Studies on adsorptions of fluoride ions by the hydroxyapatite nanoparticles
CHEN Peihu, LIU Yang, WU Wenjie, CUI Haiwei, CUI Meng, WANG Runwei, XU Qinghong*
(1. State Key Laboratory of Chemical Resource Engineering, Beijing University of Chemical Technology, Beijing 100029, China; 2. State Key Laboratory of Inorganic Synthesis and Preparative Chemistry, College of Chemistry, Jilin University, Changchun 130012, Jilin, China)
Abstract: How to remove excessive F— ions from water effectively is always a hot issue in academia and industry because it is very harmful to human body. In this paper, hydroxyapatite (HAP) nanoparticles with an average particle size about 10 nm, synthesized by the reaction between CaCl2 and (NH4)2HPO4 solutions under the existence of glucose as restricted domain reagent, were used as adsorbent to adsorb F- from waste water, and excellent adsorption capacity to F- of the adsorbent was possibly from its super strong surface polarity. Maximum adsorption capacity of the nanoparticles to F- in neutral aqueous solution is about 86.43 mg/g at 25 ℃, more than twice of HAP with average diameter about 100 nm (34.01 mg/g). 86.43 mg/g ranks the second adsorption capacity among all data reported. The adsorbent has a good application prospect in the removal of F— in aqueous solution due to its simple synthesis method, environmentally friendly characteristics and super large adsorption capacity.
Keywords: hydroxyapatite (HAP); nanoparticles; fluoride ions; adsorption; neutral aqueous solution
F-is an essential trace element in human body, and it is beneficial to form skeleton and dentin, especially to prevent dental caries. However, too much F-in the body, mainly from drinking water, will lead to hardening and brittleness of bone, fragility of tooth and other bad symptoms. Thus, removal of F-from aqueous solution effectively is an important work[1].
In 2009, titanium hydroxide was found to have good property on adsorption of F-(maximum capacity about 55.1 mg/g)[2], which attracted great attentions. Later,γ-Al2O3/MgO[3], natural clays modified by La and Al[4], Ce3+-implanted tripolyaluminium organic skeleton[5], and CeO2/SiO2were all studied as adsorbents to F-[6]. In 2015, a new kind of nanoporous material Fe-Ce-Ni was found to have adsorption capacity of 285.7 mg/g to F-, which is the largest reported datum at present[7]. However, the complex preparation and high cost limits the material to be industrialized.
HAP with micrometer scale, a non-toxic material and an inorganic component of the bone, was ever found to have adsorption property to F-in aqueous solution[8-10]. However, the adsorption capacity is small for the small surface area and poor surface polarity of the micrometer scale HAP. Thus, the synthesis of HAP with smaller diameter and strong surface polarity, in improving adsorption performance of the green adsorbent, is needed.
Traditional synthetic methods, such as hydrothermal synthesis, sol-gel synthesis and direct precipitation synthesis, are difficult to synthesize HAP with super smaller nanometer size. Hydrothermal synthesis can effectively improve the crystallinity of HAP, but the size of product will be larger. HAP with micrometer size is also easily obtained in sol-gel and precipitation methods. In 2017, HAP particles with 7 nm diameter were successfully synthesized using glucose as restricted domain reagent by JING et al.[11], and the particles were found to have super-strong adsorptions to some heavy metal ions in water for its larger specific surface area and stronger surface activity. In this paper, HAP particle was synthesized with an average diameter of 10 nm using JING’s method[11], and adsorption behaviors to F-of the particles were systematically studied. Research results indicate that the maximum adsorption capacity of F-by the HAP particles with 10 nm diameter was 86.43 mg/g in neutralaqueous solution at 25 ℃, twice than that of HAP with average diameter of 100 nm under the same conditions. This adsorption capacity ranks second datum among all the data reported. Owing to the green synthetic preparation (all the reagents are non-toxic) and environmentally-friendly characteristics, the super-small HAP particle has a good application prospect in F-removal.
1 Experiments
1.1 Materials and instruments
Glucose, CaCl2, (NH4)2HPO4, NaOH, NaF were all purchased from Beijing Chemical Reagent Company (Beijing, China). All chemicals are analytical grade and used without further purification. Deionized water was used throughout the experiments.
