Efference ect of diets on the feeding behavior and physiological properties of suspension-feeding sea cucumber Cucumaria frondosa*
YU Zonghe , , ROBINSON Shawn , MACDONALD Bruce , LANDER Terralynn , SMITH Craig
1 College of Marine Sciences, South China Agricultural University, Guangzhou 510642, China
2 Fisheries and Oceans Canada, St. Andrews Biological Station, St. Andrews, NB E5B 2L9, Canada
3 Biology Department and Centre for Coastal Studies and Aquaculture, University of New Brunswick, Saint John, NB E2L 4L5, Canada
Received Jul. 19, 2019; accepted in principle Aug. 21, 2019; accepted for publication Sep. 25, 2019 © Chinese Society for Oceanology and Limnology, Science Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
A bstract The suspension-feeding sea cucumber Cucumaria frondosa has become commercially important in recent years. Finding proper diets is the fi rst important step for intensive aquaculture of this sea cucumber. In this study, adult C. frondosa were exposed to one of the following diet treatments: control (no diet provided), two powdered seaweeds ( Ascophyllum nodosum and Saccharina latissima), a commercially available microalgal diet (shellfi sh diet) and natural seston. The efference ects of diets on the feeding behavior and physiological properties of sea cucumbers were investigated after a 5-week rearing period. Results show that sea cucumbers fed with shellfi sh diet exhibited a signifi cantly higher tentacle insertion rate (1.80± 0.20 insertion/min) than these fed with seaweed powders, and there was no signifi cant difference erent between the two groups fed by seaweed powders. No signifi cant difference erence was found on the fecal production rate among the feeding groups. The minimum oxygen consumption rate was observed in the control group (5.76±0.99 μg O 2/(g∙h)), which is signifi cantly lower than individuals fed with A. nodosum, shellfi sh diet, and natural seston; however, no signifi cant difference erence was shown between those of control and S. latissimi groups. The maximum ammonium excretion rate was found in the A. nodosum group (0.03±0.01 μmol/(g∙h)), which is signifi cantly higher than other groups. The minimum O/N ratio was observed in the A. nodosum group (14.57±1.04), which is signifi cantly lower than the S. latissima, shellfi sh diet, and natural seston groups. Individuals fed with seaweed powders had similar physiological properties with these fed with microalgae diet and natural seston, indicating that A. nodosum and S. latissima can be explored as promising diets for intensive aquaculture of C. frondosa.
K eyword: sea cucumber; Cucumaria frondosa; physiological property; feeding behavior; nutritional condition
1 INTRODUCTION
The orange-footed sea cucumber Cucumaria frondosa (Gunnerus, 1767) is widely distributed along the sub-tidal zone of North Atlantic and Arctic Ocean (Hamel and Mercier, 2008; Nelson et al., 2012a, b). In recent years, this sea cucumber has been accepted by the Asian food market, and price of the C. frondos a is US$ 0.25 per animal (fresh) (Purcell et al., 2012). The anti-hyperglycemic and antiproliferative properties of extracts from this species may increase its market value as a medicinal source (Al Shemaili et al., 2014; Hu et al., 2014). In addition, C. frondosa has been shown to be a potentially important species that can work as an extractive species in the integrated multi-trophic aquaculture (IMTA) system (Nelson et al., 2012a, b).
Sea cucumber fi sheries throughout the world are generally characterized by overexploitation and boom and bust cycles (Uthicke and Conand, 2005; Nelson et al., 2012a). The developing fi shery for C. frondosa on the east coast of North America has expanded rapidly since the 1990s. However, the future, largescale exploitation of this fi shery is still uncertain, for the slow growth rate and the slow rate of resource renewal may make C. frondosa more vulnerable to exploitation than other sea cucumber species (Hamel and Mercier, 1996a; Therkildsen and Petersen, 2006; Nelson et al., 2012a; Gianasi et al., 2016). Although some local management efference orts have already been made for this fi shery resource, signs of overfi shing have already been reported along the coast of North America, and the sustainable utilization of this species is still a big concern (Bruckner, 2005; Therkildsen and Petersen, 2006; Hamel and Mercier, 2008).
