Effects of chicory (Cichorium intybus L.) on nonalcoholic fatty liver disease
Samira Faraji, Sevana Daneghian, Mohammad Alizadeh
Effects of chicory (L.) on nonalcoholic fatty liver disease
Samira Faraji1, 2, Sevana Daneghian3*, Mohammad Alizadeh3
1Student Research Committee, Urmia University of Medical Sciences,Urmia, Iran.2Department of Nutrition, School of Medicine, Urmia University of Medical Sciences, Urmia, Iran.3Food and Beverages Safety Research Center, Urmia University of Medical Sciences, Urmia, Iran.
There is a dramatic increase in the prevalence of nonalcoholic fatty liver disease, which is slowly turning into a pandemic as well as a major challenge across the world. Nonalcoholic fatty liver disease is described as a range of liver conditions such as fat accumulation, hepatic steatosis, or end-stage liver disease. Patients with nonalcoholic fatty liver disease are asymptomatic and their mortality is higher than people without nonalcoholic fatty liver disease. The pathogenesis of nonalcoholic fatty liver disease has not been clearly determined yet. The “two hits” hypothesis is designed to explain the pathogenesis of nonalcoholic fatty liver disease. Dyslipidemia, oxidative stress, insulin resistance, obesity, and chronic inflammation are some of the morbidities involved in the progression of nonalcoholic fatty liver disease. Chicory (L.) is an herbaceous perennial, known as chicory. Chicory contains various compounds, such as vitamins, sonchuside A, caffeic acid derivatives, fructo-oligosaccharides, chlorogenic acid, magnolialide, polysaccharides, coumarins, phenolic acids, terpenoids, flavonoids, polyphenol, cichoriosides, ixerisosides, eudesmanolides, inulin, bitter sesquiterpene lactones, and alkaloids. Current research has revealed that chicory supplementation might be effective in the treatment of nonalcoholic fatty liver disease. The anti-inflammatory, antihepatotoxic, antihyperlipidemic, antidiabetic, antihyperglycemic, and antioxidant properties of chicory provide plausible mechanisms by which chicory may affect the various steps of disease progression and severity. Existing studies have shown that chicory supplementation has beneficial effects on nonalcoholic fatty liver disease, but the existence of only one human study and possible side effects of chicory necessitate further studies.
Nonalcoholic fatty liver disease, Two hits, Chicory (L.), Inflammation, Oxidative stress
The purpose of this study is to investigate the recent studies and findings on the effects of chicory (L.) on nonalcoholic fatty liver disease-related factors, such as dyslipidemia, oxidative stress, insulin resistance, obesity, and chronic inflammation, and disclose their underlying mechanisms.
Chicory (L.) is a part of the Asteraceae family (tribe of Lactuceae) and a medicinal food known as chicory now. The use of chicory dates to ancient Egypt, but the plant was used even before it was identified. The Egyptians cultivated chicory as a medicinal plant 4,000 years ago. They believed that the plant could help in purifying the blood and liver and treat heart disease. Horace’s Roman poem is one of the first references that recommended chicory consumption (65−8 B.C.E.). Its great importance can attribute to the fact that Avicenna had written a treatise on chicory and its properties. Chicory was transported from Europe to North America in the 1700s. In the early 17th century, chicory was started as an animal feed in Northern Europe. In 2000, the French Food Safety Agency confirmed that inulin is an ingredient of chicory, and it increases the proliferation of intestinal flora. Chicory was used as a coffee substitute during the Napoleonic Era. Evidence suggests that the soldiers used it in the American Civil War. This plant has been introduced as a native plant to the regions of Western Asia, Europe, and North Africa by the Food and Agriculture Organization.

Background
Nonalcoholic fatty liver disease (NAFLD) is gradually turning into a pandemic as well as a major challenge across the world, because of its high prevalence, difficult diagnosis, multifactor pathogenesis, and lack of suitable therapies [1]. The prevalence of this disease is 25% around the world, and its highest prevalence (31.8%) is reported in the Middle East[2]. The National Health and Nutrition Examination Survey, an eight-year study, revealed that the overall mortality in patients with NAFLD (35%) was about sevenfold as compared with the patients without NAFLD (5%) [3]. NAFLD has two different types: the first one is nonalcoholic fatty liver that is known as the accumulation of fat without hepatocellular injury; whereas the second one is the nonalcoholic steatohepatitis (NASH), which is worse than nonalcoholic fatty liver and is characterized by the accumulation of fat, as well as inflammation and injury of hepatocytes, with or without any fibrosis. NASH can progress into cirrhosis or may lead to hepatocellular carcinoma [4].
NAFLD is associated with chronic diseases such as type 2 diabetes mellitus (T2DM), metabolic syndrome, and cardiovascular diseases [5, 6]. Also, this chronic disease has a relationship with other metabolic disorders such as obesity, oxidative stress, inflammation, dysglycemia, and dyslipidemia [7, 8]. One of the most crucial hypotheses in the pathogenesis of NAFLD is the “two hits” hypothesis [9]. The “first hit” is the accumulation of lipids in the liver cells. Insulin resistance plays a role in the development of fat accumulation. Steatosis increases the uptake of fatty acids and consequently increases the production of triglycerides (TG) [10]. The “first hit” causes the disease to progress to the “second hit”. Lipid peroxidation, oxidative stress, dysfunction of mitochondria, adipokines, and cytokines manifest in the second hit (Figure 1) [11].
The most commonly used medications for the treatment of NAFLD are liraglutide, dipeptidyl peptidase-4 inhibitors, metformin, thiazolidinediones, sodium-glucose transport protein 2 inhibitor, vitamin E, and sulfonylurea [12, 13]. Despite the positive therapeutic effects of abovementioned treatments, various side effects have also been reported. A study has reported gastrointestinal side effects of using liraglutide [14]. Weight gain, cardiovascular side effects, bone fractures, and peripheral edema are some of the side effects that have been demonstrated in the use of thiazolidinediones [12]. Sodium-glucose transport protein-2 inhibitors can increase the infection of urinary and genital tract [15]. Headache, hypoglycemia, infection of respiratory and urinary tracts, and nasopharyngitis have been reported after using dipeptidyl peptidase-4 inhibitors [16, 17]. Nausea, diarrhea, renal problems, and lactic acidosis are the adverse reactions of metformin [12]. Finally, sulfonylurea has a direct relationship with the risk of hepatocellular carcinoma [17].
