代谢综合征及其组分与前列腺增生手术病人下尿路症状相关性
袁星辰 王文杰 姚榆 夏瑞琴 张桂铭 孙立江
[摘要] 目的 探讨代谢综合征(MetS)及其组分与良性前列腺增生(BPH)病人下尿路症状(LUTS)的相关性。方法 选取2015年10月—2018年12月因LUTS就诊于青岛大学附属医院的908例BPH病人,根据是否合并MetS分为两组,其中合并MetS组132例,不合并MetS组(对照组)776例。采集两组病人空腹血检测前列腺特异性抗原、血糖及血脂水平,并比较两组间国际前列腺症状评分(IPSS)、储尿期评分和排尿期评分的差异。结果两组间前列腺特异性抗原水平差异无统计学意义(P>0.05),血糖、三酰甘油及高密度脂蛋白胆固醇水平比较差异有统计学意义(t=2.357~7.183,P<0.05)。合并MetS组病人的IPSS及储尿期评分显著高于对照组,差异有统计学意义(t=5.940、10.114,P<0.01),两组间排尿期评分差异无统计学意义(P>0.05)。在MetS的组成成分中,是否合并腹型肥胖和高血压与IPSS具有相关性(r=-0.035、0.168,P<0.05)。结论 MetS可加重BPH病人的LUTS,预防治疗MetS可能对BPH病人的LUTS的改善有一定作用。
[关键词] 代谢综合征;前列腺增生;下尿路症状;症状评估
[中图分类号] R697.32 [文献标志码] A [文章编号] 2096-5532(2020)02-0194-05
doi:10.11712/jms.2096-5532.2020.56.040 [开放科学(资源服务)标识码(OSID)]
[网络出版] http://kns.cnki.net/kcms/detail/37.1517.R.20200320.1519.004.html;2020-03-23 11:43:43
[ABSTRACT] Objective To investigate the association of metabolic syndrome (MetS) and its components with lower urinary tract symptoms (LUTS) in patients with benign prostatic hyperplasia (BPH). Methods A total of 908 patients with BPH who attended The Affiliated Hospital of Qingdao University due to LUTS from October 2015 to December 2018 were enrolled, and according to the presence or absence of MetS, they were divided into MetS group with 132 patients and control group (without MetS) with 776 patients. Fasting blood samples were collected to measure prostate-specific antigen, blood glucose and blood lipids, and the two groups were compared in terms of International Prostate Symptom Score (IPSS), urine storage score, and micturition score. Results There was no significant difference in prostate-specific antigen between the Mets group and the control group (t=0.284,P>0.05), and there were significant differences between the two groups in blood glucose, triglyceride, and high-density lipoprotein cholesterol (t=2.357-7.183,P<0.05). The MetS group had significantly higher IPSS and urine storage score than the control group (t=5.940,10.114;P<0.01), and there was no significant difference in urination score between the two groups (P>0.05). Among the components of MetS, the presence or absence of abdominal obesity and hypertension was associated with IPSS (r=-0.035, 0.168;P<0.05). Conclusion MetS can aggravate LUTS in patients with BPH and prevention and treatment of MetS may help to improve LUTS in BPH patients.