Aquaculture has been shown to be an eき cient way to satisfy the increasing demand for sea cucumber products. The production of some traditionally consumed beche-de-mer species that are sufference ering from overexploitation (e.g., Apostichopus japonicus), are now relying heavily upon aquaculture (Hamel et al., 2001; Nelson et al., 2012a). The sea cucumber C. frondosa has potential as an aquaculture species, and culture may be a viable choice to protect the natural resource while providing a constant supply to meet the demand for this species, however, not enough has been done yet to the aquaculture of this sea cucumber (Nelson et al., 2012a; Gianasi et al., 2016).
Since C. frondosa is a passive suspension-feeder that feeds upon particles suspended in the water column, ocean-based aquaculture of C. frondosa would be limited by seasonal phytoplankton blooms, for this sea cucumber feeds mainly during spring and summer, when food is more available (Singh et al., 1998; Nelson et al., 2012a). It takes approximately 3.3 years for the C. frondosa to obtain a size of 107 mm and 5.5 years to reach a length of 120 mm in the St. Lawrence Estuary (Hamel and Mercier, 1996a). Intensive aquaculture is often used as an eき cient method for the mass production of sea cucumbers (Yuan et al., 2006; Eriksson et al., 2012). In the natural habitat, C. frondosa is usually patchily distributed, and the abundance in some area is very high, e.g., the density of C. frondosa varied between 5 and 18 ind./m2in St. Lawrence Estuary, which corresponding to a biomass of about 3 to 15 kg/m2(Hamel and Mercier, 1996b). The density could reach 50 ind./m2on the hard rocky substrates in the Passamaquoddy Bay (Singh et al., 2001). All these observations suggest this sea cucumber is able to withstand high stocking density in culture, and the intensive aquaculture of C. frondosa may be possible.
Obtaining high-quality diets with a low relative cost is one of the most important steps for sea cucumber aquaculture. Previous studies found that the natural seston, microalgae diets and fi sh eggs could be consumed eき ciently by C. frondosa (Nelson et al., 2012b; Gianasi et al., 2016), however, the aforementioned diets may not be suitable for the intensive aquaculture of C. frondosa due to limitations of seasonally low concentration or high prices. Powders made from seaweeds, such as Laminaria japonica and Sargassum thunbergii, have a high nutrient content and are usually used as high-quality diets for the deposit-feeding sea cucumber A. japonicus in China (Yuan et al., 2006; Liu et al., 2009). It is unknown whether seaweeds can be used as eき cient and cost-efference ective diets for a suspensionfeeding sea cucumber.
The feeding and physiological properties, such as respiration and excretion, of deposit-feeding sea cucumbers can be signifi cantly infl uenced by diets, and these parameters associated well with the nutritional status of sea cucumbers (Yuan et al., 2006; Liu et al., 2009; Maxwell et al., 2013). So far, however, little related information is available on the suspension-feeding sea cucumber C. frondosa. In the present study, we fed C. frondosa with seaweed powders, a mixed concentrated microalgal diet and natural seston, and tested the nutritional potential of these diets by examining the feeding behaviour and physiological properties, such as tentacle insertion, fecal production, oxygen consumption and ammonium excretion rates.
2 MATERIAL AND METHOD
2.1 Sea cucumber collection and acclimation
Sea cucumbers were collected by SCUBA diving from the subtidal zone of the Passamaquoddy Bay, southwest New Brunswick, Canada (45°03′37″N, 67°00′41″W) on July 7, 2014. After collection, they were transferred to the laboratory at the St. Andrews Biological Station (St. Andrews, New Brunswick, Canada), and acclimated in 100 L tanks with fl owthrough sand-fi ltered sea water (2-3 L/min) for four weeks during which the sea cucumbers were fed with commercial concentrated marine microalgae, the microalgae diet and water parameters were similar to the laboratory trials (see below). A simulated natural photoperiod (12 h light/12 h dark) was used throughout the period of experiment. After acclimation, 25 adult sea cucumbers with similar wet weight (261.07± 29.33 g, mean±SD) were selected for the experiment. All individuals used in this study were free of any obvious injury.
