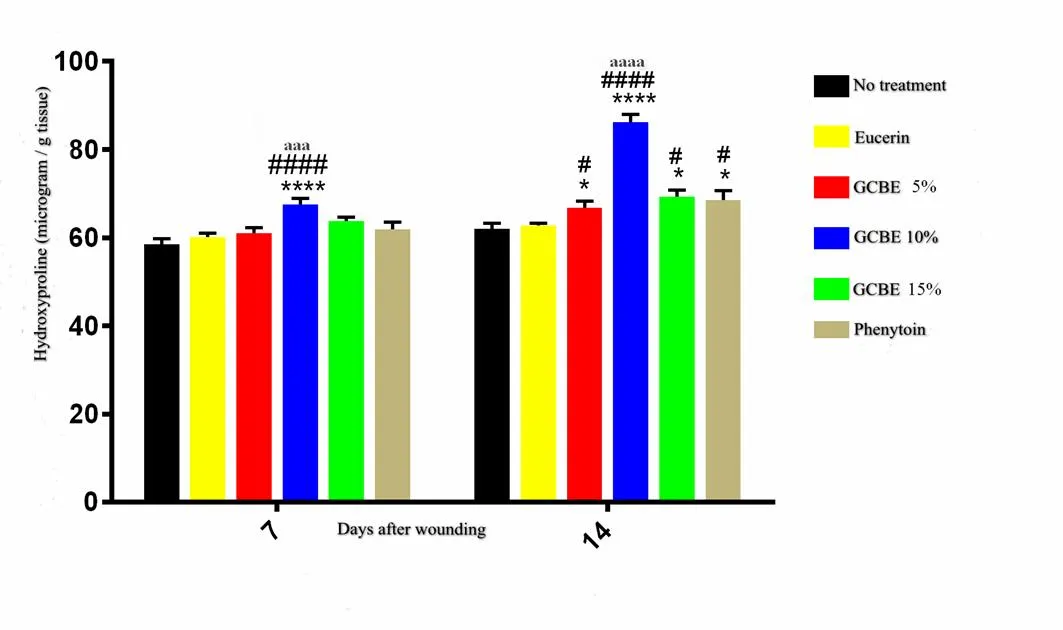
Figure 4 Effect of GCBE on hydroxyproline levels of wound-tissue samples from different groups on days 7 and 14 post-injury. Values are shown as mean ± standard deviation. *< 0.05 and ****< 0.0001 indicate the significant differences from the no-treatment group.#< 0.05 and####< 0.0001 indicate the significant differences from the eucerin-treated group.aaa< 0.001 andaaaa< 0.0001 indicate the significant differences from phenytoin-treated group. GCBE, green coffee bean extract.
Lipid peroxidation
The results of the lipid peroxidation assay on day 7 post-wounding showed significantly (< 0.05) lower levels of malondialdehyde (MDA) in wounds treated with GCBE 10% ointment compared to those of the negative control and vehicle-treated groups. There were no significant differences in MDA levels among other studied groups on day 7 post-injury. The MDA levels were decreased significantly (< 0.001) in skin samples obtained from the 5% GCBE, 10% GCBE and 15% GCBE treatment groups compared to the no-treatment and eucerin-treated groups groups on day 14 post-injury. GCBE treatment group showed superior effect than that of phehytoin-treated group on day 14 post-injury (Figure 5).