[KEY WORDS] metabolic syndrome; prostatic hyperplasia; lower urinary tract symptoms; symptom assessment
下尿路癥状(LUTS)包括尿频、尿急、排尿困难等,多见于60岁以上的老年人,在男性其主要病因是良性前列腺增生(BPH)[1-3]。LUTS严重影响病人的生活质量,病情严重者甚至会发生急性尿潴留,死亡风险也随之上升[4-5]。已有研究表明,BPH导致后尿道延长、受压变形、狭窄和尿道阻力增加,引起膀胱高压并出现相关排尿期症状。随着膀胱压力的增加,出现膀胱逼尿肌代偿性肥厚并引起相关储尿期症状,LUTS的出现不仅归因于BPH,它还与代谢综合征(MetS)密切相关[6-7]。MetS是指一种以多种代谢异常发生在同一个体为特点的综合征,如糖代谢紊乱、脂代谢紊乱、肥胖、高血压等[8-11]。有研究认为,MetS导致LUTS的发生可能与慢性高糖血症、氧化应激和自主神经病变有关,这些病理生理状态均会加重LUTS,而MetS中的各组分也是潜在的可控制的危险因素。因此,控制MetS的发生及进展,对促进BPH病人的LUTS控制可能具有积极的意义[12]。MetS被认为是BPH及其相关LUTS发生及发展的独立影响因素[13-14]。然而,有关MetS及其组分与LUTS程度相关性的研究较少,有待通过临床研究提供数据支持。本研究拟通过回顾性调查,探讨青岛地区BPH病人MetS及其各组分与LUTS的相关性。现将结果报告如下。
1 资料与方法
1.1 研究对象
选取2015年10月—2018年12月青岛大学附属医院收治、经手术和病理确诊为BPH病人908例,年龄48~90岁,平均(73.2±5.8)岁。病人均行手术治疗,且经病理检查确诊为BPH;合并明确的LUTS;BPH为首次手术治疗;病人临床、实验室、病理资料完善。根据《中国泌尿外科疾病诊断治疗指南》(2014版)诊断BPH,排除尿路感染、尿道狭窄、前列腺癌、急性前列腺炎、膀胱颈挛缩、膀胱肿瘤、神经源性膀胱、膀胱结石及其他影响排尿功能的疾病。根据《非酒精性脂肪性肝病防治指南》(2018更新版)中MetS的定义,存在以下3项及以上代谢性危险因素为MetS。