Preparation, characterization, and antifungal evaluation of a new type of aminourea chitooligosaccharide derivatives*
ZHU Jun , LIU Song , QIN Yukun XING Rong’e YU Huahua CHEN Xiaolin LI Pengcheng
1 Key Laboratory of Experimental Marine Biology, Center for Ocean Mega-Science, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China
2 Laboratory for Marine Drugs and Bioproducts, Pilot National Laboratory for Marine Science and Technology (Qingdao), Qingdao 266237, China
3 University of Chinese Academy of Sciences, Beijing 100049, China
Received Apr. 10, 2019; accepted in principle Aug. 22, 2019; accepted for publication Sep. 23, 2019 © Chinese Society for Oceanology and Limnology, Science Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Abstract Phytopathogenic fungi cause heavy negative impact on the agricultural economy, but most existing fungicides are toxic and pose a threat to both human health and environments. A green and eき cient fungicide is urgently needed. Chitooligosaccharides (COSs), the degradation products of natural polysaccharide chitosan, are nontoxic and biodegradable antifungal substances. In this study, a novel type of aminourea chitooligosaccharide derivatives (AUCOS) was synthesized by successively grafting a hydrazine group and an amine-carbonyl group onto a chitooligosaccharide backbone to enhance the antifungal capability of COSs. The structures of the target compounds were identifi ed by FTIR, 1 H NMR, and 13 C NMR, and the degree of substitution of each product was calculated from the results of the elemental analysis. The antifungal activities of the prepared chitooligosaccharide derivatives against Fusarium solani, Verticillium albo- atrum and Phytophthora capsici were tested in vitro. The AUCOSs had better inhibitory eき ciencies against the three plant pathogen fungi than that of chitooligosaccharide, of which aminourea chitooligosaccharide 2 (AUCOS2) was the most promising antifungal compound, whose highest inhibition rates were 60.12%, 82.95%, and 85.23% against F. solani, V. albo- atrum and P. capsici, respectively. The synthesized derivatives have good application prospects in crop protection and deserve further research.
Keyword: chitooligosaccharide (COS); aminourea; characterization; antifungal
1 INTRODUCTION
Plant pathogenic fungi do great harm to plants and have caused signifi cant decreases in crop yields in agriculture. For example, Fusarium solani result in root rot diseases in many vegetables, such as garden pea, potato, tomato, pepper etc. (Coleman, 2016). Verticillium albo- atrum and other Verticillium fungi can infect over 200 plant species and cause serious diseases named Verticillium wilts (Deketelaere et al., 2017). Phytophthora capsici is the most destructive soil borne bacteria to chili pepper. It can also infect many other plant species (cucumber, muskmelon, tomato etc.) (Barchenger et al., 2018). Chemical fungicides are the primary method for preventing and treating plant fungal diseases. Many types of chemical fungicides are efference ective against the various plant fungal diseases and are globally used in agriculture. However, most of these fungicides are highly toxic to living things, and in recent years, the large-scale use of chemical fungicides has caused serious environmental problems (Badawy et al., 2014). Chemical fungicides spread into the water and soil, causing harm to plants, animals and humans (Yuan et al., 2014), which has attracted extensive attention. At the same time, due to the high-frequency use of fungicides, fungi have developed resistances to them (Tripathi and Dubey, 2004). Therefore, it is necessary to develop new eき cient and environmentally friendly fungicides. Some marine-derived bioactive substances have been found to have good antimicrobial activity. For example, some secondary metabolites from marine Trichoderma can inhibit pathogenic microbes (Song et al., 2018, 2019).
Chitosan (CS), a deacetylation product of chitin that is widely found in crustacean shells, is a macromolecular polysaccharide consisting of D-glucosamine monomers linked through β-(1-4) glycosidic linkages. Chitosan is a marine-sourced bioactive substance that can inhibit a variety of bacteria and fungi (Vinšová and Vavříková, 2011; Xia et al., 2011). In addition, it is biodegradable and causes little harm to the environment, thus, chitosan is a suitable raw material for developing novel biological pesticides. A widely accepted reason for its antimicrobial activity is that the presence of amino groups gives chitosan unique cationic property, and its protonated amino groups (-NH3+) may interact with the negatively charged groups of microorganism cell membranes, which would lead to leakage of the intracellular contents and cell structure disruption (Rabea et al., 2003; Kong et al., 2010; Badawy and Rabea, 2011). However, chitosan is not suき ciently efference ective when compared with conventional fungicides (Qin et al., 2012), and its low water solubility has limited its application in agriculture to some extent. Thus, many researchers have tried to improve its antimicrobial activity by enhancing the strength of the positive charges on chitosan molecules. Grafting more amino groups (Meng et al., 2012; Zhang et al., 2017) or other positive electronic groups, such as quaternary ammonium (Guo et al., 2007; Badawy et al., 2014; Tan et al., 2017; Liu et al., 2018) and guanidine (Zhao et al., 2009; Sun et al., 2010), onto chitosan is the major method to increase the positive charge strength; additionally, the water solubility of chitosan has been improved following the introduction of hydrophilic cationic groups.
Chitooligosaccharide (COS) is the degradation product of chitosan and much more soluble in water. Chitooligosaccharide has a broad range of antimicrobial efference ects, similar to chitosan, and may be more powerful against some plant pathogenic microbes than chitosan (Park et al., 2004; Seyfarth et al., 2008; Benhabiles et al., 2012). Similarly, it is feasible to promote the antimicrobial capabilities of chitooligosaccharide by introducing positively charged groups, which several reports have demonstrated (Kim et al., 2003; Liu et al., 2013). However, there have been few reports connecting two difference erent electropositive groups to chitooligosaccharide in succession to improve its antimicrobial activity.
Based on the above results, we designed three aminourea chitooligosaccharide derivatives (AUCOS), and synthesized them by successively grafting two electropositive groups, hydrazine and amine-carbonyl, onto chitooligosaccharide molecules to strengthen their positive charge intensity and antifungal properties. Then, the physicochemical characteristics of the new chitooligosaccharide derivatives were characterized by FTIR,1H NMR,13C NMR, and elemental analysis. In addition, the antifungal properties of the AUCOSs against three plant-threatening fungi ( F. solani, V. albo- atrum and P. capsici) were evaluated.