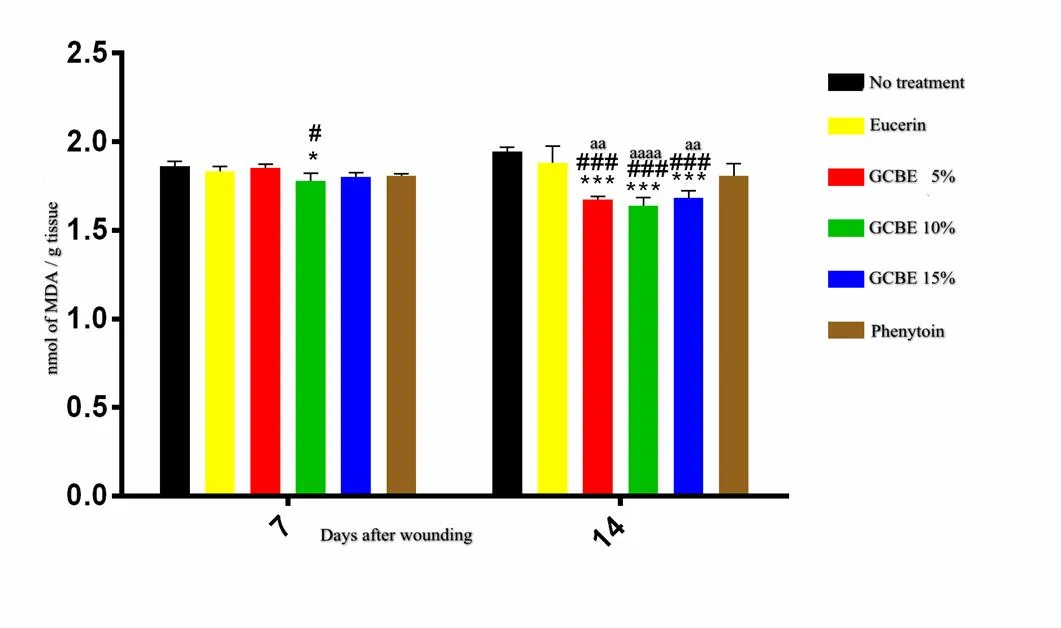
Figure 5 Effect of GCBE on lipid peroxidation of tissue samples from different experimental groups. Values are expressed as mean ± standard deviation. *< 0.05 and ***< 0.001 indicate significant differences from the no-treatment group.#< 0.05 and###< 0.001 indicate significant differences from the eucerin-treated group.aa< 0.01 andaaaa< 0.0001 indicate the differences between 5% and 15% GCBE and phenytoin-treated groups as well as 10% GCBE and phenytoin-treated groups, respectively. GCBE, green coffee bean extract; MDA, malondialdehyde.
Histopathological studies
Histological study of samples on day 7 demonstrated low levels of inflammatory cells, higher number of fibroblasts and enhanced granulation tissue formation in the 10% GCBE group compared to the negative control and vehicle-treated animals. In the negative control and vehicle-treated groups, persistent inflammation with noticeable tissue necrosis and little fibroblast proliferation was observed, indicating a poor rate of wound healing. In the 5% and 15% GCBE and phenytoin-treated wounds, there was evidence of fibroblast proliferation and granulation tissue formation. Tissue samples obtained from animals treated with 10% GCBE presented a higher area of re-epithelialization and enhanced neovascularization compared to samples of the negative control and vehicle-treated groups on day 14 post-injury. In the 5% GCBE group, re-epithelialization, dermal papillae and hair follicles were observed. In the 15% GCBE group, an absence of re-epithelialization and inflammatory cells infiltration were observed. In the phenytoin group, granulation tissue with numerous small vessels and fibroblast proliferation were observed (Figure 6).
Discussion
The main purpose of this study was to evaluate the wound-healing activity of GCBE through assessment of its effect on wound-closure rates, hydroxyproline content, lipid peroxidation and the histopathological state of the healing wounds.
Wound healing is a well orderly sequence of interacting processes categorized into four distinct but overlapping phases of hemostasis, inflammation, proliferation and remodeling. The events of wound healing must occur in a regular and precise manner. Wound healing can be impaired by multiple factors including improper oxygenation, infections, metabolic disorders, malnutrition and medication. Medicinal plants and plant-based products are a major source of wound-healing agents because of their antioxidant and antimicrobial properties [3].