①腹型肥胖:腰围>90 cm(男性)或>85 cm(女性);②高血压:动脉血压≥17.3/11.3 kPa,或者正在应用降血压药物;③高三酰甘油(TG)血症:空腹血清TG≥1.7 mmol/L或者正在服用降血脂药物;④低高密度脂蛋白胆固醇(HDL-C)血症:空腹血清HDL-C<1.0 mmol/L(男性)或<1.3 mmol/L(女性);⑤高糖血症:空腹血糖(FBG)≥5.6 mmol/L,或餐后2 h血糖≥7.8 mmol/L,或有2型糖尿病家族史。依据以上诊断标准,908例BPH病人中有132例合并有MetS(Ⅰ组),776例不合并MetS(Ⅱ组)。本研究通过青岛大学附属医院伦理委员会审批。
1.2 数据和标本采集
病人入院后收集年龄、身高、体质量、吸烟史等基本信息。禁食8 h后采集空腹静脉血,检测FBG、TG、HDL-C、前列腺特异性抗原(PSA)等指标。对病人进行国际前列腺症状评分(IPSS)、储尿期评分以及排尿期评分。行前列腺超聲检查,计算前列腺体积(PV)。PV=0.52×左右径×前后径×上下径。
1.3 统计方法
应用SPSS 22.0统计软件对数据进行处理。符合正态分布计量资料采用±s形式表示,组间比较采用两独立样本t检验;计数资料的比较采用χ2检验。检验水准为α=0.05。
2 结 果
2.1 一般资料相关指标比较
Ⅰ组和Ⅱ组病人的体质量指数(BMI)、FBG、TG、HDL-C,是否合并腹型肥胖,是否合并高血压、糖尿病、冠心病等比较,差异均有统计学意义(t=2.357~10.182,χ2=10.182~177.245,P<0.05),两组年龄、PSA、前列腺体积、吸烟及饮酒史等比较差异无统计学意义(P>0.05)。见表1。
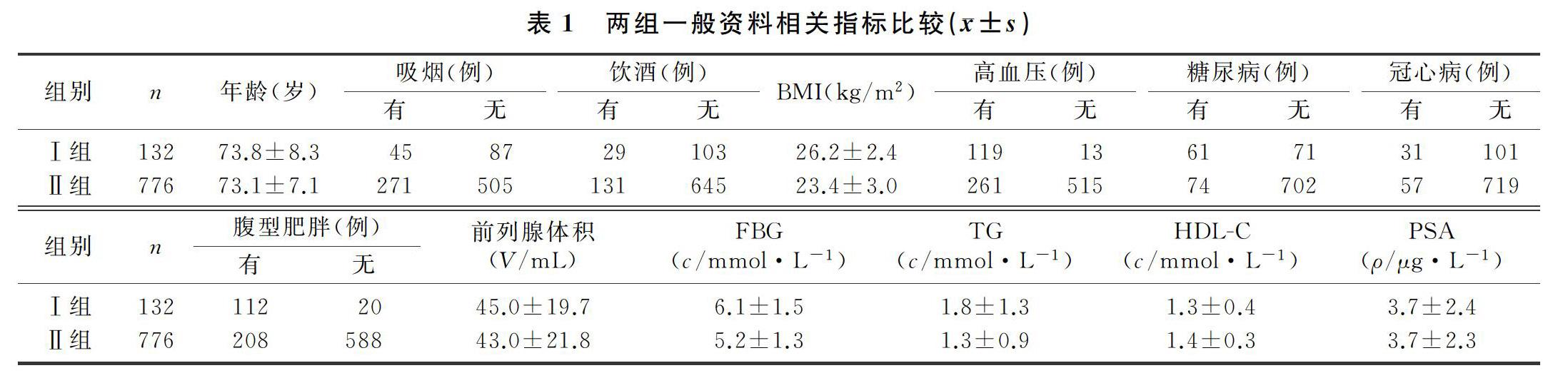
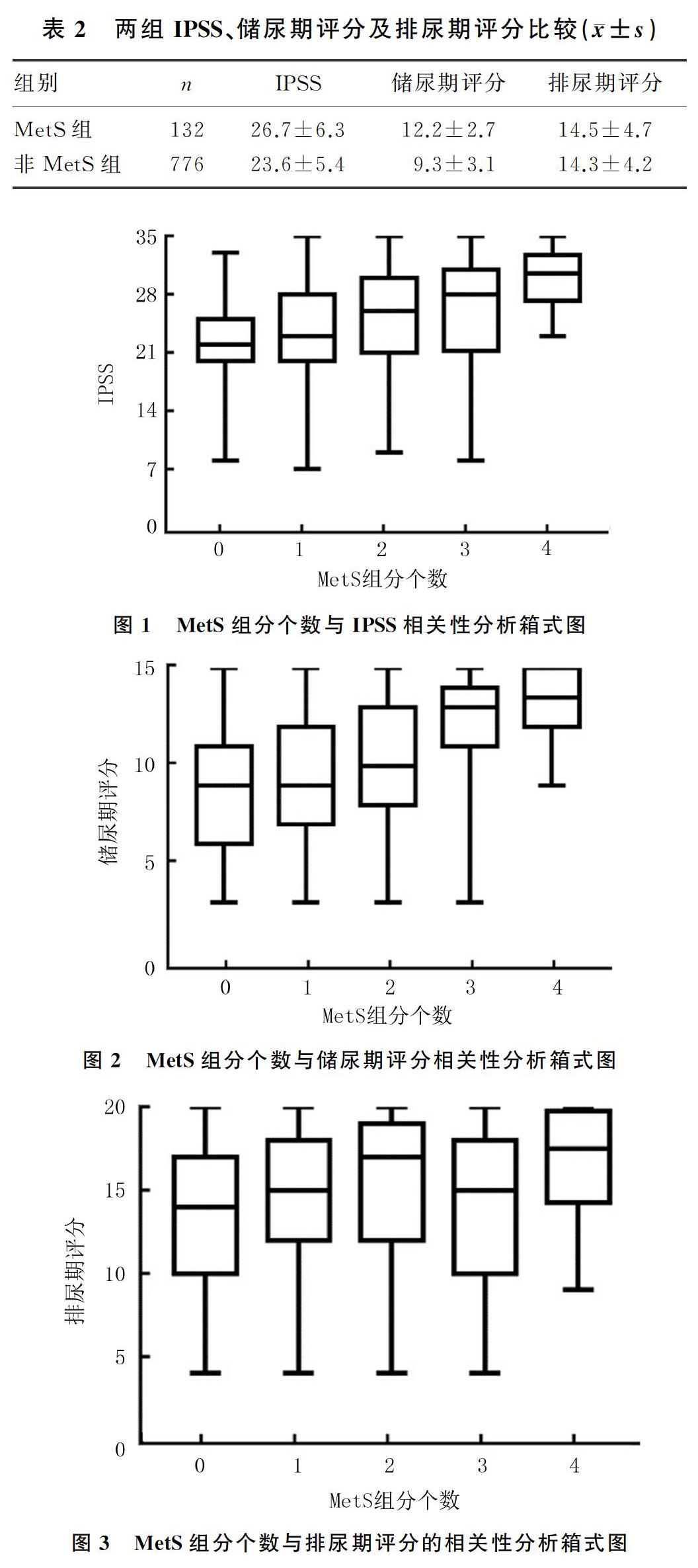
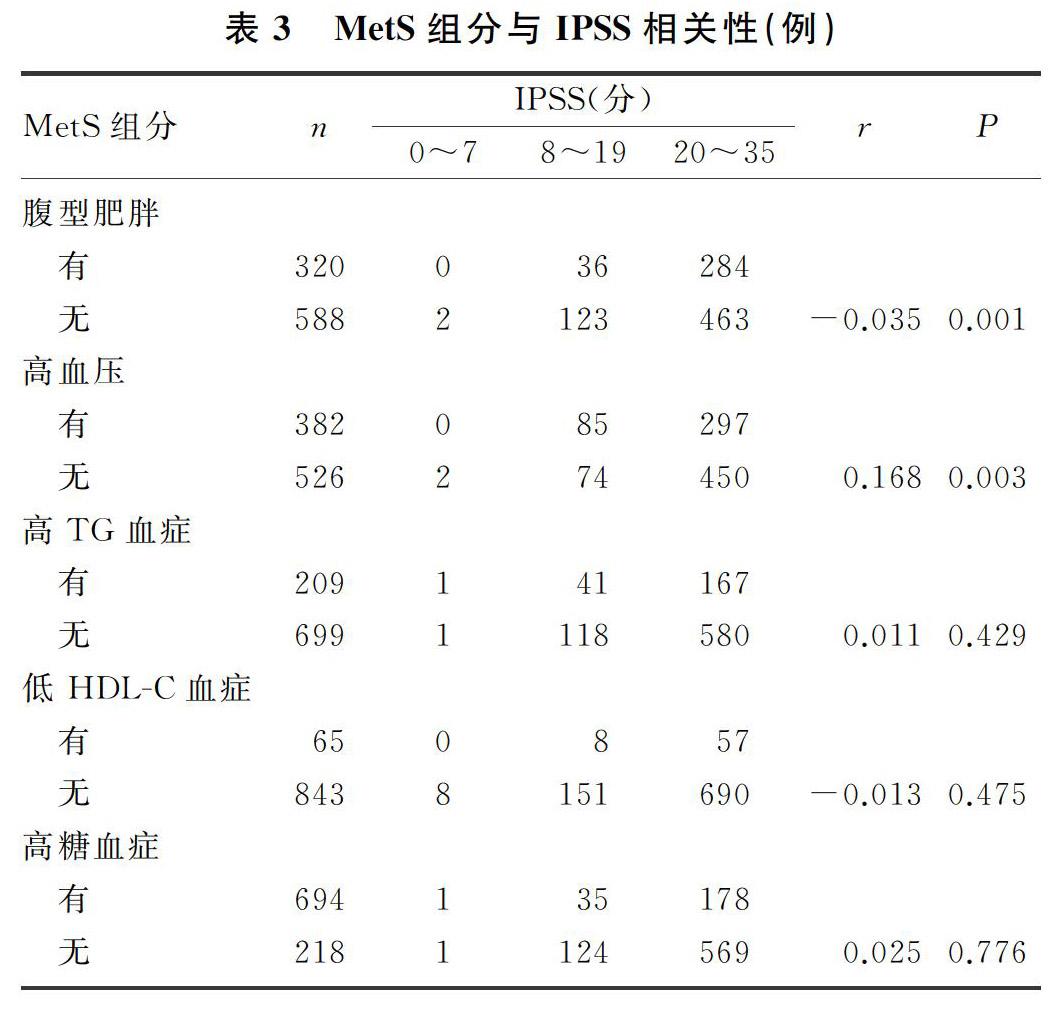
2.2 两组MetS组分个数与IPSS、储尿期评分及排尿期评分的相关性
两组IPSS、储尿期评分比较差异有统计学意义(t=5.940、10.114,P<0.01),两组间排尿期评分比较差异无统计学意义(P>0.05)。见表2。使用箱式图将MetS组分个数分别与IPSS、储尿期评分及排尿期评分进行比较,结果显示随着MetS组分个数增加,IPSS、储尿期评分升高(r=0.585、0.683,P<0.05),而排尿期评分则未见规律性变化(P>0.05)。见图1~3。
2.3 MetS组分与IPSS相关性