Crystallinities of the samples were investigated by X-ray diffraction (XRD, Bruker D8 Advance, Germany), Cu Kαradiation (λ=0.154 06 nm) was used to affirm the calcium phosphate phase. The working accelerating voltage and emission current were 40 kV and 40 mA, respectively. S-4700 field emission scanning electron microscope (SEM, Hitachi, Japan) was used to observe the morphology and size of the synthesized nanoparticles. Nicolet 8700 Fourier transform infrared spectrometer (FTIR, Thermo Electron, USA) was used to characterize the functional groups in the samples, and the spectra were acquired at a resolution of 4 cm-1in wave number ranging from 4 000 to 400 cm-1. Potentiometer (HX-3B) with calomel electrode as reference electrode and fluoride ion selective electrode as indicator electrode was used to detect fluoride ion concentrations at 25 ℃.
1.2 Synthesis of the super-small HAP particles in restricted domain conditions
Synthesis of the super-small HAP particles was accorded to the method in the reference[11].
In a typical procedure, 100 mL of CaCl2(0.01 mol/L) and 100 mL of (NH4)2HPO4(0.006 mol/L) solutions were mixed together. The above mixture was regulated to weak acidity (pH =3.0-3.5) with HCl solution (0.1 mol/L). The above solution was dropwised into a glucose alkaline solution that was prepared by mixing 10 g of glucose, 1.5 g of NaOH and 80 mL of deionized water, and a white precipitate was formed immediately. The super-small HAP particles were finally obtained after being centrifuged (12 000 rpm, 15 min), washed with deionized water 10 times and dried at 80 ℃.
1.3 Synthesis of the HAP particles without using any restricted domain reagent
All the synthetic processes were same as those of the super-small HAP particles except for using the glucose.
1.4 Drawing of F- concentration standard curve
Five standard solutions of NaF with concentrations from 1.0×10-5to 1.0×10mol/L were prepared, and their potential values (Es) were measured at room temperature with F-selective electrode. TISAB, a solution containing NaCl and Na3C6H5O72H2O, was used to reduce interference of other ions during the measurement. A standard relationship curve with slopeSof -54.8 and linear correlation coefficientR2of 0.997 2 between the concentration of standard solution and its potential value indicate that a good linear relationship between the potential values and logarithm values of F-concentrations was found.
The F-concentrations (c, mol/L) in the solutions can be calculated using slopeSof the standard curve andEvalues of the solution by formula of lgc=E/S.
The fluorine electrode was activated by soaking it in a NaF solution (0.001 mol/L) for one to two hours before used, and potential of the activated electrode in deionized water should be over 200 mV.
1.5 Adsorptions
A solution of NaF with 1.19×10mol/L content of F-was prepared, and its potential was determined in using F-selective electrode. 80 mL of the above NaF solutions were added to different beakers respectively, and the solutions were regulated to different pH values with HCl solution (0.1 mol/L). Some of the HAP with average diameter about 10 nm and HAP with average diameter about 100 nm were then added to the NaF solutions to finish adsorptions under different conditions, including temperatures, pH values, times and mass of adsorbents. The mixtures were separated by centrifugation after adsorptions, electrode potential values of the supernatant after centrifugation were measured, and the saturated adsorption capacities (q, mg/g) of HAP to F-were calculated according to the formula:
qe= [(c0-ce)VMF]/mHAP
(1)
wherec0(mol/L) andce(mol/L) are the initial and equilibrium concentrations of F-,V(L) is volume of the solution,MFis atomic weight of F andmHAP(g) is mass of HAP added.
2 Results and discussion
2.1 Synthesis and characterizations of the super-small HAP particles
In 2000, NAUTA and MILLER found that the water molecules in liquid helium could form some small clusters with dimers, trimers or tetramers[12]. However, these clusters were not stable, for example, a water molecular monomer could insert into some trimer to form a tetramer cluster, but chain-like trimers and tetramers were more easily transformed into ring-like trimers and tetramers. Opening and rearrangement of hydrogen bonds were found during the transformation process. Later, ZHANG and CHENG found that the ethanol-water solution was not a uniform system with increasing the concentration[13], and some water molecules were encapsulated by large number of ethanol molecules to form some confined regions when molar concentration of ethanol was higher than 0.68, and diffusion of the water molecules was limited by ethanol molecules.
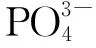
SEM images of the synthesized HAP particles in and out of using restricted domain method are shown in Fig.2. The average diameter of HAP synthesized in using restricted domain method is about 10 nm (Fig.2A); however, the average diameter of HAP particles synthesized out of using restricted domain method is about 100 nm (Fig.2B). In addition, the super-small HAP particles existed in a high dispersity.