2 MATERIAL AND METHOD
2.1 Material
Chitooligosaccharide with a 90% degree of deacetylation and an average molecular weight of 2 200 Da was provided by Shandong Weikang Biomedical Technology Co., Ltd. (Linyi, China). Hydrazine hydrate, 1,2-ethylenediamine, dimethyl carbonate (DMC), N,N-dimethylformamide (DMF), triethylamine, zinc acetate and ethanol were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Additionally, 1,2-dibromoethane was purchased from Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China), and 1,2-diaminobenzene was purchased from Chengdu Kelong Chemical Reagent Factory (Chengdu, China). Iprodione was purchased from FMC (Suzhou) Crop Care Co., Ltd. (Suzhou, China). All the chemical reagents were of analytical grade. F. solani, V. albo- atrum and P. capsici were purchased from the China General Microbiological Culture Collection Center (CGMCC).
2.2 Characterization
Fourier transform infrared (FTIR) spectra were carried out over the range from 4 000 cm-1to 400 cm-1using a Thermo Scientifi c Nicolet iS10 FTIR spectrometer.1H NMR and13C NMR spectra were measured on a JEOL JNM-ECP600 spectrometer, using D2O and CD3COOD as the solvents. The elemental analysis (C and N) was recorded by a Vario EL-III elemental analyser. The percent contents of C and N were estimated to calculate the degree of substitution (DS) of the COS derivatives.
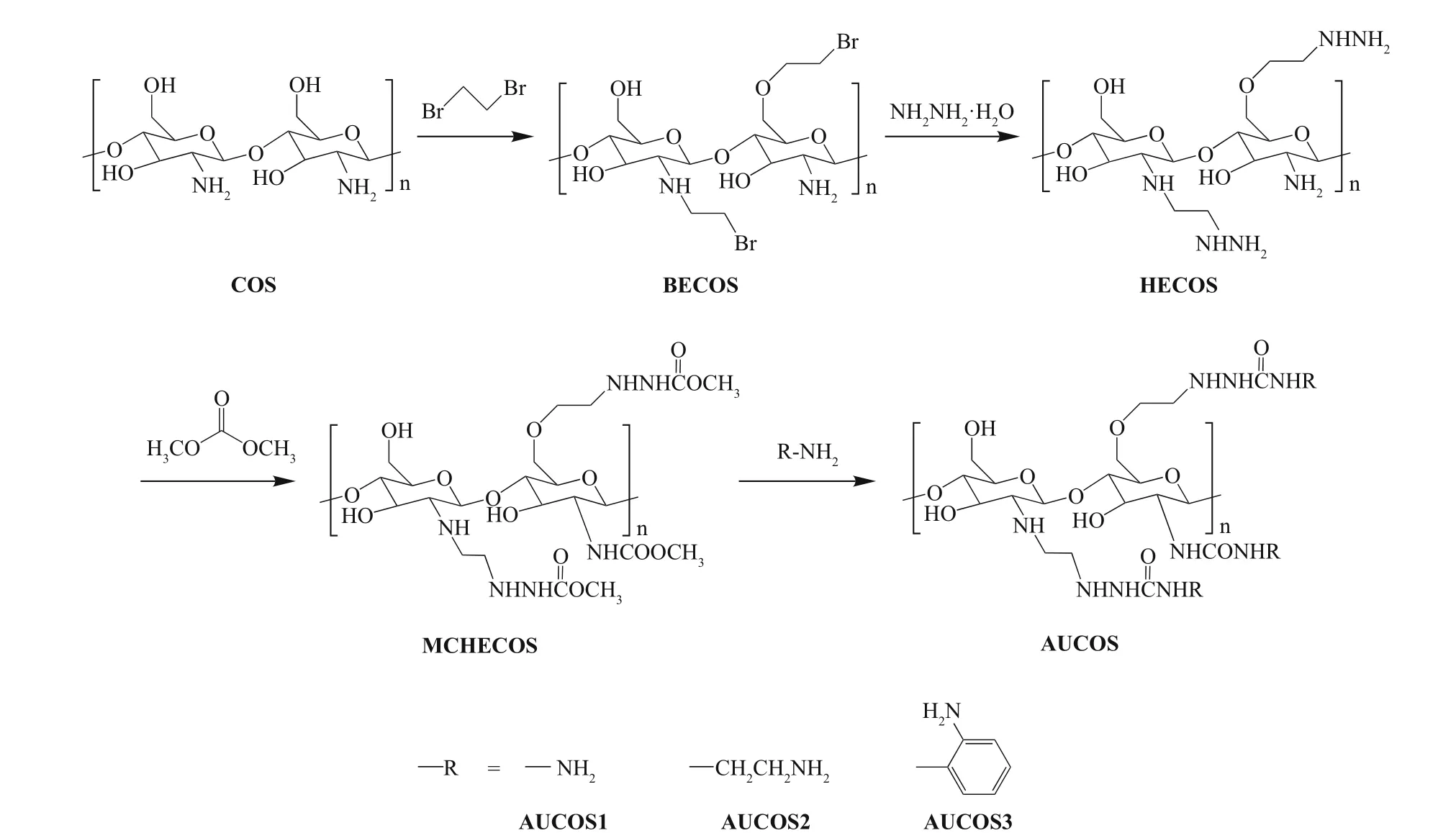
Fig.1 Synthetic route for the aminourea chitooligosaccharide derivatives
2.3 Synthesis of the aminourea chitooligosaccharide derivatives
The aminourea chitooligosaccharide derivatives were synthesized as shown in Fig.1. First, chitooligosaccharide (8.0 g), triethylamine (0.1 mol) and 1,2-dibromoethane (0.1 mol) were added to DMF (80 mL) successively, and the mixture was stirred at 50°C for 12 h. The product, labelled bromoethyl chitooligosaccharide (BECOS), was fi ltered, washed with ethanol and dried at 50°C. Then, BECOS (6.0 g) was dispersed in a mixture of ethanol (60 mL) and hydrazine hydrate (0.1 mol) and stirred at 60°C for 18 h. The precipitate was collected by fi ltration and then washed with ethanol. After drying, a light yellow powder of the hydrazino-ethyl chitooligosaccharide derivative (HECOS) was obtained.