进一步分析MetS各个组分与所有病人IPSS的相关性,结果显示,是否合并有腹型肥胖、是否合并高血压病人的IPSS差异均具有统计学意义(r=-0.035、0.168,P<0.05);而是否合并有高TG血症、高糖血症、低HDL-C血症组分IPSS比较差异无统计学意义(P>0.05)。见表3。
3 讨 论
随着社会人口的老龄化,BPH的患病率也在逐年增加,已经成为一种常见的世界性疾病,也是引起男性LUTS的主要病因[15-16]。BPH以前列腺增大和尿道狭窄为特征,部分病人由于疾病进展缓慢,病人可能会习惯这些症状而未早期进行诊治,导致出现突然的急性尿潴留,或是出现慢性尿潴留伴肾衰竭[17-18]。BPH是前列腺细胞非恶性增殖的结果,尽管增殖的病因尚不清楚,但与其相关的已知因素是衰老和睾丸分泌的雄性激素减少。另有研究表明,代谢紊乱包括胰岛素抵抗、血脂异常和肥胖等可能在BPH的发展中起作用[19-23]。本研究探讨MetS与BPH病人LUTS的相关性,并进一步分析MetS组分与IPSS的相关性,为进一步提高BPH病人的生活质量提供诊疗思路。
2016年,WANG等[20]进行的荟萃研究分析表明,BPH伴MetS病人的前列腺体积和前列腺体积生长率明显高于未伴MetS病人,LUTS持续时间明显延长,该项研究纳入了包含中国、意大利、土耳其、伊朗等5个国家的8项研究,均为高质量研究。鉴于前列腺体积增大与LUTS有直接关系,本文进一步研究了是否合并MetS与IPSS、储尿期评分及排尿期评分的相关性,结果显示合并MetS的病人IPSS和储尿期评分显著增加,两组病人的排尿期评分并无明显差异;并且随着MetS组分个数的增加,IPSS、储尿期评分及排尿期评分均升高。HAKIMI等[24]研究发现,人们通过改变生活方式可以有效地减轻MetS引起的相关病症,这样对于BPH-LUTS病人围术期LUTS的改善及后续的手术治疗会产生较大帮助,有助于病人术后身体恢复。还有研究结果显示,服用他汀类药物病人LUTS累积发生率低、尿流率高,并且BPH发生率低,推荐应用降血脂药物改善病人的MetS状态,从而减轻BPH病人的LUTS症状[25]。GRZESIAK[26]等进一步分析了BPH病人MetS与炎症脂质介质的关系,结果显示BPH合并MetS病人炎症脂质介质可能影响生化和激素水平,而炎症在BPH的发病机制中起着重要作用,提示LUTS与BPH和MetS有着密不可分的关联,而其分子机制还有待进一步研究。本文的研究结果显示,合并MetS的BPH病人IPSS评分及储尿期评分显著高于单纯BPH病人,提示MetS及其危险因素对BPH病人LUTS的发生、进展及分级等有重要影响;进一步分析MetS的组分个数与IPSS、储尿期评分及排尿期评分的相关性,结果显示MetS组分个数与病人IPSS及储尿期评分呈正相关,提示MetS的发生及进展可加重BPH病人的LUTS,而MetS的筛查及控制对BPH的防治可能具有积极意义。