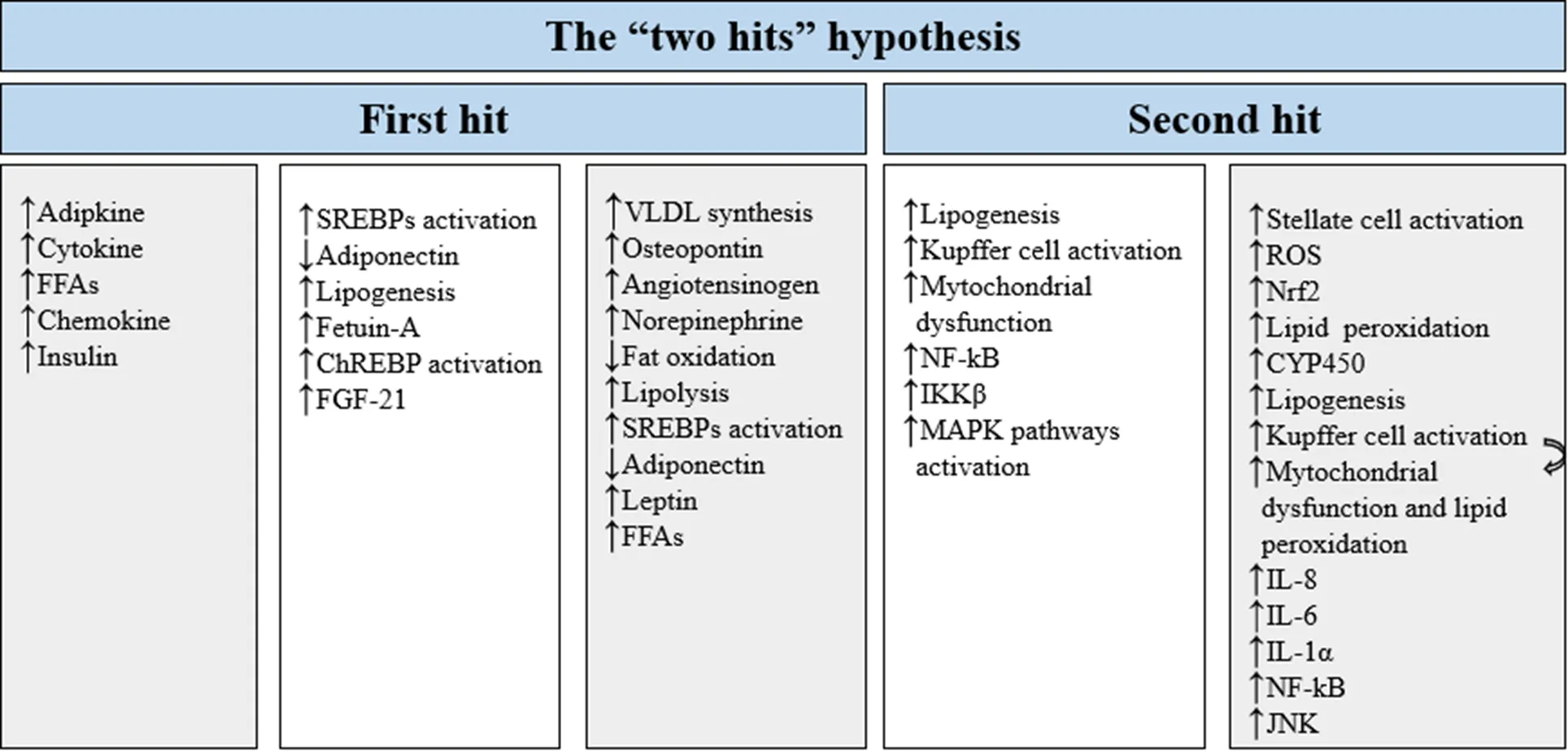
Figure 1 Two hits hypothesis. The “two hits” hypothesis is designed to explain the pathogenesis of NAFLD, with the “first hit” including fat accumulation in the liver, elevated liver enzymes, abdominal obesity, and insulin resistance; and the “second hit” including cellular stresses such as oxidative stress, fibrogenesis, and apoptosis. FFAs, free fatty acids; ChREBP,carbohydrate-responsive element-binding protein; FGF-21, fibroblast growth factor 21; SREBPs, sterol regulatory element-bindings; VLDL, very low-density lipoprotein; NF-κB, nuclear factor-κb; IKKβ, IKβ kinase β; MAPK, mitogen-activated protein kinase; ROS, reactive oxygen species; CYP450, cytochrome P450; Nrf2, nuclear factor erythroid 2-related factor 2; IL-8, interleukin 8; IL-6, interleukin 6; IL-1α, interleukin-1 alpha; JNK, c-Jun N-terminal kinase.↑shows increase, and ↓ shows decrease.
Therapeutic options for NAFLD are limited and herbal medicine may offer an alternative treatment for NAFLD because of the cultural, economic, and practical reasons as well as low side effects and benefits [18, 19]. The most commonly used herbal medicine for patients with NAFLD are resveratrol, Qianggan and Yiqi Sanjuformulas of traditional Chinese medicine, berberine,, soluble fibers, and silymarin/silybin[18, 20−24]. Herbal medications may have some side effects too. Moreover, the consumption of silybin can cause dysgeusia and pruritus [25]. Qianggan formula in capsule form consists of 16 herbs such asL.,Pall.,,,, and some other components can cause nausea, burning sensation, and diarrhea [24, 26]. The consumption of berberine led to anorexia, stomach problems, diarrhea, and constipation [27]. Yiqi Sanjuformula consists ofand other herbscancause diarrhea, as well as gastrointestinal and appetite problems [24].can cause dyspepsia, diarrhea, headache, bleeding of per-rectal and gums, chest pain, cough, decreased vision [28]; and, leucopoenia, and thrombocytopenia are some of the adverse effects of using resveratrol [24].
Chicory (L.) is a part of the Asteraceae family (tribe of Lactuceae) and a medicinal food known as chicory now [29]. The Egyptians cultivated chicory as a medicinal plant 4,000 years ago [30]. They believed that the plant could help in purifying the blood and liver and treat heart disease. Horace’s Roman poem is one of the first references that recommended the consumption of chicory (65−8 B.C.E.). Its great importance can attribute to the fact that Avicenna has written a treatise on chicory and its properties (691 C.E.). Chicory was transported from Europe to North America in the 1700s. In the early 17th century, chicory was started as an animal feed in Northern Europe. In 2000, the French Food Safety Agency confirmed that inulin is an ingredient of chicory, and it increases the proliferation of intestinal flora. Chicory was used as a substitute of coffee during the Napoleonic Era. Evidence suggests that the soldiers used it in the American Civil War. Also, the chicory root was used in the United States instead of bean drinks. This plant has been introduced as a native plant to the regions of Western Asia, Europe, and North Africa by the Food and Agriculture Organization [31]. Literature has shown that the important constituents of chicory are vitamins, sonchuside A, caffeic acid derivatives, fructo-oligosaccharides, chlorogenic acid, magnolialide, polysaccharides, coumarins, phenolic acids, terpenoids, flavonoids, polyphenol, cichoriosides, ixerisosides, eudesmanolides, inulin, bitter sesquiterpene lactones, and alkaloids [22, 23, 29]. Chicory has antioxidant, antibacterial, antipyretic, antidiabetic, antihepatotoxic, anti-inflammatory, antiulcerogenic (root), antihyperglycemic, and antihyperlipidemic, anticancer and antimalarial activities, which have been successfully demonstrated in several studies [29, 32−37]. The purpose of this study is to investigate the recent studies and findings on the effects of chicory on the NAFLD-related factors.