Subsequently, HECOS (1.5 g) and zinc acetate (0.3 mmol) were added to a mixture of dimethyl carbonate and ethanol (V:V=2:1). The mixture was reacted at 80°C for 8 h to form the methoxy acylated hydrazino-ethyl chitooligosaccharide derivative (MCHECOS). After cooling to room temperature, the solid was fi ltered, washed with ethanol and dried at 50°C. MCHECOS (0.4 g) was collected and then reacted with hydrazine hydrate (3 equivalents), 1,2-ethylenediamine (3 equivalents) and 1,2-diaminobenzene (3 equivalents) in ethanol (10 mL) with added zinc acetate (0.1 mmol) at 70°C for 10 h. After fi ltration, washing with DMF and ethanol, and drying, the target compounds were obtained.
2.4 Antifungal assay
The antifungal activity was evaluated in vitro according to a mycelial growth inhibition method in the literature (Li et al., 2016; Liu et al., 2017). In brief, COS, HECOS and the AUCOSs were dissolved in deionized water, and then the solutions were mixed with sterile molten potato dextrose agar (PDA, for F. solani and V. albo- atrum) or potato saccharose agar (PSA, for P. capsici) to produce fi nal concentrations of 0.1 mg/mL, 0.2 mg/mL, 0.4 mg/mL and 0.8 mg/mL. Iprodione, which contains a urea group in its molecular structure, was used as a positive control and mixed with PDA/PSA to prepare the same concentrations of drug-loading plates. The test plates were incubated at 28°C for 2 d after transferring the mycelia of the fungi. The antifungal index was calculated according to Eq.1:
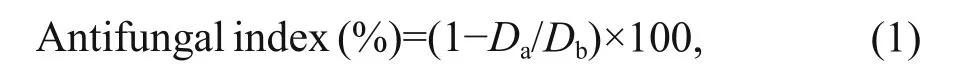
where Dais the diameter of the growth zone in the test plates and Dbis the diameter of the growth zone in the blank control plate.
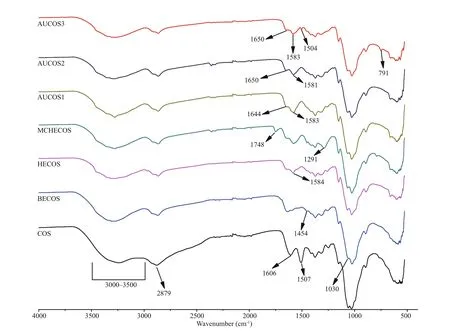
Fig.2 The FTIR spectra of COS and the COS derivatives
Three replicates for each test were carried out, and all the data were processed using Excel (version 2010) and SPSS software (version 17.0). The results are presented as the mean±SD. For P <0.05, the results were considered statistically signifi cant.
3 RESULT
3.1 Preparation and characterization
As shown in Fig.1, the AUCOSs were prepared through a four-step approach. First, we attached a bromoethyl group to the chitooligosaccharide molecule to act as a linker, but the introduction of this hydrophobic branched chain led to poor water solubility for the product of the fi rst step. Then, the fi rst cationic group (-NHNH2) was introduced through a nucleophilic substitution reaction, and water-soluble HECOS was formed. Next, dimethyl carbonate was used to link HECOS to the second positively charged group. The ester groups in the MCHECOS molecules were ammonifi ed by the amino groups of three diamines (hydrazine hydrate, 1,2-ethylenediamine and 1,2-diaminobenzene), and the AUCOSs were obtained.
The abovementioned chitooligosaccharide derivatives were characterized by FTIR,1H NMR,13C NMR, and elemental analysis.
3.2 FTIR
As shown in Fig.2, there are difference erences between the FTIR spectra of COS and its derivatives. For COS, the broad band ranging from 3 000 cm-1to 3 500 cm-1was considered to be the absorption peaks of O-H and N-H. The weak band at 2 879 cm-1was attributed to the characteristic absorption band of C-H. The peak at 1 606 cm-1was associated with the C=O (the amide I) stretching vibration, the -NH2absorption peak was at 1 507 cm-1, and the signals of the C-O stretching vibration were at 1 030 and 1 060 cm-1. Compared with COS, the -NH2absorption peak at 1 507 cm-1disappeared in the FTIR spectra of BECOS, and the new peak at 1 454 cm-1could be attributed to the -C-H bond of the bromoethyl group. These changes indicated that the hydrogen on the amino group was replaced by the bromoethyl group. The weaker signal at 1 030 cm-1suggested that nucleophilic substitution occurred at the -OH. In the spectra of HECOS, a new signal at 1 584 cm-1could be assigned to the N-H bond of -NHNH2. This result indicated that the hydrazine group was grafted onto the COS backbone successfully.