Fig.1 A: X-ray diffractions of the synthesized HAP nanoparticles; B: FT-IR spectra of the HAPs with nanometer scale (a) and micrometer scale (b)

Fig.2 SEM images of the HAP with nanometer scale (A) and micrometer scale (B)
2.2 Effect on different dosages of HAP nanopar-ticles to the adsorption of F- ions
10, 20, 30, 40 and 50 mg of HAP nanoparticles were added to 80 mL of neutral NaF solutions (1.19 mmol/L) in different beakers, respectively. The mixtures were stirred at 25 ℃, and 10 mL of the mixture was taken out from every beaker at an interval, centrifuged and contents of F-in the supernatants were determined. All the adsorption process lasted 200 min.
Relationships between the concentrations (F-) and adsorption times under the existence of different dosages of HAP nanoparticles at 25 ℃ were examined, and most of the adsorptions was found in reaching saturation states after 150 min (Fig.3A). Adsorption capacities of F-in different conditions were found decreasing with increase of the adsorbent dosage and the time lasted, and amount of F-ions adsorbed by HAP nanoparticles with different dosages had difference at the beginning. Fig.3B shows the maximum adsorption capacity of different adsorbent added. The maximum adsorption capacity was found to be gradually decreased with increasing amount of the adsorbent added. Considering the mass effect on adsorption, 20 mg was affirmed to be an appropriate amount of the adsorbent although the largest adsorption capacity of 86.43 mg/g appeared when the adsorbent was 10 mg. Some data relating to the adsorption capacities with different adsorbent added and adsorption times are listed in Table 1.
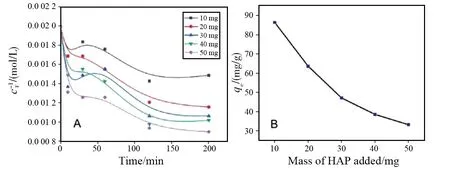
Fig.3 Effect on the different dosages of HAP nanoparticles to the adsorption of F- (A) and the maximum adsorption capacity of F- by different dosages of the HAP nanoparticles (B)
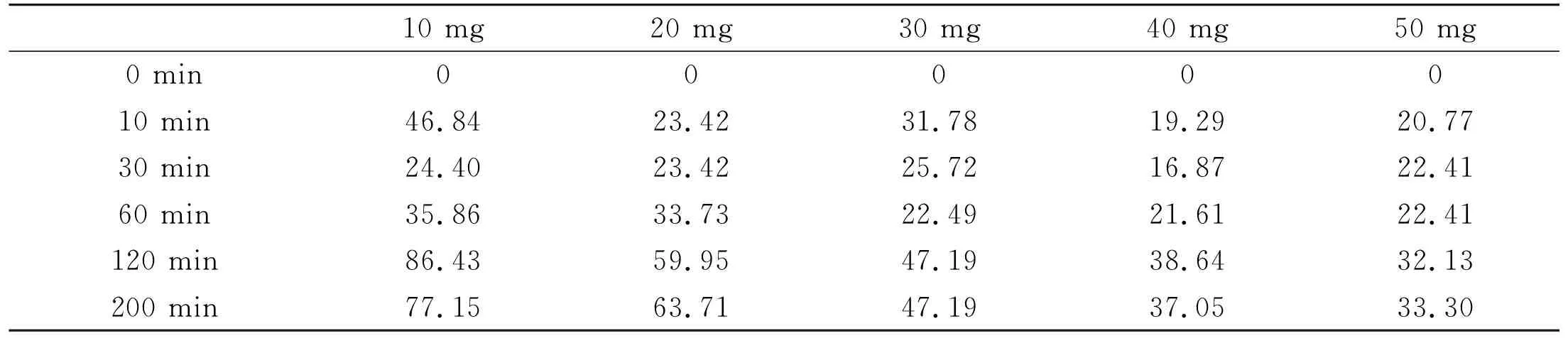
Table 1 Adsorption capacities (mg/g) with different adsorbent added and the times passed at 25 ℃
2.3 Effect on pH to the adsorption of HAP nanoparticles to F-
The adsorption capacities of HAP nanoparticles (20 mg) to F-under different pH value solutions, regulated by NaOH solution (0.1 mol/L) or HCl solution(0.1 mol/L), were studied at 25 ℃, and the results are shown in Fig.4A. Maximum adsorption capacity of the nanoparticles was found to be the smallest when pH of the F-solution was at 9.0 (Fig.4B), and adsorption performance of the nanoparticles was poor under a slight acidic environment. The adsorption capacity reached its maximum when the solution was near to neutrality (Fig.4B).