2012年6月19日,中国煤炭加工利用协会、中国煤炭工业协会选煤分会在北京21世纪饭店联合举办了2012′中国选煤发展论坛。论坛由中国煤炭加工利用协会副理事长、中国煤炭工业协会选煤分会会长张绍强主持,中国煤炭加工利用协会副理事长蔡明华,中国煤炭工业协会选煤分会副会长魏丕杰、符东旭、李明辉,高级顾问周少雷,秘书长马剑,乌克兰和印度选煤协会主席出席了论坛,来自全国选煤行业的140多名代表参加了本次论坛。
The effect of chicory on obesity and NAFLD
Obesity is an increasingly significant public health issue in the world[38, 39].The degree of obesity (excess weight, obese, and severely obese) is closely associated with NAFLD, and it is a strong risk factor for a lot of entities, including NAFLD [8, 40]. The prevalence of NAFLD in people with obesity is reported to be about 80%[41, 42]. Weight loss and exercise are the most essential lifestyle changes for the management and treatment of patients with NAFLD[43, 44]. The effect of body weight loss on the treatment of NAFLD is related to the amount of weight loss. Up to 7−10% of weight loss should be the primary aim of patients but even 3−5% of weight loss can improve steatosis, more than 5% can decrease inflammation, more than 7% can cause a notable decrease in steatohepatitis, and 10% or more weight reduction is suitable for the treatment of fibrosis[45].
In addition, obesity is strongly linked to insulin resistance and high inflammatory markers such as interleukin-1 and tumor necrosis factor alpha (TNF-α); therefore, weight loss may decrease these factors and can improve NAFLD in this way [5]. Also, Sookoian et al. discovered that fibrosis score and NAFLD activity score were higher in patients with both obesity and NAFLD [46].
Many researchers have studied the effects of consumption of chicory in patients with NAFLD on body composition [33, 47−50]. In some human and animal studies, the authors have shown that chicory can lead to weight loss [47, 48]. Presumably, there is no human randomized control trial (RCT) study, except the study of Ghaffari et al. that examined the effects of chicory seed and turmeric supplementation on patients with NAFLD [21]. In the abovementioned study of 2019, there was a decrease in waist circumference, weight, and body mass index in patients who used chicory and turmeric plus chicory as compared to placebo (< 0.05) [21]. In only two animal studies, chicory supplementation in rats significantly reduced the weight. A study conducted by Cho et al. in 2010, showed that after an intervention of 14 days, the supplementation of chlorogenic acid and caffeic acid, the two important constituents of chicory, decreased weight and visceral fat mass in eight male mice[47]. In addition, weight had a correlation with insulin (< 0.01) and leptin (< 0.01). Even in this study, the effect of chlorogenic acid was more effective than caffeic acid in reducing the body weight and regulating the lipid metabolism. In the study of Wu et al., 48 male Sprague Dawley rats received chicory supplementation and a high fat diet (fat = 45%) for four days [33]. After the intervention, their body weights changed significantly. However, a study of 46 patients with diabetes showed that there was not any difference in the body weight between two groups after supplementing a daily dose of 10 g of chicory for 2 months [48]. According to the inconsistent results of the abovementioned and some other studies, chicory supplementation is likely to have a positive effect on weight [49, 50].
The effect of chicory on insulin resistance and NAFLD
The development of NASH conforms to the “two hits” hypothesis. The “first hit” involves the accumulation of fat in the liver and dysglycemia, whereas the “second hit” includes cellular stresses such as oxidative stress-mediated inflammation, fibrogenesis, and apoptosis [9, 10]. The prevalence of NAFLD in patients with diabetes has not exactly been known. Recent studies have shown that the prevalence of NAFLD in patients with diabetes has been increased rapidly over the past two decades worldwide as compared with the individuals without diabetes [51, 52]. The several lines of evidence have estimated that the prevalence rates of NASH and fibrosis in patients with diabetes and NAFLD are approximately 69.2% and 41.0%, respectively [53]. There is a bidirectional association between NAFLD and T2DM, in which NAFLD appears to enhance the risk for T2DM, and T2DM may promote NAFLD progression; however, additional investigation is needed to determine accurate results [8]. Putting together, it can be stated that there is a relationship between NAFLD and insulin resistance [54].
There is no human RCT study, except the study by Ghaffari et al. that examined the effects of consumption of chicory seed and turmeric on insulin resistance in patients with NAFLD [21]. After 12 weeks of intervention, homeostatic model assessment for insulin resistance decreased in both groups that received chicory seed only (9 g/d (4.5 g/100 mL)) and that received turmeric with chicory seed (3 g/d turmeric plus infused 9 g/d chicory seed). It should be noted that in one animal study by Ghamarian et al., the level of glycated hemoglobin A1c and fasting blood sugar decreased significantly after 28 days of chicory extract injections (125 mg/kg body weight), but in the group of rats with early-stage diabetes, unlike late-stage diabetes, fasting serum insulin concentration were higher at the end of the study [55]. Apart from that, in another animal study that was conducted on 32 high-fat-diet-induced obese mice, the insulin levels decreased after 14 days supplementation of caffeic acid and chlorogenic acid [47]. In another study, 46 female patients with diabetes were divided into two groups: the first was placebo (n = 22) and the second was intervention group that received enriched treatment of chicory (n = 27) in form of a daily dose of 10 g of chicory. In this study after two months, the fasting serum glucose levels decreased in the intervention group [48]. As previously suggested by others, the antihyperglycemic effect of chicory has been reported in other experiments about ferulic, caffeic, and chicoric acids in roots of chicory [56, 57]. Because of the lack of human studies to investigate the effect of this traditional medicine plant on fatty liver, there is an urgent need of more human studies. However, it should be noted that the studies should consider all the possible side effects of chicory.
The effect of chicory on dyslipidemia and NAFLD
According to the “two hits” hypothesis, NAFLD is related to the excess levels of triacylglycerol in liver, which is known as steatosis [58]. This condition may occur because of a problem in any of the pathways that are related to the delivery, synthesis, export, or oxidation of triacylglycerol, synthesis of very low-density lipoprotein, increased lipolysis, and liver free fatty acids (FFAs) uptake, which had mentioned in the “first hit”[54, 59]. Circulating FFAs, which represent the main source of hepatic fat accumulation, are originated from lipolysis[60].