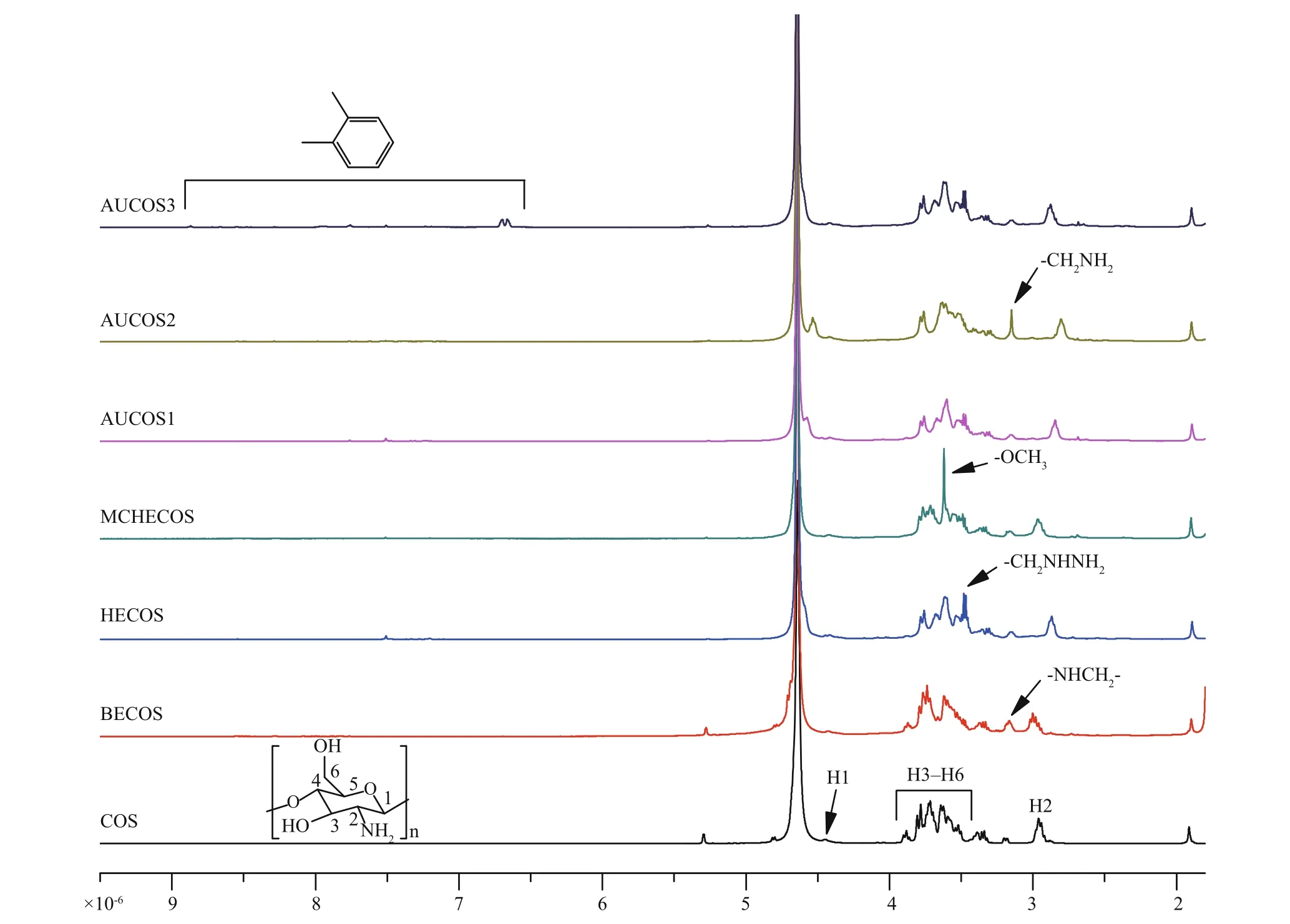
Fig.3 The 1 H NMR spectra of COS and the COS derivatives
For the next intermediate product, MCHECOS, the two new absorption peaks at 1 748 cm-1and 1 291 cm-1belonged to -COO- and C-O, respectively, indicating that -COOCH3was successfully connected to -NH-. However, in the spectra of the three aminourea COS derivatives, the -COO- absorption peaks at 1 748 cm-1disappeared. The new absorption peaks for C=O (urea) were at 1 644 cm-1, 1 650 cm-1, and 1 650 cm-1, respectively, and the N-H absorption peaks were at 1 583 cm-1, 1 581 cm-1, and 1 583 cm-1, respectively. In addition, two new absorption peaks at 1 504 cm-1and 791 cm-1in the spectrum of AUCOS3 could be attributed to the C=C and C-H bonds of the phenyl group. All of the above results support the successful synthesis of the AUCOSs.
3.3 1 H NMR
The1H NMR spectra of COS and its derivatives are shown in Fig.3. Signals in the spectrum of COS were in accord with those of earlier reports (Yue et al., 2017; Fan et al., 2018). The peak at 2.96×10-6was assigned to H2, the multiplet at (3.5-3.8)×10-6was due to H3, H4, H5 and H6 of COS, and the peak at 4.42×10-6was attributed to H1. In contrast to the spectrum of COS, there was a new peak at 3.17×10-6in the spectrum of BECOS, which belonged to the hydrogen atom on the -NHCH2- group. The new peaks at 3.47×10-6and 3.48×10-6in the HECOS spectrum represented the protons of -CH2NHNH2. The single peak of MCHECOS at 3.62×10-6originated from the protons of -COOCH3. However, this peak disappeared in the spectra of the AUCOSs, which indicated that -OCH3had been replaced. The new peak at 3.15×10-6was derived from the hydrogen atoms of aminoethyl, confi rming the structure of AUCOS2. In the spectrum of AUCOS3, signals at 6.66×10-6, 6.70×10-6, 7.92×10-6and 8.87×10-6showed that the benzene ring had been introduced, proving the successful synthesis of AUCOS3.