Fig.4 Effect on different acidic and alkaline environments to the adsorption of HAP nanoparticles to F- (A) and maximum adsorption capacity of F- by the HAP nanoparticles under different acidic and alkaline environments (B)
2.4 Effect on temperatures and times to the adso-rptions of HAP nanoparticles to F-
HAP is not suitable to be used as adsorbent in an acidic environment due to its alkaline property and it will be decomposed when environmental pH is less than 6.4. Although HAP is stable in an alkaline environment, large amount of OHgroups in the solution will compete with other anions (including F-) during the adsorption process. Part of the surface area of HAP will be occupied by OH-ions despite of their larger volumes. In a neutral NaF solution, amount of OHions in solution is few, and the adsorption mainly happens among the adsorbent, F-ions and Na+ions. Some data relating to adsorption capacities of the adsorbent to F-in different pH solutions are listed in Table 2.
Four of 20 mg of HAP nanoparticles were added to four 80 mL of NaF solutions (1.83 mol/L) respectively, and pH of the mixtures were regulated to 7.0 with HCl solution (0.1 mol/L). The mixtures were stirred at 30 ℃,40 ℃, 50 ℃ and 60 ℃, respectively. 10 mL of the solution in every mixture was taken out at intervals during the adsorption process and centrifuged, and contents of F-in the supernatants were determined. All the adsorption process lasted 400 min.
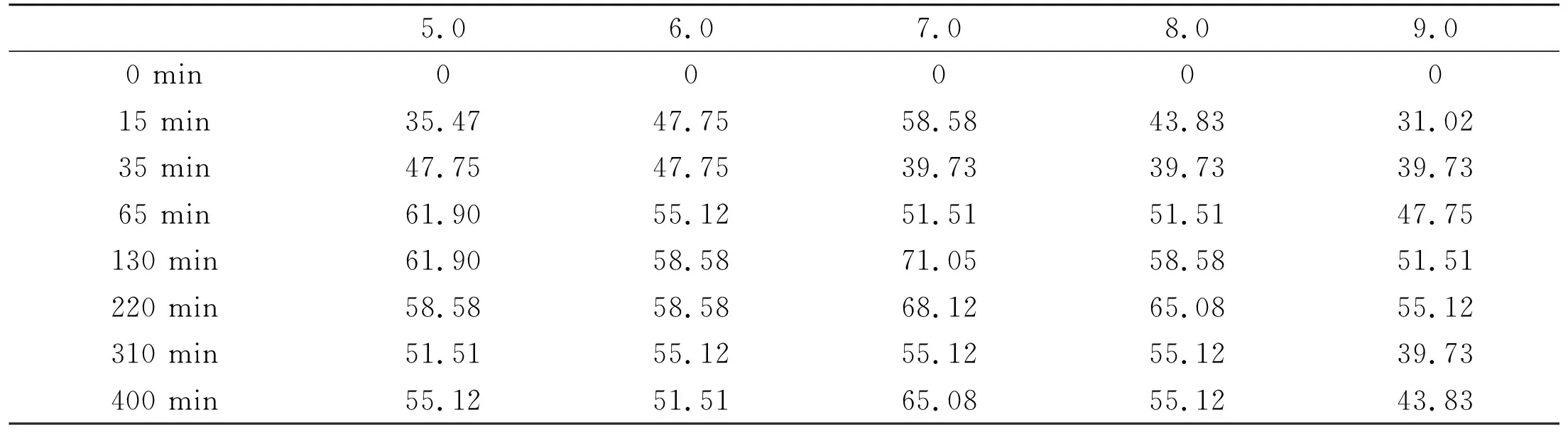
Table 2 Adsorption capacities (mg/g) with different pH and the times passed at 25 ℃
The relationships between F-concentrations and adsorption times at different temperatures (Fig.5A) indicate that the concentrations of F-in the solutions were decreased rapidly at beginning of the adsorption under different temperatures, and the adsorptions reached saturation when the adsorptions lasted for 300 min. Fig.5C shows that maximum adsorption capacities of the adsorbent (about 73.85 mg/g) appeared at about 40 ℃ and the capacities were not varied when the temperature was higher. Thus, optimum pH, temperature and time for the adsorption are at about 7.0, 40 ℃ and 300 min, respectively. Some data in this part are listed in Table 3.