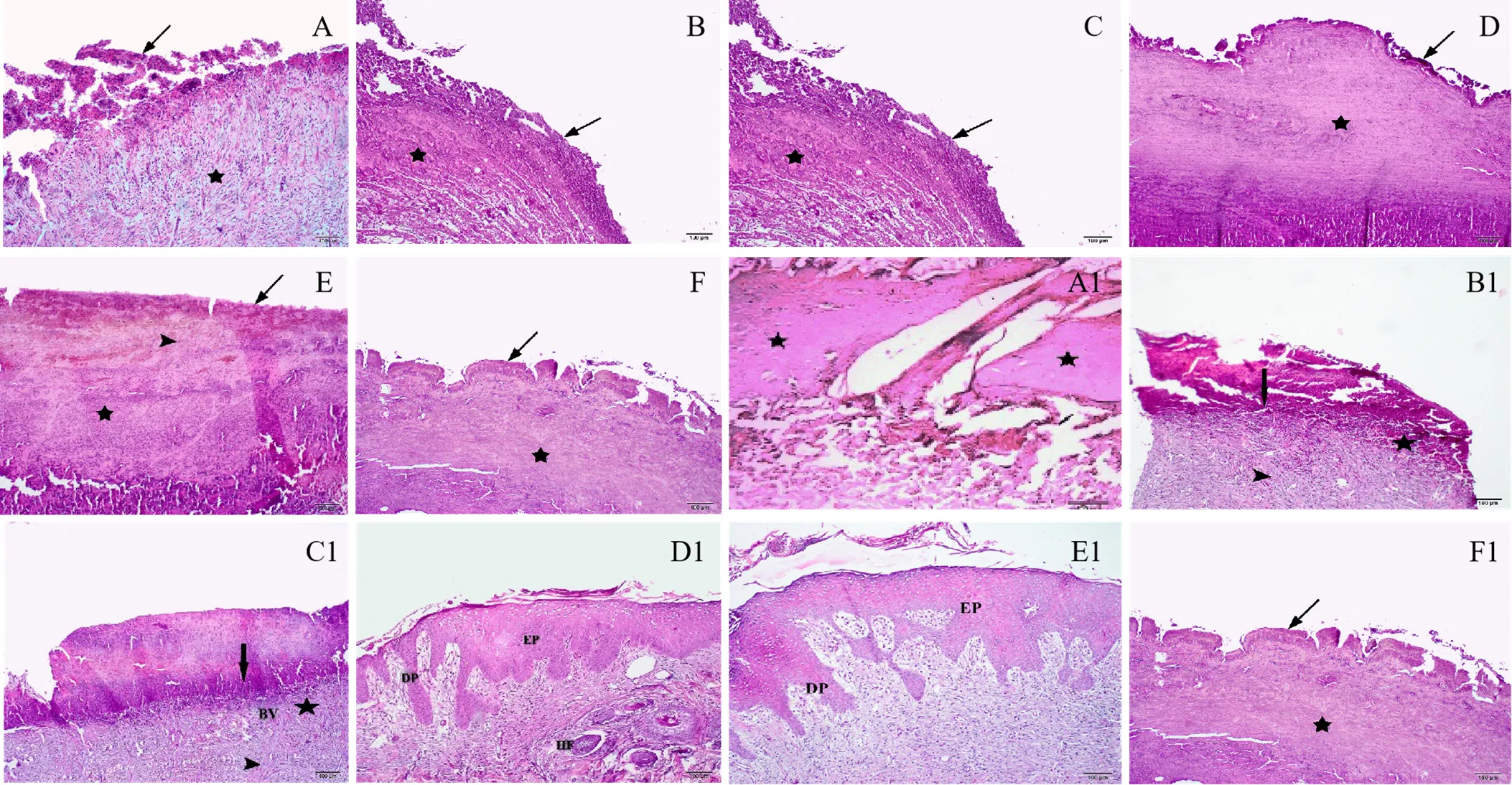
Figure 6 Histopathological changes of tissue samples from different groups on days 7 and 14 post-injury (hematoxylin-eosin). A to F: no-treatment group, eucerin group, phenytoin group, GCBE 5% group, GCBE 10% and GCBE 15%, respectively, on day 7 post-wounding. A1 to F1: no-treatment group, eucerin group, phenytoin group, GCBE 5% group, GCBE 10%, and GCBE 15%, respectively, on day 14 post-wounding. Arrow, coagulum; star, granulation tissue; arrowhead, inflammatory infiltration and fibroblasts. EP, epiderm; DP, dermal papillae; BV, blood vessel; HF, hair follicle.