As a consequence, patients with NAFLD usually have high TG, increased very low-density lipoprotein, and high apolipoprotein B-to-apolipoprotein A‐1 ratio, as well as a higher concentration of small dense LDL coupled with low high‐density lipoprotein concentration [7, 61]. On the contrary, saturated fatty acids activate the path of the c-Jun terminal kinase and contribute to the development of hepatic steatosis and insulin resistance [62].
The prevalence of dyslipidemia (hyper- cholesterolemia, hypertriglyceridemia, or both) in individuals with NAFLD has been estimated to be between 20% and 80% [63]. Other studies on prevalence of NAFLD in individuals with dyslipidemia had demonstrated, more than 50% of individuals with abnormality in amount of lipids have NAFLD[8, 33]. Recently, it is presented that the “two hits” hypothesis is outdated, but still it can explain the mechanism of lipotoxicity in NAFLD, in the best way.
Based on the evidence, several studies have shown that chicory can affect lipid profiles[21, 33, 47, 50]. But, in the study conducted by Ghaffari et al., chicory seed supplementation as long as 12 weeks had no effect on the lipid profile in the chicory seed group [21]. In this study, subjects were divided into four groups: the first group received (3 g/d) turmeric, the second group received (9 g/d) chicory seed, the third group received turmeric (3 g/d) plus chicory seed supplementation (9 g/d), and the fourth group received placebo. In the turmeric group and turmeric plus chicory seed group, total cholesterol, LDL-cholesterol, and TG levels decreased significantly after the end of the treatment. The significance of lipid profile changes in turmeric group and turmeric plus chicory group as compared to chicory group can be attributed to the turmeric effect in both groups.
In another study, after supplementation with caffeoylquinic acid as a chicory extracted ingredient, no effect was observed on the lipid profile[66]. The method of investigation that lasted for 28 days was as follows: (1) during the first two weeks of feeding, rats were on a high cholesterol and fructose diet; (2) in the second two weeks, the diet of rats was high on caffeoylquinic acid. Probably other components of chicory have the ability to change lipid profile. In two studies related to this issue, Zhu et al. in 2019 and Wu et al. in 2018 found that chicory supplementation in rats decreased the total cholesterol, TG, and LDL and increased high‐density lipoprotein levels [33, 50]. In another study by Cho et al., caffeic acid and chlorogenic acid caused a positive effect on TG and cholesterol levels after 14 days [47]. According to the results reported above and other available studies, the positive effects of chicory have been observed on lipid metabolism [55, 67]. In summary, there is considerable evidence that links chicory supplementation with lipid homeostasis and NAFLD.
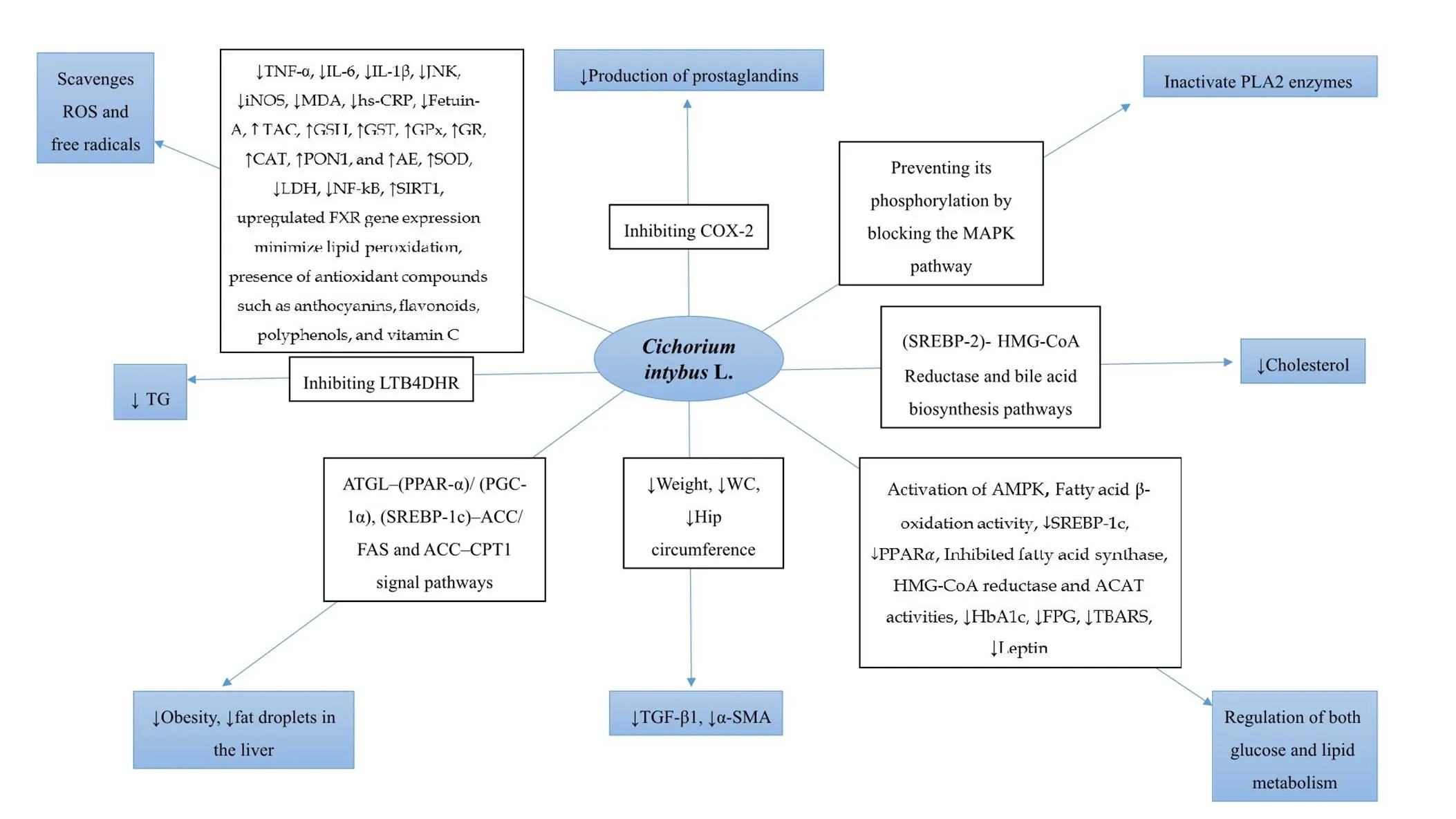
Figure 2 Relationship betweenL. and the NAFLD-related factors. ROS, reactive oxygen species; TNF-α, tumor necrosis factor alpha; IL-6, interleukin 6; IL-1β, interleukin 1 beta; JNK, c-Jun N-terminal kinase; iNOS, inducible nitric oxide synthase; MDA, malondialdehyde; hs-CRP, high-sensitivity C-reactive protein; TAC, total antioxidant capacity; GSH, glutathione; GST, glutathione S-transferase; GPx, glutathione peroxidase; GR, glutathione reductase; CAT, catalase; PON1, paraoxonase 1; AE, arylesterase; SOD, superoxide dismutase; LDH, lactate dehydrogenase; NF-κB, nuclear factor-κb; SIRT1, sirtuin 1; FXR, farnesoid‐X‐receptor; Cox-2, cyclooxygenase-2; PLA2, phospholipase A2; MAPK, mitogen-activated protein kinase; SREBP-2, sterol regulatory element-binding protein 2; HMG-CoA, 3-hydroxy-3-methyl-glutaryl-coenzyme A; AMPK, adenosine 5'-monophosphate-activated protein kinase; SREBP-1c, sterol regulatory element-binding protein-1c; PPAR-α, peroxisome proliferator-activated receptor alpha; ACAT, acyl-coenzyme A cholesterol acyltransferase; HbA1c, glycosylated hemoglobin; FPG, fasting plasma glucose; TBARS, thiobarbituric acid-reactive substances; TGF-β1, transforming growth factor-β1; α-SMA, alpha-smooth muscle actin; WC, waist circumference; ATGL, adipose triglyceride lipase; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; ACC, acetyl-CoA carboxylase; FAS, fatty acid synthase; CPT1, carnitine palmitoyl transferase I; TG, triacylglycerol; LTB4DHR, leukotriene B4 dehydrogenase. ↑shows increase, and ↓ shows decrease.