Compared to the adsorbent of super-small HAP particles, maximum adsorption capacity of HAP particles with micrometer diameter is only about 35.47 mg/g under all the same conditions (Fig.5B).
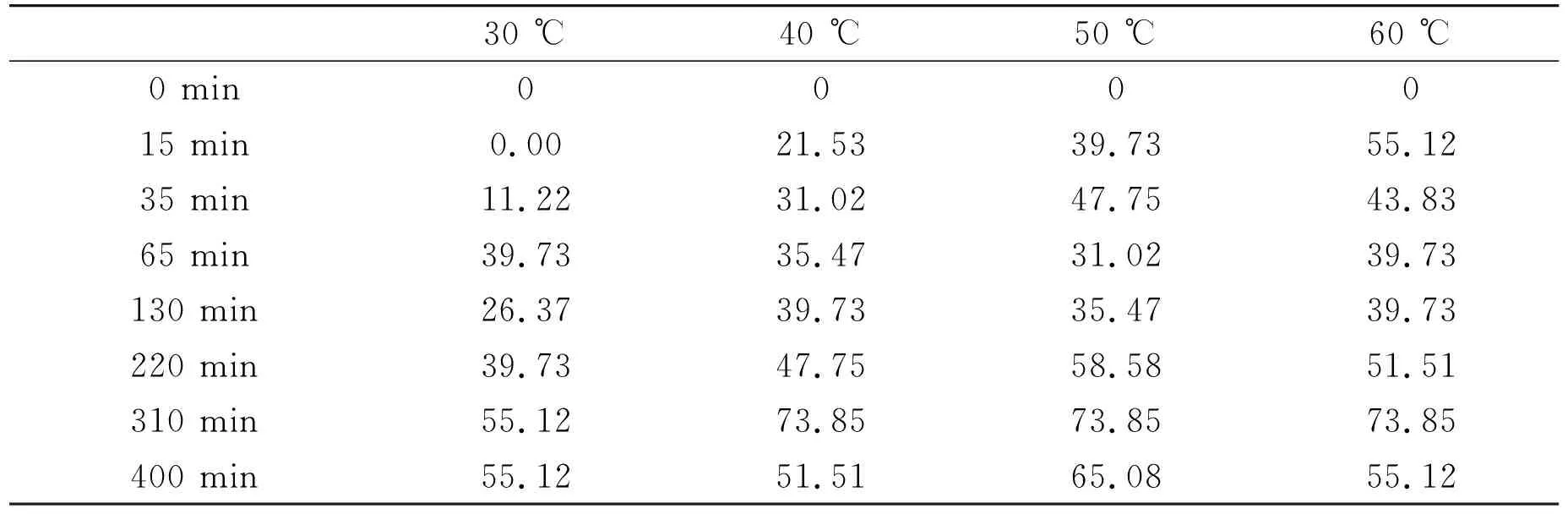
Table 3 Adsorption capacities (mg/g) with different temperatures and the times passed at pH 7.0

Fig.5 Relationships between F- concentrations and adsorption times during the adsorption process for HAP nanoparticles to F- under pH 7.0 and at different temperatures (A); the relationship between F- concentrations and adsorption times during the adsorption process for HAP with micrometer scale to F- at 40 ℃ and pH 7.0 (B); maximum adsorption capacity of F- by the HAP nanoparticles at different temperatures (C)
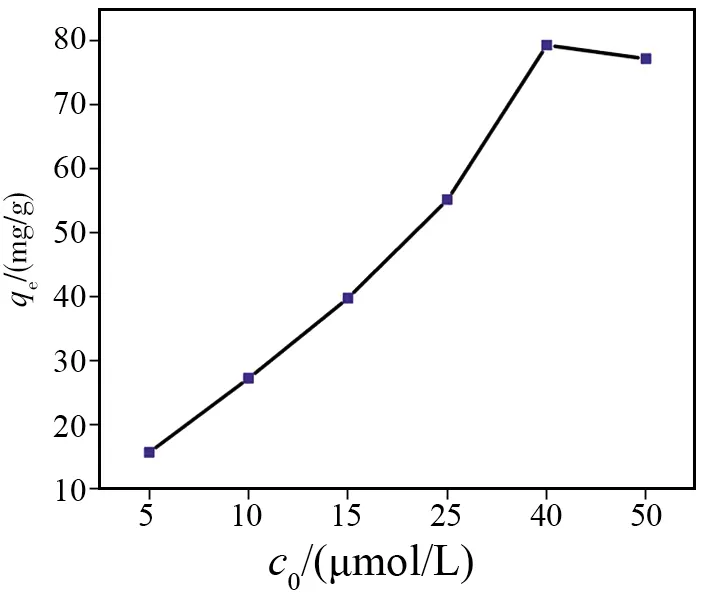
Fig.6 Adsorption capacities (mg/g) of F- with different initial concentrations by the HAP nanoparticles at pH 7.0 and 4 h
2.5 Effect on initial concentration to the adsorp-tions of HAP nanoparticles to F-
Under room temperature (25 ℃) and neutral environment, 20 mg HAP was added to the solutions with different initial F-concentrations (5, 10, 15, 25, 40 and 50 μmol/L) and stirred for 4.0 h. The solutions were then centrifuged to obtain supernatants for measuring adsorption capacities of the adsorbent to F-. Results indicate that the adsorption capacity of HAP nanoparticles to F-increased with F-concentration increasing until 40 μmol/L (Fig.6 and Table 4) and remained unchangeable later. Maximum adsorption capacity of F-adsorbed by the super-small HAP particle is 79.40 mg/g.

Table 4 Adsorption capacities (mg/g) with different initial concentrations passed at pH 7.0 and reaction 4 h
2.6 Discussion on mechanism of the adsorptions
Langmuir and Freundlich adsorption models were used to investigate the adsorption behaviors of an adsorbent. Langmuir equation is expressed follow as