3.4 13 C NMR
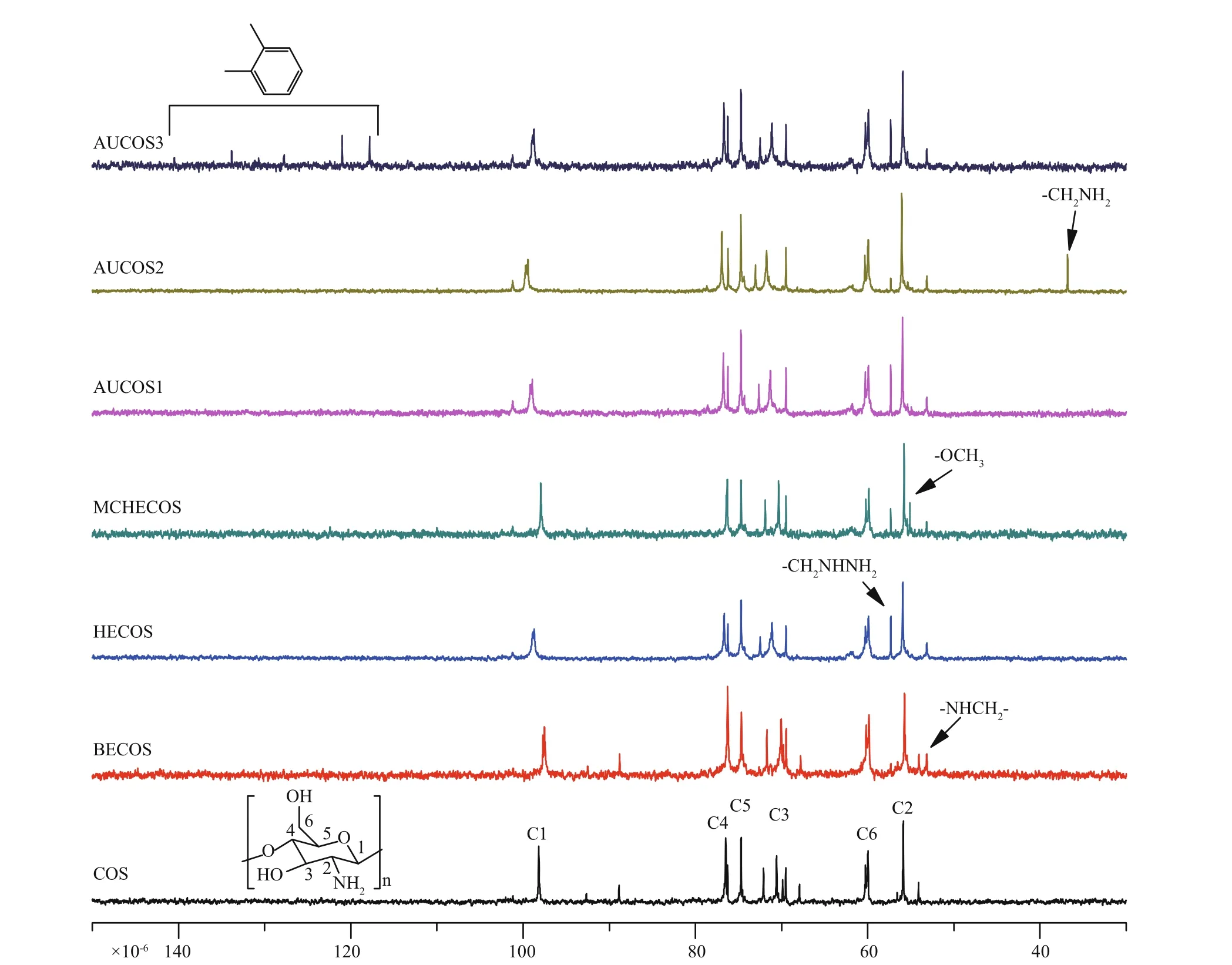
Fig.4 The 13 C NMR spectra of COS and the COS derivatives
Figure 4 shows the13C NMR spectra of COS and its derivatives. The signals of COS at 55.87×10-6, 59.97×10-6, 70.38×10-6, 74.69×10-6, 76.48×10-6and 99.18×10-6were attributed to C2, C6, C3, C5, C4 and C1, respectively. There was a new peak at 53.15×10-6(BECOS) representing the carbon of -NH-CH2-. A new signal for -CH2NHNH2(HECOS) appeared at 57.32×10-6, and for MCHECOS, the carbon in the methyl group of -COOCH3was at 55.11×10-6. Additionally, the peak at 55.11×10-6decreased in the spectrum of AUCOS1, suggesting the formation of ureido. Similar changes were observed in the spectra of AUCOS2 and AUCOS3. Moreover, a new peak at 36.80×10-6was assigned to the -CH2NH2of AUCOS2, and the six peaks at 117.81×10-6, 121.00×10-6, 127.73×10-6, 130.69×10-6, 133.82×10-6and 137.84×10-6were derived from the six carbon atoms of the benzene ring, demonstrating the structure of AUCOS3 again.
3.5 Elemental analysis and degree of substitution
The degree of substitution was estimated based on the elemental analysis data; all the results are shown in Table 1.
The equations for the DS are given below, and the DS of BECOS and HECOS were calculated according to Eq.2:
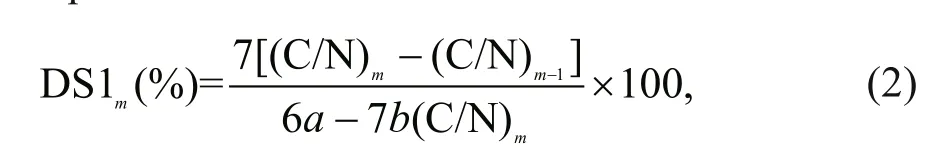
where (C/N)mis the mass ratio of the carbon and nitrogen in the compounds, m is the serial number of the compounds given in Table 1 ( m=2-3), and a and b are the numbers of carbon and nitrogen atoms introduced after modifi cation, respectively.
The DS of MCHECOS and the AUCOSs were calculated according to Eq.3:
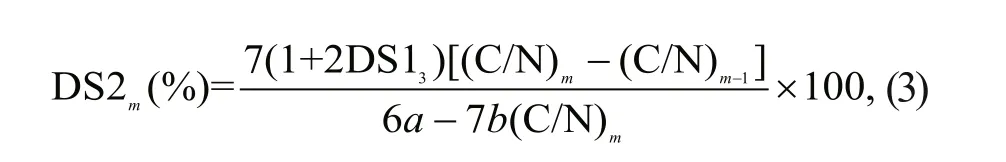
where (C/N)mis the mass ratio between the carbon and nitrogen in the compounds, m is the serial number of each compound given in Table 1 ( m=4-7), DS13is the degree of substitution of HECOS, calculated according to Eq.2, and a and b are the numbers of carbon and nitrogen atoms introduced after modifi cation, respectively.