The effect of chicory on inflammation and NAFLD
According to the second hit in the “two hits” model, systemic chronic inflammation plays a basic role in the pathogenesis of NAFLD, because the increasing levels of proinflammatory cytokines can increase the risk of NAFLD [68]. The accumulation of lipids in the hepatocytes leads to redox imbalance and fat oxidation, thereby causing the inflammatory response of proinflammatory cytokines such as high-sensitivity C-reactive protein and TNF-α[69]. Among the inflammatory markers, TNF-α and interleukin 6 (IL-6) play a basic role in causing the inflammation [70−73]. TNF-α plays a role in both the early and later stages of NAFLD [73−76]. Different studies have shown a direct association of TNF-α expression with the severity of NAFLD and degree of fibrosis in patients with NASH [21, 69, 70]. Studies conducted on humans have showed that the expression of IL-6 is related to the degree of inflammation and fibrosis[21, 77]. It should be noted that the contribution of liver in the construction of the IL-6 is presently unclear. Moreover, the adipose tissue is currently one of the main production sites for IL-6 [78].
Different animal studies have shown the role of IKβ Kinase β and NF-κB signaling in NAFLD, but these studies do not have strong findings for humans [79]. One clinical study found that the expression of nuclear factor-Κb p65 was increased in the patients with NAFLD (20 patients with NASH, 40 patients with alcoholic steatohepatitis); however, further research is needed to determine the role of this pathway in the patients with NAFLD[80].
In searching for the anti-inflammatory activity of chicory, Cavin et al. found that the extract of chicory and ethyl acetate can suppress TNF-α and prostaglandin E2 in the human colon HT29 cells, respectively [81]. The study of Ghaffari et al. demonstrated that the supplementation of chicory seeds (infused 9 g/d (4.5 g/100 mL)) for 12 weeks significantly decreased the level of TNF-α [21]. The levels of high-sensitivity C-reactive protein and IL-6 decreased significantly in the participants that received turmeric and chicory seed supplements as well. The individuals were 92 patients with NAFLD aged 20−60 years old (body mass index = 24.9−40 kg/m2). Various mechanisms suggest that chicory appears to have an anti-inflammatory activity, but there is no study on the effects of different parts of chicory on the inflammation; therefore, further human and animal studies are needed.
The effect of chicory on oxidative stress and NAFLD
The role of oxidative stress in NASH and NAFLD is described in the “two hits” hypothesis[68]. Oxidative stress is caused by a cellular imbalance and production of reactive oxygen species [82, 83]. In these conditions, the increased lipid peroxidation leads to the activation of liver stellate cells and, thus, to fibrogenesis[82, 83]. Reactive oxygen species are main intermediates of inflammation [82]. As a consequence, FFAs, inflammatory factors, and TNF-α lead to oxidative stress [53, 84]. Indeed, several studies have a strong relation between the severity of NASH and the degree of oxidative stress[85].
Ghaffari et al.’s study is the only study that found the relationship between chicory supplementation and oxidative stress indicators [21]. Their mentioned study found that malondialdehyde were decreased and the total antioxidant capacity levels were increased significantly after chicory intake. The relationship between chicory and improvement in the blood antioxidant status are noted in a majority of animal studies [33, 34, 50, 66, 86, 87]. Only in one study conducted by Saggu et al., rats that used 4-tert-octylphenol for 8 weeks had decreased glutathione, superoxide dismutase, and catalase levels, whereas a reduction in thiobarbituric acid-reactive substances recorded in the rats that received chicory fruit extract with 4-tert-octylphenol [49]. The observed results mean that the chicory fruit extract could improve the abnormalities resulting from 4-tert-octylphenol by decreasing thiobarbituric acid-reactive substances and the pronounced improvement of the investigated biochemical and antioxidant parameters. These mentioned studies justify and validate the antilipid peroxidation and antioxidant effects of chicory.
Possible side effects of chicory
Despite the positive effects of chicory on NAFLD, like other treatments for NAFLD, the possibility of adverse effects for this herbal treatment is not unthinkable, but only one study by Farhangi et al. has shown some side effects of using this plant after two months [48]. They evaluated the effects of chicory inulin on the enzymes of liver, metabolism of calcium, and blood factors in women with T2DM. In this study, chicory significantly decreased hematocrit and mean corpuscular volume (< 0.05).
Conclusion
Due to the “two hits” hypothesis of NAFLD pathogenesis, NAFLD is related to dysglycemia, dyslipidemia, oxidative stress, inflammation, and obesity[88]. The anti-inflammatory, antihepatotoxic, antihyperlipidemic, antidiabetic, antihyperglycemic, and antioxidant properties of chicory provide plausible mechanisms by which chicory may affect the various steps of disease progression and severity (Figure 2). The current research has suggested that chicory supplementation might be effective in the treatment of NAFLD. However, there are two reasons that justify the need for a more well-designed research in humans. First, RCTs investigating the effects of chicory on NAFLD are limited and there is only one study that had been conducted on patients with NAFLD [21]. Second, despite the positive effects of chicory, the evaluation of possible side effects by RCTs is necessary.