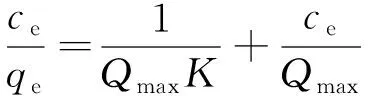
(2)
whereqeis the equilibrium adsorption capacity,ceis the equilibrium concentration (ppm) of F-,Qmaxis the max adsorption capacity,Kis the Langmuir constant relative to the adsorption ability. A linear plot can be obtained whence/qeis plotted againstceover the concentration range of F-investigated.
Freundlich equation is expressed follow as

(3)
Whereqeis the equilibrium adsorption capacity,ceis the equilibrium concentration (ppm) of F-,Kfandnare the Freundlich model constants that can be determined by plotting lnqeversus lnce.
ce/qeandcewere calculated according to the adsorption results of HAP nanoparticles at different initial concentrations of F-. Linear fitting was performed according to the equation of Langmuir adsorption model, and the linear correlation coefficient is 0.981 11 (Fig.7A). That is to say, a linear graph can be obtained whence/qeis plotted withce.
At the same time,qeandcewere calculated also according to the experimental results. Linear fitting was performed according to the equation of Freundlich adsorption model, and the linear correlation coefficient is 0.977 74 (Fig.7B).
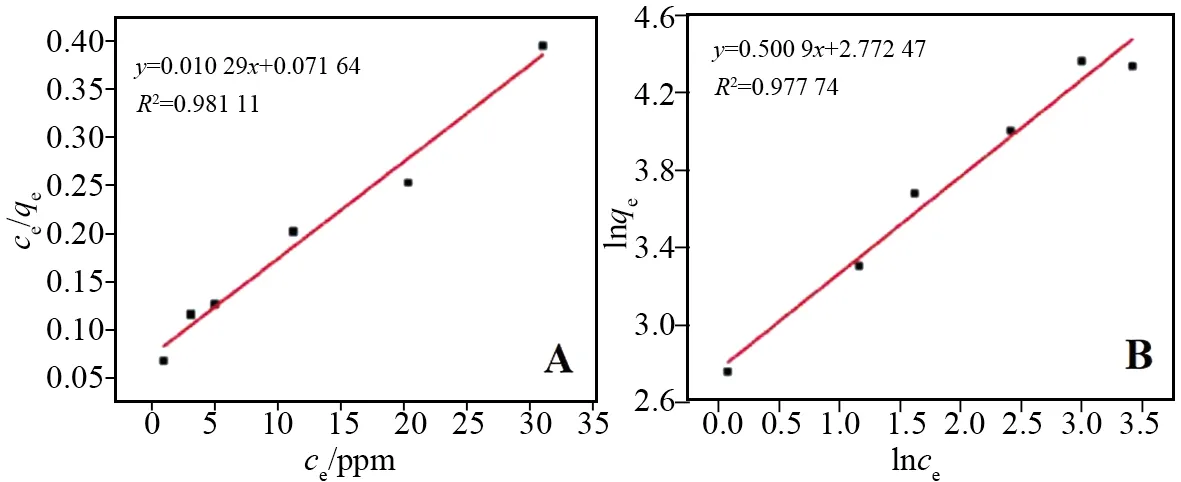
Fig.7 Results of Langmuir (A) and Freundlich (B) adsorption model analysis
Adsorption behavior of the super-small HAP particle to F-in this study conforms to both Langmuir and Freundlich adsorption models. As surface polarity of the particle is very strong due to its super-small scale, and the sodium and fluorine ions adsorbed arrayed alternatively in monolayer in initial adsorption stage and in multilayer later on the surface. On the other hand, the HAP particles after adsorption were easily to be aggregated and arrayed in regular multilayer with each other for their spatial stacking effect. Thus, the two models all exist in this adsorption. Schematic diagram of the adsorption process is drawn as Scheme 1.