FU等[27]對525例患有LUTS的病人进行了3年的随访研究,结果表明MetS组分中的高糖血症和高血压会加速老年男性BPH的临床进展。但本文研究结果表明,MetS组分中的腹型肥胖和高血压会增加IPSS分数,即加速BPH的临床进展。也有研究结果表明,因MetS而导致的LUTS病人人数正在增加,然而MetS并不是LUTS恶化的决定因素[28]。还有研究纳入了经前列腺摘除术后的BPH病人,分析其LUTS仍然存在的原因可能是多种代谢危险因素引起的系统性疾病[29]。关于MetS是否能加重LUTS,以及是哪种组分起主导作用,尚存在争议。此外,本研究纳入的对象均为经手术治疗的BPH病人,并未包含未就诊的以及保守治疗的轻症BPH病人;本文病人的症状相对较重,其IPSS评分也偏高,不能代表全部的BPH病人,可能会对结果造成一定的影响。因此,本研究结果有待扩大样本量进一步证实。
由于老龄人口的增加和饮食习惯的改变,MetS作为一种复杂的全球性疾病,其患病率正在逐年上升[30]。MetS与包括泌尿生殖系统在内的多器官系统的损害、功能障碍和衰竭有关,它与需要治疗的BPH显著相关[31-32]。本文结果显示,MetS组分中的腹型肥胖、高血压等诱导的代谢紊乱在加重BPH所导致的LUTS中具有重要的作用。通过促进饮食结构的改变和经常的体育锻炼可以预防和减轻MetS,增加体力活动可显著降低LUTS的发病风险,尽管其机制尚未明确,但其临床意义已经得到了较为广泛的认可。MetS病人进行血压控制、脂代谢调节及血糖调节可显著改善LUTS及MetS,对提高BPH病人的生活质量,减少尿潴留发生,降低手术干预的风险均有重要的临床意义。
[参考文献]
[1] PAPAEFSTATHIOU E, MOYSIDIS K, SARAFIS P, et al. The impact of diabetes mellitus on lower urinary tract symptoms (LUTS) in both male and female patients[J]. Diabetes & Metabolic Syndrome, 2019,13(1):454-457.