is the most important species of thegenus as it accounts for about 70% of world coffee production. Coffee is an important source of polyphenols. Polyphenols have antioxidant activity that reduces oxidative stress [21]. The results of this study indicated that topical application of GCBE could suppress lipid peroxidation in wound tissue. Lipid peroxidation can contribute to wound-healing impairment through affecting fibroblasts, keratinocytes and endothelial cells’ metabolism and collagen synthesis [28]. Lipid peroxidation also may impair wound healing by altering vascular endothelial growth factor expression thus, inhibition of lipid peroxidation by GCBE may restore impaired vascular endothelial growth factor expression and stimulate angiogenesis and wound healing [29].
The wound-contraction rate was significantly higher in animals treated with 10% GCBE than in other experimental groups. Wound contraction functions to reduce the wound size and subsequently the wound area that needs repair. In full-thickness wounds, contraction is an important part of the wound-healing process through which collagen is synthesized by fibroblasts and results in connective tissue formation. After that, fibroblasts differentiate to myofibroblasts which function to pull the wound edges toward the center of the defect, reducing the size of the wound [30, 31]. GCBE may enhance the fibroblast proliferation and collagen synthesis that result in increased wound-closure rates. In addition, our results indicated that the hydroxyproline content of tissue samples from animals treated with 10% GCBE was significantly higher than other experimental groups on post-wounding and these results agreed with the results of the wound-contraction evaluation. Also, the significantly shorter period of epithelialization in animals treated with GCBE might be due to possible ability of GCBE to induce keratinocytes and fibroblast proliferation and migration, and to enhance collagen synthesis as well as to the antimicrobial properties of its phytochemicals [19, 32, 33]. However, further studies on the action of GCBE in the wound-healing process are necessary to clarify its role.
One of the main functions of intact skin is to prevent microbes that live on the skin surface from reaching underlying tissues. After dermal injury, exogenous microorganisms can gain access to subcutaneous tissues which provide a favorable (warm, moisture and nutrient-rich) environment for their growth and proliferation, potentially leading to severe infections [34]. There are supporting data for the treatment of wounds with natural antibiotics. GCBE possesses antimicrobial properties and these may be contributing to its wound-healing activity [19, 32].
The wound-healing activity of GCBE was also supported by histopathological studies. The histopathological analysis revealed a better wound-healing pattern in wounds treated with GCBE 10% compared to other experimental groups in this study. The histopathological examination showed that GCBE 15%-treated wounds had a slower rate of healing compared to 5% and 10% GCBE, and it indicated that GCBE in concentration ranges of 5% to 10% had a better effect on the wound-healing process in rabbits. Enhanced re-epithelialization in wounds treated with 5% and 10% GCBE supported the results of the wound-contraction assessment.
Conclusion
The results of the present study demonstrated that the hydroalcoholic extract of green coffee beans enhanced wound-healing activity in rabbits, which might be attributed to its antioxidant and antimicrobial properties. The GCBE and its chemicals possibly promote keratinocytes and fibroblast proliferation and migration which are critical during the wound-healing process. Further studies are needed to elucidate the precise mechanism of GCBE in the wound-healing process.
1. Atala A, Irvine DJ, Moses M, et al. Wound healing versus regeneration: role of the tissue environment in regenerative medicine. MRS Bull 2010, 35: 10.1557/mrs2010.1528.
2. Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med 2014, 6: 265sr266.