1. Neuschwander-Tetri BA. Non-alcoholic fatty liver disease. BMC Med 2017, 15: 45.
2. Iqbal U, Perumpail BJ, Akhtar D, et al. The epidemiology, risk profiling and diagnostic challenges of nonalcoholic fatty liver disease. Medicines (Basel) 2019, 6: 41.
3. Bakhshimoghaddam F, Shateri K, Sina M, et al. Daily consumption of synbiotic yogurt decreases liver steatosis in patients with nonalcoholic fatty liver disease: a randomized controlled clinical trial. J Nutr 2018, 148: 1276−1284.
4. Khan FZ, Perumpail RB, Wong RJ, et al. Advances in hepatocellular carcinoma: nonalcoholic steatohepatitis-related hepatocellular carcinoma. World J Hepatol 2015, 7: 2155−2161.
5. Dietrich P, Hellerbrand C. Non-alcoholic fatty liver disease, obesity and the metabolic syndrome. Best Pract Res Clin Gastroenterol 2014, 28: 637−653.
6. Moazzen H, Alizadeh M. Effects of pomegranate juice on cardiovascular risk factors in patients with metabolic syndrome: a double-blinded, randomized crossover controlled trial. Plant Foods Hum Nutr 2017, 72: 126−133.
7. Katsiki N, Mikhailidis DP, Mantzoros CS. Non-alcoholic fatty liver disease and dyslipidemia: an update. Metabolism 2016, 65: 1109−1123.
8. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012, 55: 2005−2023.
9. Fernando DH, Forbes JM, Angus PW, et al. Development and progression of non-alcoholic fatty liver disease: the role of advanced glycation end products. Int J Mol Sci 2019, 20: 5037.
10. Marzuillo P, Grandone A, Perrone L, et al. Understanding the pathophysiological mechanisms in the pediatric non-alcoholic fatty liver disease: The role of genetics. World J Hepatol 2015, 7: 1439−1443.
11. Dowman JK, Tomlinson JW, Newsome PN. Pathogenesis of non-alcoholic fatty liver disease. QJM 2009, 103: 71−83.
12. Tacelli M, Celsa C, Magro B, et al. Antidiabetic drugs in NAFLD: the accomplishment of two goals at once? Pharmaceuticals (Basel) 2018, 11: 121.
13. Hardy T, Anstee QM, Day CP. Nonalcoholic fatty liver disease: new treatments. Curr Opin Gastroenterol 2015, 31: 175−183.
14. Guss DA, Mohanty SR. Liraglutide’s use in treatment of non-alcoholic fatty liver: an evaluation of the non-alcoholic steatohepatitis study. Hepatobiliary Surg Nutr 2016, 5: 515−518.
15. Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2013, 159: 262−274.
16. Cox ME, Rowell J, Corsino L, et al. Dipeptidyl peptidase-4 inhibitors in the management of type 2 diabetes: safety, tolerability, and efficacy. Drug Healthc Patient Saf 2010, 2: 7−19.
17. Singh S, Singh PP, Singh AG, et al. Anti-diabetic medications and the risk of hepatocellular cancer: a systematic review and meta-analysis. Am J Gastroenterol 2013, 108: 881−891; quiz 892.
18. Xiao J, Fai So K, Liong EC, et al. Recent advances in the herbal treatment of non-alcoholic fatty liver disease. J Tradit Complement Med 2013, 3: 88−94.
19. Zhang L, Yao Z, Ji G. Herbal extracts and natural products in alleviating non-alcoholic fatty liver disease via activating autophagy. Front Pharmacol 2018, 9: 1459.
20. El-Din SH, Sabra AN, Hammam OA, et al. Pharmacological and antioxidant actions of garlic and.or onion in non-alcoholic fatty liver disease (NAFLD) in rats. J Egypt Soc Parasitol 2014, 44: 295−308.
21. Ghaffari A, Rafraf M, Navekar R, et al. Turmeric and chicory seed have beneficial effects on obesity markers and lipid profile in non-alcoholic fatty liver disease (NAFLD). Int J Vitam Nutr Res 2019, 89: 293−302.
22. Hussain M, Habib Ur R, Akhtar L. Therapeutic benefits of green tea extract on various parameters in non-alcoholic fatty liver disease patients. Pak J Med Sci 2017, 33: 931−936.
23. Lulu SS, Thabitha A, Vino S, et al. Naringenin and quercetin--potential anti-HCV agents for NS2 protease targets. Nat Prod Res 2016, 30: 464−468.
24. Bagherniya M, Nobili V, Blesso CN, et al. Medicinal plants and bioactive natural compounds in the treatment of non-alcoholic fatty liver disease: A clinical review. Pharmacol Res 2018, 130: 213−240.
25. Loguercio C, Andreone P, Brisc C, et al. Silybin combined with phosphatidylcholine and vitamin E in patients with nonalcoholic fatty liver disease: a randomized controlled trial. Free Radic Biol Med 2012, 52: 1658−1665.
26. Zhu M, Li M, Zhou W, et al. Qianggan extract improved nonalcoholic steatohepatitis by modulating lncRNA/circRNA immune ceRNA networks. BMC Complement Altern Med 2019, 19: 156.
27. Yan HM, Xia MF, Wang Y, et al. Efficacy of berberine in patients with non-alcoholic fatty liver disease. PLoS One 2015, 10: e0134172.
28. Wong VW, Wong GL, Chan AW, et al. Treatment of non-alcoholic steatohepatitis with Phyllanthus urinaria: a randomized trial. J Gastroenterol Hepatol 2013, 28: 57−62.
29. Satmbekova D, Srivedavyasasri R, Orazbekov Y, et al. Chemical and biological studies onL. Nat Prod Res 2018, 32: 1343−1347.
30. Street RA, Sidana J, Prinsloo G.: traditional uses, phytochemistry, pharmacology, and toxicology. Evid Based Complement Alternat Med 2013, 2013: 579319.
31. Bahmani M, Shahinfard N, Rafieian-kopaei M, et al. Chicory: a review on ethnobotanical effects ofL. Int J Curr Pharm Res 2015, 8: 672−682.
32. Ning C, Wang X, Gao S, et al. Chicory inulin ameliorates type 2 diabetes mellitus and suppresses JNK and MAPK pathways in vivo and in vitro. Mol Nutr Food Res 2017, 61.
33. Wu Y, Zhou F, Jiang H, et al. Chicory (L.) polysaccharides attenuate high-fat diet induced non-alcoholic fatty liver disease via AMPK activation. Int J Biol Macromol 2018, 118: 886−895.
34. El-Sayed YS, Lebda MA, Hassinin M, et al. Chicory (L.) root extract regulates the oxidative status and antioxidant gene transcripts in CCl4-induced hepatotoxicity. PLoS One 2015, 10: e0121549.