[2] RUSSO G I, REGIS F, SPATAFORA P, et al. Association between metabolic syndrome and intravesical prostatic protrusion in patients with benign prostatic enlargement and lower urinary tract symptoms (MIPS Study)[J]. BJU International, 2018,121(5):799-804.
[3] WU Yu, PAN Hong, WANG Weiming, et al. A possible relationship between serum sex hormones and benign prostatic hyperplasia/lower urinary tract symptoms in men who underwent transurethral prostate resection[J]. Asian Journal of Andrology, 2017,19(2):230-233.
[4] ARMITAGE J N, SIBANDA N, CATHCART P J, et al. Mortality in men admitted to hospital with acute urinary retention: database analysis[J]. BMJ (Clinical Research ed.), 2007,335(7631):1199-1202.
[5] ALAWAMLH O A, GOUELI R, LEE R K. Lower urinary tract symptoms, benign prostatic hyperplasia, and urinary retention[J]. Medical Clinics of North America, 2018,102(2):301.
[6] CHOL C M, SONG S H, YONG C S, et al. Patient-reported ejaculatory function and satisfaction in men with lower urinary tract symptoms/benign prostatic hyperplasia[J]. Asian Journal of Andrology, 2018,20(1):69-74.
[7] KAPLANS A. Re: serenoa repens for lower urinary tract symptoms/benign prostatic hyperplasia:current evidence and its clinical implications in naturopathic medicine[J]. The Journal of Urology, 2018,199(6):1372-1373.
[8] WOYESA S B, HIRIGO A T, WUBE T B. Hyperuricemia metabolic syndrome in type 2 diabetes mellitus patients at Hawassa university comprehensive specialized hospital, South West Ethiopia[J]. BMC Endocrine Disorders, 2017,17(1):76.
[9] DE NUNZIO C, ARONSON W, FREEDLAND S J, et al. The correlation between metabolic syndrome and prostatic di-seases[J]. European Urology, 2012,61(3):560-570.
[10] SUN Xue, JIANG Feng, ZHANG Rong, et al. Serum uric acid levels are associated with polymorphisms in the SLC2A9, SF1, and GCKR genes in a Chinese population[J]. Acta Pharmacologica Sinica, 2014,35(11):1421-1427.
[11] VIGNOZZI L, GACCI M, MAGGI M. Lower urinary tract symptoms, benign prostatic hyperplasia and metabolic syndrome [J]. Nature Reviews Urology, 2016,13(2):108-119.
[12] CHEN Jiawei, ZHAO Jie, CAO Ying, et al. Relationship between alterations of urinary microbiota and cultured negative lower urinary tract symptoms in female type 2 diabetes patients[J]. BMC Urology, 2019,19(1):78.
[13] CHEN I H, TONG Y C, CHENG J T. Metabolic syndrome decreases tissue perfusion and induces glandular hyperplasia in the fructose-fed rat prostate[J]. Neurourology and Urodyna-mics, 2012,31(4):600-604.
[14] CHEN I H, TSAI Y S, TONG Y C. Correlations among cardiovascular risk factors, prostate blood flow, and prostate vo-lume in patients with clinical benign prostatic hyperplasia[J]. Urology, 2012,79(2):409-414.