As shown in Table 1, the DS values of AUCOSs were difference erent from each other. It was assumed that the reactivities of the three diamines with ester were difference erent, which might result from their difference erent steric hindrances.
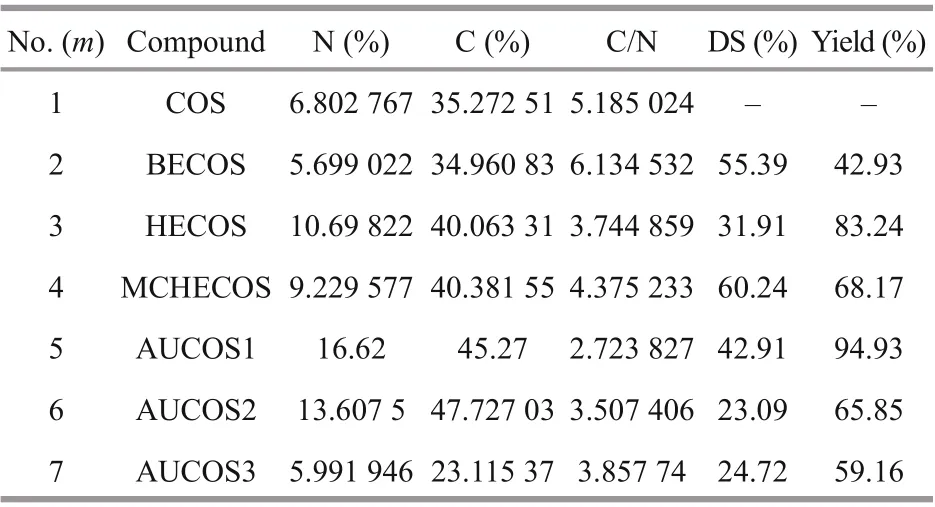
Table 1 The elemental analysis results, degrees of substitution and yields of COS and the COS derivatives
3.6 Antifungal activity
According to the literature (Seyfarth et al., 2008; Hussain et al., 2012; Rahman et al., 2015), chitooligosaccharide has inhibitory efference ects on various fungi. In this study, we tested the antifungal activities of COS and its derivatives against three plant pathogenic fungi, F. solani, V. albo- atrum and P. capsici. The samples were tested at concentrations ranging from 0.2 to 0.8 mg/mL for F. solani and V. albo- atrum and from 0.1 to 0.4 mg/mL for P. capsici. All the results are shown in Table 2.
It is obvious that the antifungal capabilities of all compounds increased with increasing concentration. The antifungal activities of COS and the COS derivatives against P. capsici were much better than those against F. solani and V. albo- atrum. Therefore, we tested the antifungal eき ciency against P. capsici at lower concentrations. As shown in Table 2, COS and its derivatives had good inhibitory efference ects on P. capsici, and the most eき cient compound was AUCOS2, whose highest inhibitory value was 85.23%. The intermediate product HECOS exhibited a slightly better inhibitory efference ect against P. capsici than that of the original chitooligosaccharide, and the AUCOSs showed relatively higher inhibitory efference ects against P. capsici than that of HECOS, but the increase in the inhibition was not high. Furthermore, it was interesting that iprodione showed weak activity against P. capsici, much weaker than that of COS.
The results show that the antifungal activities of HECOS and the AUCOSs signifi cantly increased compared with that of COS against F. solani and V. albo- atrum, with the highest inhibitory values ranging from 50.29% to 60.69% against F. solani and from 70.45% to 82.95% against V. albo- atrum. The three AUCOSs had stronger antifungal capabilities than that of HECOS against F. solani, especially AUCOS3, for which the inhibition was approximately 10% higher than that of HECOS at every concentration. The highest inhibitory rate of AUCOS3 was 60.69%, while it was only 29.48% for COS. This indicated that increasing the amount of amino groups in the COS molecules is helpful for strengthening the antifungal properties of COS, which is consistent with the results of previous reports (Meng et al., 2012). However, the inhibitory activities of the AUCOSs against V. albo- atrum were complicated. Both AUCOS1 and AUCOS3 had lower antifungal indices than that of HECOS, and only AUCOS3 showed a higher inhibitory eき ciency against V. albo- atrum than that of HECOS. There were signifi cant difference erences among the three aminourea chitooligosaccharide derivatives. The antifungal activity may be related not only to the amount of amino groups but also to the whole structure of the introduced amine fragments.
The maximum antifungal indices against F. solani were 60.69% (AUCOS3) and 60.12% (AUCOS2); the most powerful compounds against V. albo- atrum were AUCOS2 (82.95%) and HECOS (79.55%), while for P. capsici, they were AUCOS2 (85.23%) and AUCOS1 (84.66%). Hence, a desirable compound, AUCOS2, which could efference ectively inhibit F. solani, V. albo- atrum and P. capsici, was obtained.
4 DISCUSSION
Based on the above results and analysis, HECOS is more powerful against plant pathogenic fungi than COS. It can be summarized that introducing the hydrazine group can improve the antifungal activity of COS, which can be explained by the fact that the hydrazine group, which is composed of two amino groups, might enhance the strength of the positive charge of COS. Strongly electropositive compounds can interact with the electronegative fungal cell membranes and disturb their function, eventually causing death (Je and Kim, 2006; Li et al., 2010; Qin et al., 2014).