3. Guo S, DiPietro LA. Factors affecting wound healing. J Dent Res 2010, 89: 219–229.
4. Trostrup H, Bjarnsholt T, Kirketerp K, et al. What is new in the understanding of non healing wounds epidemiology, pathophysiology, and therapies. Ulcers 2013, 2013: 1–8.
5. Budovsky A, Yarmolinsky L, Ben-Shabat S. Effect of medicinal plants on wound healing. Wound Repair Regen 2015, 23: 171–183.
6. Patay ÉB, Bencsik T, Papp N. Phytochemical overview and medicinal importance ofspecies from the past until now. Asian Pac J Trop Med 2016, 9: 1127–1135.
7. Ross IA. Medicinal plants of the world. New Jersey: Humana Press, 2005.
8. Tabuti JRS, Lye KA, Dhillion SS. Traditional herbal drugs of Bulamogi, Uganda: plants, use and administration. J Ethnopharmacol 2003, 88: 19–44.
9. Belayneh A, Bussa NF. Ethnomedicinal plants used to treat human ailments in the prehistoric place of Harla and Dengego valleys, eastern Ethiopia. J Ethnobiol Ethnomed 2014, 10: 18.
10. Gruenwald J, Jaenicke C. Physicians’ desk reference for herbal medicines. New Jersey: Medical Economics Company, 2000.
11. Khorasani MḤA. Makhzan-ol-Advieh (Storehouse of Medicaments). Sabz Arang Tehran, 2009. (originally was written in 1771)
12. Perry LM. Medicinal plants of East and Southeast Asia. Massachusetts: Massachusetts Institute of Technology Press Cambridge, 1980.
13. Affonso RCL, Voytena APL, Fanan S, et al. Phytochemical composition, antioxidant activity, and the effect of the aqueous extract of coffee (L.) bean residual press cake on the skin wound healing. Oxid Med Cell Longev 2016, 2016: 1923754.
14. Bradbury AGW, Halliday DJ. Chemical structures of green coffee bean polysaccharides. J Agric Food Chem 1990, 38: 389–392.
15. Mussatto SI, Ballesteros LF, Martins S, et al. Extraction of antioxidant phenolic compounds from spent coffee grounds. Sep Purif Technol 2011, 83: 173–179.
16. Naidu MM, Sulochanamma G, Sampathu SR, et al. Studies on extraction and antioxidant potential of green coffee. Food Chem 2008, 107: 377–384.
17. Mazzafera P. Chemical composition of defective coffee beans. Food Chem 1999, 64: 547–554.
18. Brezová V, Šlebodová A, Staško A. Coffee as a source of antioxidants: an EPR study. Food Chem 2009, 114: 859–868.
19. Duangjai A, Suphrom N, Wungrath J, et al. Comparison of antioxidant, antimicrobial activities and chemical profiles of three coffee (L.) pulp aqueous extracts. Integr Med Res 2016, 5: 324–331.
20. Yi T, Shah M, Raulji D, et al. Comparative evaluation of antimicrobial efficacy of coffee extract and 0.2% chlorhexidine mouthwash on the periodontal pathogens,,, and: an in vitro study. Adv Hum Biol 2016, 6: 99–103.
21. Zain MZM, Baba AS, Shori AB. Effect of polyphenols enriched from green coffee bean on antioxidant activity and sensory evaluation of bread. J King Saud Univ Sci 2018, 30: 278–282.
22. Funakoshi-Tago M, Nonaka Y, Tago K, et al. Pyrocatechol, a component of coffee, suppresses LPS-induced inflammatory responses by inhibiting NF-κB and activating Nrf2. Sci Rep 2020, 10 (Suppl 1): 297–303.
23. Pergolizzi S, D'Angelo V, Aragona M, et al. Evaluation of antioxidant and anti-inflammatory activity of green coffee beans methanolic extract in rat skin. Nat Prod Res 2020, 34: 1535–1541.