[15] SHIN Y S, KARNA K K, CHOI B R, et al. Finasteride and erectile dysfunction in patients with benign prostatic hyperplasia or male androgenetic alopecia[J]. The World Journal of Mens Health, 2019,37(2):157-165.
[16] LLOYD G L, MARKS J M, RICKE W A, et al. Benign prostatic hyperplasia and lower urinary tract symptoms:what is the role and significance of inflammation[J]? Current Urology Reports, 2019,20(9):1-8.
[17] FOO K T. What is a disease? What is the disease clinical benign prostatic hyperplasia (BPH)[J]? World Journal of Urology, 2019,37(7):1293-1296.
[18] DESAI M M, SINGH A, ABHISHEK S, et al. Aquablation therapy for symptomatic benign prostatic hyperplasia: a single-centre experience in 47 patients[J]. BJU International, 2018,121(6):945-951.
[19] TELLI O, DEMIBAS A, EKICI M, et al. Does metabolic syndrome or its components correlate with lower urinary tract symptoms in benign prostatic hyperplasia patients[J]? Nephro-urology Monthly, 2015,7(3):e27253.
[20] WANG Jianye, FU Yanyan, KANG Deying. The association between metabolic syndrome and characteristics of benign prostatic hyperplasia: a systematic review and Meta-analysis[J]. Medicine, 2016,95(19):e3243.
[21] LIM K B. Epidemiology of clinical benign prostatic hyperplasia[J]. Asian Journal of Urology, 2017,4(3):148-151.
[22] FOSTER H E, BARRY M J, DAHM P, et al. Surgical ma-nagement of lower urinary tract symptoms attributed to benign prostatic hyperplasia: AUA guideline amendment 2019[J]. The Journal of Urology, 2019,202(3):592-598.
[23] PATTANAIK S, MAVUDURU R S, PANDA A, et al. Phosphodiesterase inhibitors for lower urinary tract symptoms consistent with benign prostatic hyperplasia[J]. The Cochrane Database of Systematic Reviews, 2018,11(1): CD010060.
[24] HAKIMI Z, HERDMAN M, PAVESI N, et al. Using EQ-5D-3L and OAB-5D to assess changes in the health-related quality of life of men with lower urinary tract symptoms associated with benign prostatic hyperplasia[J]. Quality of Life Research, 2017,26(5):1187-1195.
[25] BECHER E, ROEHRBORN G, SIAMI P, et al. The effects of dutasteride, tamsulosin, and the combination on storage and voiding in men with benign prostatic hyperplasia and prostatic enlargement:2-year results from the Combination of Avodart and Tamsulosin study[J]. Prostate Cancer and Prostatic Diseases, 2009,12(4):369-374.
[26] GRZESIAK K, RYL A, STACHOWSKA E, et al. The relationship between eicosanoid levels and serum levels of metabolic and hormonal parameters depending on the presence of metabolic syndrome in patients with benign prostatic hyperplasia[J]. Int J Environ Res Public Health, 2019,16(6):e1006.
[27] FU Yongqiang, ZHOU Zhe, YANG Bing, et al. The relationship between the clinical progression of benign prostatic hyperplasia and metabolic syndrome: a prospective study[J]. Urologia Internationalis, 2016,97(3):330-335.
[28] RUSSO G I, CASTELLI T, URZ D, et al. Connections between lower urinary tract symptoms related to benign prostatic enlargement and metabolic syndrome with its components: a systematic review and meta-analysis[J]. Aging Male, 2015,18(4):207-216.
[29] PLATA M, CAICEDO J I, TRUJILLO C G, et al. Prevalence of metabolic syndrome and its association with lower urinary tract symptoms and sexual function[J]. Actas Urologicas Espanolas, 2017,41(8):522-528.
[30] GACCI M, CORONA G, VIGNOZZI L, et al. Metabolic syndrome and benign prostatic enlargement: a systematic review and meta-analysis[J]. BJU International, 2015,115(1):24-31.
[31] THOR P J, MAZUR M, FURGALA A, et al. The autonomic nervous system function in benign prostatic hyperplasia[J]. Folia Med Cracov, 2006,47(1/4):79-86.
[32] HAMMARSTEN J, HOGSTEDT B. Calculated fast-growing benign prostatic hyperplasia-a risk factor for developing clinical prostate cancer[J]. Scandinavian Journal of Urology and Nephrology, 2002,36(5):330-338.
(本文編辑 黄建乡)