Similarly, the three AUCOSs, which contained more amino groups, are more powerful than HECOS against F. solani and P. capsici because of their higher positive charge intensity. However, the increasing rates of the antifungal indices are not high. It can be inferred that the formation of amide bonds resulted in a decrease in the strength of the positive charge of the amine, but the overall positive charge of the AUCOSs might still be slightly increased because of the presence of the additional primary amino groups. Furthermore, we speculated that the higher the degrees of substitution of the electropositive groups, the stronger the antimicrobial activities of the compounds, which can be proved by the fact that HECOS (DS=31.91%) is more efference ective than COS (DS=0) against the fungi. Interestingly, the results show that AUCOS1 has the highest DS among the AUCOSs but no the strongest antifungal activity. Although the DS of AUCOS2 is relatively low, AUCOS1 and AUCOS3 are less active than AUCOS2. These may be related to the difference erent structures of the three compounds. We assumed that the difference erent substituents caused difference erent cationic properties of the AUCOSs, and further afference ect their antifungal properties. To be more specifi c, the primary amino group contained in the -R group of AUCOS2 is far away from the electron-withdrawing group (carbonyl group), and the positive charge intensity of the primary amino group is less afference ected. As for AUCOS1 and AUCOS3, the primary amino groups in their molecules are close to the carbonyl group and phenyl group, respectively. The electron-withdrawing efference ect of the two groups reduces the abilities of the amino groups to bind protons. Once the amino group becomes inactive, it cannot react with the microbial cell membranes, limiting its ability to resist fungi. However, AUCOS3 was slightly more efference ective against F. solani than AUCOS2, perhaps because F. solani is also sensitive to phenyl groups, which are molecular fragments of many fungicides, such as iprodione.
However, both AUCOS1 and AUCOS2 were less efference ective than HECOS against V. albo- atrum, and it seems that a more positive charge strength is not the only factor infl uencing the antifungal abilities. The mechanism of the interaction between the active groups and fungi needs to be further explored. In addition, the antimicrobial activities of the mentioned COS derivatives are still relatively weak in comparison with the positive control against F. solani and V. albo- atrum, which deserves further research.
5 CONCLUSION
In this study, novel aminourea chitooligosaccharide derivatives (AUCOS) were synthesized, several methods (FTIR,1H NMR,13C NMR, and elemental analysis) were used to identify their structures, and the target compounds were prepared successfully. Moreover, the antifungal abilities of the obtained COS derivatives were evaluated, the aminourea chitooligosaccharide derivatives (AUCOS) and the intermediate product (HECOS) had stronger antifungal activities against F. solani, V. albo- atrum and P. capsici than those of chitooligosaccharide. In addition, AUCOS2 was the most promising antifungal compound, which had a greater inhibitory efference ect than that of HECOS for all the tested fungi. It was inferred that the sequential introduction of the hydrazine group and amine-carbonyl groups endowed the AUCOSs with an increased density of the cationic charge, which is an important and infl uential factor for antifungal capacity. Our research provides a new strategy for the derivatization of chitooligosaccharide, which can be used as a reference for the development of novel biological fungicides through the modifi cation of chitooligosaccharide.
6 DATA AVAILABILITY STATEMENT
The original data used to support the fi ndings of this study are available from the corresponding author upon request.
References
Badawy M E I, Rabea E I, Taktak N E M. 2014. Antimicrobial and inhibitory enzyme activity of N-(benzyl) and quaternary N-(benzyl) chitosan derivatives on plant pathogens. Carbohydrate Polymers, 111: 670-682.
Badawy M E I, Rabea E I. 2011. A biopolymer chitosan and its derivatives as promising antimicrobial agents against plant pathogens and their applications in crop protection. International Journal of Carbohydrate Chemistry, 2011: 460381.
Barchenger D W, Lamour K H, Bosland P W. 2018. Challenges and strategies for breeding resistance in Capsicum annuum to the multifarious pathogen, Phytophthora capsici. Frontiers in Plant Science, 9: 628.
Benhabiles M S, Salah R, Lounici H, Drouiche N, Goosen M F A, Mameri N. 2012. Antibacterial activity of chitin, chitosan and its oligomers prepared from shrimp shell waste. Food Hydrocolloids, 29(1): 48-56.
Coleman J J. 2016. The Fusarium solani species complex: ubiquitous pathogens of agricultural importance. Molecular Plant Pathology, 17(2): 146-158.
Deketelaere S, Tyvaert L, Franca S C, Höfte M. 2017. Desirable traits of a good biocontrol agent against verticillium wilt. Front iers in Microbiol ogy, 8: 1 186.
Fan Z Q, Qin Y K, Liu S, Xing R E, Yu H H, Chen X L, Li K C, Li P C. 2018. Synthesis, characterization, and antifungal evaluation of diethoxyphosphoryl polyaminoethyl chitosan derivatives. Carbohydrate Polymers, 190: 1-11.
Guo Z Y, Xing R E, Liu S, Zhong Z M, Ji X, Wang L, Li P C. 2007. The infl uence of the cationic of quaternized chitosan on antifungal activity. International Journal of Food Microbiology, 118(2): 214-217.
Hussain I, Singh T, Chittenden C. 2012. Preparation of chitosan oligomers and characterization: their antifungal activities and decay resistance. Holzforschung, 66(1): 119-125.
Je J Y, Kim S K. 2006. Chitosan derivatives killed bacteria by disrupting the outer and inner membrane. Journal of Agricultural and Food Chemistry, 54(18): 6 629-6 633.
Kim J Y, Lee J K, Lee T S, Park W H. 2003. Synthesis of chitooligosaccharide derivative with quaternary ammonium group and its antimicrobial activity against Streptococcus mutans. International Journal of Biological Macromolecules, 32(1-2): 23-27.
Kong M, Chen X G, Xing K, Park H J. 2010. Antimicrobial properties of chitosan and mode of action: a state of the art review. International Journal of Food Microbiology, 144(1): 51-63.
Li X F, Feng X Q, Yang S, Fu G Q, Wang T P, Su Z X. 2010. Chitosan kills Escherichia coli through damage to be of cell membrane mechanism. Carbohydrate Polymers, 79(3): 493-499.
Li Y, Liu S, Qin Y K, Xing R E, Chen X L, Li K C, Li P C. 2016. Synthesis of novel pyrimethanil grafted chitosan derivatives with enhanced antifungal activity. Biomed Research International, 2016: 8196960.
Liu B, Wang X Y, Pang C S, Luo J W, Luo Y Q, Sun R C. 2013. Preparation and antimicrobial property of chitosan oligosaccharide derivative/rectorite nanocomposite. Carbohydrate Polymers, 92(2): 1 078-1 085.