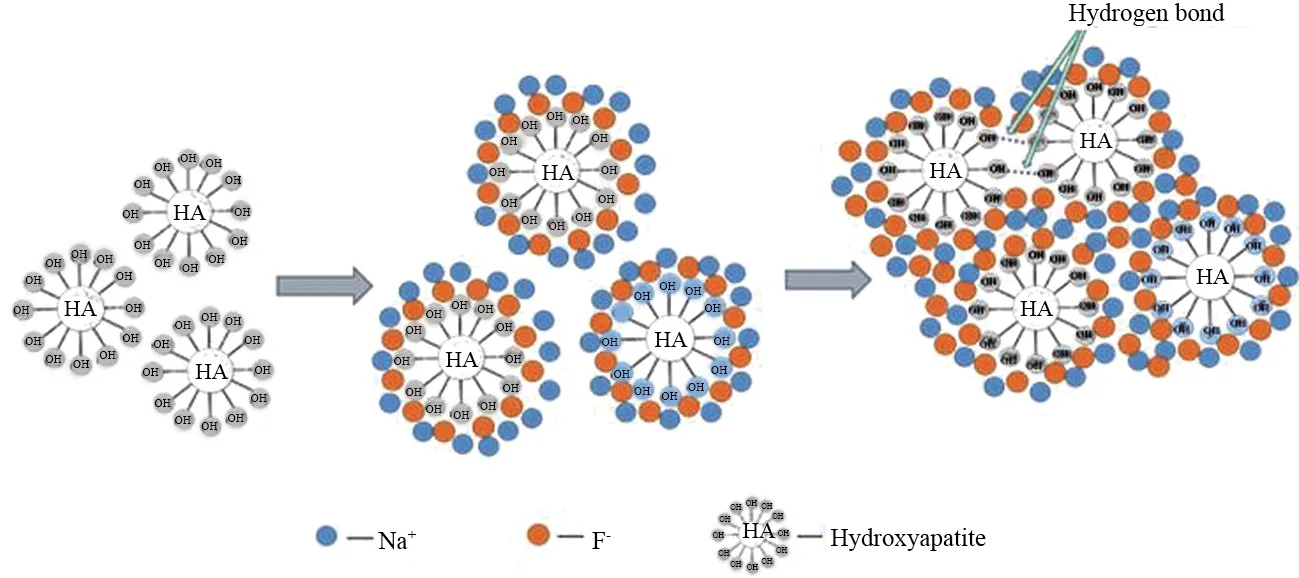
Scheme 1 Schematic diagram of F- adsorption on HAP nanoparticles
3 Conclusions
In this paper, the adsorptions of F-in aqueous solution by HAP nanoparticles with an average diameter of 10 nm were studied systematically. Results indicate that the HAP nanoparticles have good adsorption properties to F-with a maximum adsorption capacity about 86.43 mg/g at 25 ℃ and pH=7.0, more than twice as that of the HAP with micrometer scale. This adsorption capacity ranks the second adsorption capacity among all data reported. In view of its non-toxic and harmless properties, the HAP particle with super-small diameter has good application prospects in F-ions removal from water.