35. Jackson KMP, Rathinasabapathy T, Esposito D, et al. Structural constraints and importance of caffeic acid moiety for anti-hyperglycemic effects of caffeoylquinic acids from chicory. Mol Nutr Food Res 2017, 61.
36. Wang Y, Lin Z, Zhang B, et al.L. extract suppresses experimental gout by inhibiting the NF-κB and NLRP3 signaling pathways. Int J Mol Sci 2019, 20: 4921.
37. Rasmussen MK, Klausen CL, Ekstrand B. Regulation of cytochrome P450 mRNA expression in primary porcine hepatocytes by selected secondary plant metabolites from chicory (L.). Food Chem 2014, 146: 255−263.
38. Wong RJ, Ahmed A. Obesity and non-alcoholic fatty liver disease: disparate associations among Asian populations. World J Hepatol 2014, 6: 263−273.
39. Alizadeh M, Daneghian S, Ghaffari A, et al. The effect of hypocaloric diet enriched in legumes with or without L-arginine and selenium on anthropometric measures in central obese women. J Res Med Sci 2010, 15: 331−343.
40. Chongmelaxme B, Phisalprapa P, Sawangjit R, et al. Weight reduction and Pioglitazone are cost-effective for the treatment of non-alcoholic fatty liver disease in Thailand. Pharmacoeconomics 2019, 37: 267−278.
41. Bellentani S, Saccoccio G, Masutti F, et al. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med 2000, 132: 112−117.
42. Milić S, Lulić D, Štimac D. Non-alcoholic fatty liver disease and obesity: biochemical, metabolic and clinical presentations. World J Gastroenterol 2014, 20: 9330−9337.
43. European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016, 64: 1388−1402.
44. Eslamparast T, Tandon P, Raman M. Dietary composition independent of weight loss in the management of non-alcoholic fatty liver disease. Nutrients 2017, 9: 800.
45. Ratziu V. Non-pharmacological interventions in non-alcoholic fatty liver disease patients. Liver Int 2017, 37 Suppl 1: 90−96.
46. Sookoian S, Pirola CJ. Systematic review with meta-analysis: the significance of histological disease severity in lean patients with nonalcoholic fatty liver disease. Aliment Pharmacol Ther 2018, 47: 16−25.
47. Cho AS, Jeon SM, Kim MJ, et al. Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice. Food Chem Toxicol 2010, 48: 937−943.
48. Farhangi MA, Javid AZ, Dehghan P. The effect of enriched chicory inulin on liver enzymes, calcium homeostasis and hematological parameters in patients with type 2 diabetes mellitus: A randomized placebo-controlled trial. Prim Care Diabetes 2016, 10: 265−271.
49. Saggu S, Sakeran MI, Zidan N, et al. Ameliorating effect of chicory (L.) fruit extract against 4-tert-octylphenol induced liver injury and oxidative stress in male rats. Food Chem Toxicol 2014, 72: 138−146.
50. Zhu H, Wang Z, Wu Y, et al. Untargeted metabonomics reveals intervention effects of chicory polysaccharide in a rat model of non-alcoholic fatty liver disease. Int J Biol Macromol 2019, 128: 363−375.
51. Amiri Dash Atan N, Koushki M, Motedayen M, et al. Type 2 diabetes mellitus and non-alcoholic fatty liver disease: a systematic review and meta-analysis. Gastroenterol Hepatol Bed Bench 2017, 10: S1−S7.
52. Dai W, Ye L, Liu A, et al. Prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus: a meta-analysis. Medicine (Baltimore) 2017, 96: e8179.
53. Engin A. Non-alcoholic fatty liver disease. Adv Exp Med Biol 2017, 960: 443−467.
54. Shojaei Zarghani S, Abbaszadeh S, Alizadeh M, et al. The effect of metformin combined with calcium-vitamin D3 against diet-induced nonalcoholic fatty liver disease. Adv Pharm Bull 2018, 8: 97−105.
55. Ghamarian A, Abdollahi M, Su X, et al. Effect of chicory seed extract on glucose tolerance test (GTT) and metabolic profile in early and late stage diabetic rats. Daru 2012, 20: 56.
56. Azay-Milhau J, Ferrare K, Leroy J, et al. Antihyperglycemic effect of a natural chicoric acid extract of chicory (L.): a comparative in vitro study with the effects of caffeic and ferulic acids. J Ethnopharmacol 2013, 150: 755−760.
57. Pushparaj PN, Low HK, Manikandan J, et al. Anti-diabetic effects ofin streptozotocin-induced diabetic rats. J Ethnopharmacol 2007, 111: 430−434.
58. Carr RM, Ahima RS. Pathophysiology of lipid droplet proteins in liver diseases. Exp Cell Res 2016, 340: 187−192.
59. Fruci B, Giuliano S, Mazza A, et al. Nonalcoholic fatty liver: a possible new target for type 2 diabetes prevention and treatment. Int J Mol Sci 2013, 14: 22933−22966.
60. Sahini N, Borlak J. Recent insights into the molecular pathophysiology of lipid droplet formation in hepatocytes. Prog Lipid Res 2014, 54: 86−112.
61. Wu KT, Kuo PL, Su SB, et al. Nonalcoholic fatty liver disease severity is associated with the ratios of total cholesterol and triglycerides to high-density lipoprotein cholesterol. J Clin Lipidol 2016, 10: 420−425.e1.
62. Gadang V, Kohli R, Myronovych A, et al. MLK3 promotes metabolic dysfunction induced by saturated fatty acid-enriched diet. Am J Physiol Endocrinol Metab 2013, 305: E549−556.
63. Gaggini M, Morelli M, Buzzigoli E, et al. Non-alcoholic fatty liver disease (NAFLD) and its connection with insulin resistance, dyslipidemia, atherosclerosis and coronary heart disease. Nutrients 2013, 5: 1544−1560.
64. Ahmed MH, Byrne CD. Modulation of sterol regulatory element binding proteins (SREBPs) as potential treatments for non-alcoholic fatty liver disease (NAFLD). Drug Discov Today 2007, 12: 740−747.
65. Ziamajidi N, Khaghani S, Hassanzadeh G, et al. Amelioration by chicory seed extract of diabetes- and oleic acid-induced non-alcoholic fatty liver disease (NAFLD)/non-alcoholic steatohepatitis (NASH) via modulation of PPARalpha and SREBP-1. Food Chem Toxicol 2013, 58: 198−209.
66. Jurgonski A, Juskiewicz J, Zdunczyk Z, et al. Caffeoylquinic acid-rich extract from chicory seeds improves glycemia, atherogenic index, and antioxidant status in rats. Nutrition 2012, 28: 300−306.