Liu W X, Qin Y K, Liu S, Xing R E, Yu H H, Chen X L, Li K C, Li P C. 2017. Synthesis, characterization and antifungal eき cacy of C-coordinated O-carboxymethyl chitosan Cu(II) complexes. Carbohydrate Polymers, 160: 97-105.
Liu W X, Qin Y K, Liu S, Xing R E, Yu H H, Chen X L, Li K C, Li P C. 2018. Synthesis, characterization and antifungal eき cacy of chitosan derivatives with triple quaternary ammonium groups. International Journal of Biological Macromolecules, 114: 942-949.
Meng X T, Xing R E, Liu S, Yu H H, Li K C, Qin Y K, Li P C. 2012. Molecular weight and pH efference ects of aminoethyl modifi ed chitosan on antibacterial activity in vitro. International Journal of Biological Macromolecules, 50(4): 918-924.
Park P J, Je J Y, Byun H G, Moon S H, Kim S K. 2004. Antimicrobial activity of hetero-chitosans and their oligosaccharides with difference erent molecular weights. Journal of Microbiology and Biotechnology, 14(2): 317-323.
Qin Y K, Liu S, Xing R E, Yu H H, Li K C, Meng X T, Li R F, Li P C. 2012. Synthesis and characterization of dithiocarbamate chitosan derivatives with enhanced antifungal activity. Carbohydrate Polymers, 89(2): 388-393.
Qin Y K, Xing R E, Liu S, Yu H H, Li K C, Hu L F, Li P C. 2014. Synthesis and antifungal properties of (4-tolyloxy)-pyrimidyl-α-aminophosphonates chitosan derivatives. International Journal of Biological Macromolecules, 63: 83-91.
Rabea E I, Badawy M E T, Stevens C V, Smagghe G, Steurbaut W. 2003. Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules, 4(6): 1 457-1 465.
Rahman H, Hjeljord L G, Aam B B, Sørlie M, Tronsmo A. 2015. Antifungal efference ect of chito-oligosaccharides with difference erent degrees of polymerization. European Journal of Plant Pathology, 141(1): 147-158.
Seyfarth F, Schliemann S, Elsner P, Hipler U C. 2008. Antifungal efference ect of high- and low-molecular-weight chitosan hydrochloride, carboxymethyl chitosan, chitosan oligosaccharide and N-acetyl-D-glucosamine against Candida albicans, Candida krusei and Candida glabrata. International Journal of Pharmaceutics, 353(1-2): 139-148.
Song Y P, Miao F P, Liu X H, Ji N Y. 2019. Responses of marine-derived Trichoderma fungi to seawater and their potential antagonistic behaviour. Journal of Oceanology and Limnology, 37(2): 525-534.
Song Y P, Shi Z Z, Miao F P, Fang S T, Yin X L, Ji N Y. 2018. TricholuminA, a highly transformed ergosterol derivative from the alga-endophytic fungus Trichoderma asperellum. Organic Letters, 20(19): 6 306-6 309.
Sun S L, An Q Z, Li X, Qian L Y, He B H, Xiao H N. 2010. Synergistic efference ects of chitosan-guanidine complexes on enhancing antimicrobial activity and wet-strength of paper. Bioresource Technology, 101(14): 5 693-5 700.
Tan W Q, Zhang J J, Luan F, Wei L J, Chen Y, Dong F, Li Q, Guo Z Y. 2017. Design, synthesis of novel chitosan derivatives bearing quaternary phosphonium salts and evaluation of antifungal activity. International Journal of Biological Macromolecules, 102: 704-711.
Tripathi P, Dubey N K. 2004. Exploitation of natural products as an alternative strategy to control postharvest fungal rotting of fruit and vegetables. Postharvest Biology and Technology, 32(3): 235-245.
Vinšová J, Vavříková E. 2011. Chitosan derivatives with antimicrobial, antitumour and antioxidant activities—a review. Current Pharmaceutical Design, 17(32): 3 596-3 607.
Xia W S, Liu P, Zhang J L, Chen J. 2011. Biological activities of chitosan and chitooligosaccharides. Food Hydrocolloids, 25(2): 170-179.
Yuan B, Xu P Y, Zhang Y J, Wang P P, Yu H, Jiang J H. 2014. Synthesis of biocontrol macromolecules by derivative of chitosan with surfactin and antifungal evaluation. International Journal of Biological Macromolecules, 66: 7-14.
Yue L, Li J R, Chen W W, Liu X L, Jiang Q X, Xia W S. 2017. Geraniol grafted chitosan oligosaccharide as a potential antibacterial agent. Carbohydrate Polymers, 176: 356-364.
Zhang Y B, Dang Q F, Liu C S, Yan J Q, Cha D S, Liang S N, Li X L, Fan B. 2017. Synthesis, characterization, and evaluation of poly(aminoethyl) modifi ed chitosan and its hydrogel used as antibacterial wound dressing. International Journal of Biological Macromolecules, 102: 457-467.
Zhao X, He J X, Zhan Y Z. 2009. Synthesis and characterization of chitosan biguanidine hydrochloride under microwave irradiation. Polymer Journal, 41(12): 1 030-1 035.
 Journal of Oceanology and Limnology2020年3期
Journal of Oceanology and Limnology2020年3期
- Journal of Oceanology and Limnology的其它文章
- List of the Most Outstanding Papers Published by CJOL/JOL in 2017-2018
- Who is the “murderer” of the bloom in coastal waters of Fujian, China, in 2019?*
- The investigation of internal solitary waves over a continental shelf-slope*
- Efference ect of diets on the feeding behavior and physiological properties of suspension-feeding sea cucumber Cucumaria frondosa*
- Efference ects of light quality on growth rates and pigments of Chaetoceros gracilis (Bacillariophyceae)*
- Marine bacterial surfactin CS30-2 induced necrosis-like cell death in Huh7.5 liver cancer cells*