24. Fukagawa S, Haramizu S, Sasaoka S, et al. Coffee polyphenols extracted from green coffee beans improve skin properties and microcirculatory function. Biosci Biotechnol Biochem 2017, 81: 1814–1822.
25. Tamri P, Hemmati A, Boroujerdnia MG. Wound healing properties of quince seed mucilage: in vivo evaluation in rabbit full-thickness wound model. Int J Surg 2014, 12: 843–847.
26. Pastar I, Stojadinovic O, Yin NC, et al. Epithelialization in wound healing: a comprehensive review. Adv Wound Care 2014, 3: 445–464.
27. Kondo T, Ishida Y. Molecular pathology of wound healing. Forensic Sci Int 2010, 203: 93–98.
28. Kim YW, Byzova TV. Oxidative stress in angiogenesis and vascular disease. Blood 2014, 123: 625–631.
29. Altavilla D, Saitta A, Cucinotta D, et al. Inhibition of lipid peroxidation restores impaired vascular endothelial growth factor expression and stimulates wound healing and angiogenesis in the genetically diabetic mouse. Diabetes 2001, 50: 667–674.
30. Manske RC. Postsurgical orthopedic sports rehabilitation. Saint Louis: Mosby Press, 2006, 3–18.
31. Albanna MZ, Holmes Iv JH. Skin Tissue Engineering and Regenerative Medicine. Boston: Academic Press, 2016, 83–108.
32. Runti G, Pacor S, Colomban S, et al. Arabica coffee extract shows antibacterial activity againstepidermidis andfaecalis and low toxicity towards a human cell line. LWT- Food Sci Technol 2015, 62: 108–114.
33. Velazquez Pereda Mdel C, Dieamant Gde C, Eberlin S, et al. Effect of greenL. seed oil on extracellular matrix components and water-channel expression in in vitro and ex vivo human skin models. J Cosmet Dermatol 2009, 8: 56–62.
34. Negut I, Grumezescu V, Grumezescu AM. Treatment strategies for infected wounds. Molecules 2018, 23: 2392.
:
Reza Shahriari contributed to data collection and analysis; Pari Tamri contributed to study design and supervision of research, data analysis and writing of manuscript; Amir Larki contributed to data collection and manuscript editing and proofreading; Alireza Nourian contributed to data collection and manuscript proofreading.
:
This study was financially supported by Vice-Chancellor of Research and Technology, Hamadan University of Medical Sciences, Hamadan, Iran (Gran no, 9509095210).
:
GCBE, green coffee bean extract; MDA, malondialdeyde.
:
The authors declare that there is no conflict of interest.
:
Reza Shahriari, Pari Tamri, Amir Larki Harchegani, et al. Green coffee bean hydroalcoholic extract accelerates wound healing in full-thickness wounds in rabbits. Traditional Medicine Research 2020, 5 (6): 433–441.
:Jing-Na Zhou.
: 05 March 2020,
18 May 2020,
:20 September 2020.
Pari Tamri, Department of Pharmacology & Toxicology, School of Pharmacy, Hamadan University of Medical Sciences, No. 417 Shahid Fahmideh Blvd, Hamadan 6517838678, Iran. Email: ptamri@gmail.com.
10.12032/TMR20200603191
 Traditional Medicine Research2020年6期
Traditional Medicine Research2020年6期
- Traditional Medicine Research的其它文章
- Jadwar (Delphinium denudatum Wall.): a medicinal plant
- Gastrointestinal effects of Artemisia absinthium Linn. based on traditional Persian medicine and new studies
- Evaluation of scientific evidence for abortifacient medicinal plants mentioned in traditional Persian medicine
- Effects of chicory (Cichorium intybus L.) on nonalcoholic fatty liver disease
- Is “Pangolin (Manis Squama) is not used in medicine" an improvement in the protection of precious and rare species or an improvement in the safety of using medicine?
- The gap between clinical practice and limited evidence of traditional Chinese medicine for COVID-19