67. Abd El-Mageed NM. Hepatoprotective effect of feeding celery leaves mixed with chicory leaves and barley grains to hypercholesterolemic rats. Pharmacogn Mag 2011, 7: 151−156.
68. Gentile CL, Pagliassotti MJ. The role of fatty acids in the development and progression of nonalcoholic fatty liver disease. J Nutr Biochem 2008, 19: 567−576.
69. Wong VW, Hui AY, Tsang SW, et al. Metabolic and adipokine profile of Chinese patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2006, 4: 1154−1161.
70. Haukeland JW, Damås JK, Konopski Z, et al. Systemic inflammation in nonalcoholic fatty liver disease is characterized by elevated levels of CCL2. J Hepatol 2006, 44: 1167−1174.
71. Paredes-Turrubiarte G, Gonzalez-Chavez A, Perez-Tamayo R, et al. Severity of non-alcoholic fatty liver disease is associated with high systemic levels of tumor necrosis factor alpha and low serum interleukin 10 in morbidly obese patients. Clin Exp Med 2016, 16: 193−202.
72. Ziemianski P, Domienik-Karlowicz J, Cylke R, et al. A high-sensitivity C-reactive protein as a new predictor of the course of non-alcoholic fatty liver disease in patients after bariatric surgery. Pol Arch Intern Med 2019,129: 556−558.
73. Manco M, Marcellini M, Giannone G, et al. Correlation of serum TNF-alpha levels and histologic liver injury scores in pediatric nonalcoholic fatty liver disease. Am J Clin Pathol 2007, 127: 954−960.
74. Crespo J, Cayon A, Fernandez-Gil P, et al. Gene expression of tumor necrosis factor alpha and TNF-receptors, p55 and p75, in nonalcoholic steatohepatitis patients. Hepatology 2001, 34: 1158−1163.
75. Ding WX, Yin XM. Dissection of the multiple mechanisms of TNF-alpha-induced apoptosis in liver injury. J Cell Mol Med 2004, 8: 445−454.
76. Poniachik J, Csendes A, Diaz JC, et al. Increased production of IL-1alpha and TNF-alpha in lipopolysaccharide-stimulated blood from obese patients with non-alcoholic fatty liver disease. Cytokine 2006, 33: 252−257.
77. Wieckowska A, Papouchado BG, Li Z, et al. Increased hepatic and circulating interleukin-6 levels in human nonalcoholic steatohepatitis. Am J Gastroenterol 2008, 103: 1372−1379.
78. Bernstein LE, Berry J, Kim S, et al. Effects of etanercept in patients with the metabolic syndrome. Arch Intern Med 2006, 166: 902−908.
79. Rezagholizadeh L, Pourfarjam Y, Nowrouzi A, et al. Effect ofL. on the expression of hepatic NF-κB and IKKβ and serum TNF-α in STZ− and STZ+ niacinamide-induced diabetes in rats. Diabetol Metab Syndr 2016, 8: 11.
80. Ribeiro PS, Cortez-Pinto H, Sola S, et al. Hepatocyte apoptosis, expression of death receptors, and activation of NF-kappaB in the liver of nonalcoholic and alcoholic steatohepatitis patients. Am J Gastroenterol 2004, 99: 1708−1717.
81. Cavin C, Delannoy M, Malnoe A, et al. Inhibition of the expression and activity of cyclooxygenase-2 by chicory extract. Biochem Biophys Res Commun 2005, 327: 742−749.
82. Ashraf NU, Sheikh TA. Endoplasmic reticulum stress and oxidative stress in the pathogenesis of non-alcoholic fatty liver disease. Free Radic Res 2015, 49: 1405−1418.
83. Tariq Z, Green CJ, Hodson L. Are oxidative stress mechanisms the common denominator in the progression from hepatic steatosis towards non-alcoholic steatohepatitis (NASH)? Liver Int 2014, 34: e180−190.
84. Fischer R, Maier O. Interrelation of oxidative stress and inflammation in neurodegenerative disease: role of TNF. Oxid Med Cell Longev 2015, 2015: 610813.
85. Chalasani N, Deeg MA, Crabb DW. Systemic levels of lipid peroxidation and its metabolic and dietary correlates in patients with nonalcoholic steatohepatitis. Am J Gastroenterol 2004, 99: 1497−1502.
86. Hassan HA, Yousef MI. Ameliorating effect of chicory (L.)-supplemented diet against nitrosamine precursors-induced liver injury and oxidative stress in male rats. Food Chem Toxicol 2010, 48: 2163−2169.
87. Li GY, Gao HY, Huang J, et al. Hepatoprotective effect ofL., a traditional Uighur medicine, against carbon tetrachloride-induced hepatic fibrosis in rats. World J Gastroenterol 2014, 20: 4753−4760.
88. Takaki A, Kawai D, Yamamoto K. Multiple hits, including oxidative stress, as pathogenesis and treatment target in non-alcoholic steatohepatitis (NASH). Int J Mol Sci 2013, 14: 20704−20728.
:
Samira Faraji and Mohammad Alizadeh conceived the study, Samira Faraji conducted literature review and wrote the manuscript, Mohammad Alizadeh and Sevana Daneghian critically revised the manuscript; and all authors read and approved the final manuscript.
:
NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis, T2DM, type 2 diabetes mellitus; TG, triglycerides; TNF-α, tumor necrosis factor alpha; RCT, randomized control trial; FFAs, free fatty acids; IL-6, interleukin 6; LDL, low-density lipoprotein.
:
The authors declare that there is no conflict of interest.
:
Samira Faraji, Sevana Daneghian, Mohammad Alizadeh. Effects of chicory (L.) on nonalcoholic fatty liver disease. Traditional Medicine Research 2020, 5 (6): 476–486.
:Jing-Na Zhou.
:25 February 2020,
04 May 2020,
:31 August 2020
Sevana Daneghian. School of Medicine, Serow Highway, Nazloo, Urmia, Iran. PO Box: 5756115111, Tel: 00984432752372, Fax: 00984432780800. E-mail: Sevana_d@yahoo.com.
10.12032/TMR20200603192
 Traditional Medicine Research2020年6期
Traditional Medicine Research2020年6期
- Traditional Medicine Research的其它文章
- Green coffee bean hydroalcoholic extract accelerates wound healing in full-thickness wounds in rabbits
- Gastrointestinal effects of Artemisia absinthium Linn. based on traditional Persian medicine and new studies
- Evaluation of scientific evidence for abortifacient medicinal plants mentioned in traditional Persian medicine
- Jadwar (Delphinium denudatum Wall.): a medicinal plant
- Is “Pangolin (Manis Squama) is not used in medicine" an improvement in the protection of precious and rare species or an improvement in the safety of using medicine?
- The gap between clinical practice and limited evidence of traditional Chinese medicine for COVID-19