2.2 Seaweeds diet preparation
Two common seaweeds of the Canadian coast were used in this study. The seaweed knotted wrack ( Ascophyllum nodosum) was collected from the intertidal zone ofference the St. Andrews Biological Station, and the seaweed sugar kelp ( Saccharina latissima) was collected from mooring lines on a local fi sh farm in Passamaquoddy Bay, and both were prepared as diets for C. frondosa. All the seaweeds were washed with fresh water and oven-dried at 60°C to constant weight, ground into fi ne particles with a cofference ee grinder, passed through a 200-μm screen sieve and stored in plastic ziplock bags at 4°C for further use. The particle sizes of the seaweed diets were within the diameter range that could be fully ingested by C. frondosa (<350 μm) (Hamel and Mercier, 1998; Holtz and MacDonald, 2009).
2.3 Experimental design
The feeding experiment was carried out from August 7 to September 16, 2014. Sea cucumbers were randomly selected and divided into fi ve groups (fi ve individuals per group), of which, four groups were used in the laboratory trials, and one group was used in the fi eld trial.
2.3.1 Laboratory trials
Each sea cucumber used in the laboratory trials was held individually in a 132-L cylindrical tank (diameter 65 cm, height 40 cm) with fl owing sandfi ltered seawater (3 L/min) and aeration. They were exposed to one of the four dietary treatments: (1) control (no diet provided); (2) A. nodosum powder; (3) S. latissima powder; or (4) a commercial microalgae diet (Instant Algae Shellfi sh Diet 1800, Reed Mariculture, Campbell, CA, USA) that contained a mix of 40% Isochrysis, 15% Pavlova, 25% Tetraselmis and 20% Thalassiosira pseudonana (dry weight 8%). There were fi ve replicate tanks for each treatment. A continuous feeding method was used to feed the C. frondosa of each tank. Each sea cucumber was fed at a rate of 10 g per day (i.e., 10 g pre-weighed seaweed powder (dry weight) or 125 g shellfi sh diet (wet weight) was mixed with seawater in a 20-L bucket with continual aeration to disperse food particles and prevent clumping) and the suspension was delivered to each rearing tank by using a variable fl ow peristaltic mini-pump (Fisher Scientifi c, Waltham, MA, USA). The total particulate matter (TPM) in the water column of each feeding tank was always kept at a concentration of 10-15 mg/L during the daytime for ~10 h. Feedings and fl owing waters were stopped during the night, and sea cucumbers were continued to be fed by the residual diet in the tanks during this period. Diet in each tank was assumed to be suき cient for the experimental sea cucumber, as the TPM provided in the experiment was higher than that in the fi eld (2.18-3.21 mg/L) (Nelson et al., 2012b). Feces and uneaten diets were removed from the tanks by siphoning every three days.
2.3.2 Field trial
Sea cucumbers used for the fi eld trial were placed in fi ve 5-L square plastic aquaponic baskets (Finofi l, UK) (length and width 24 cm, height 14 cm; one individual per basket), suspended at a depth of 2 m beside the wharf of St. Andrews Biological station (45°04′58″N, 67°05′05″W) and exposed to the natural seston. Each basket was covered with 0.6 cm mesh net and weighted with a 1-kg weight on the bottom to reduce disturbance by the current, and the horizontal distance between the baskets was 1 m. The culture density of sea cucumbers here was much lower than that found in the natural habitat, therefore the seston concentration in the fi eld trial was assumed suき cient for the experimental individuals. The baskets were retrieved and cleaned once a week during the experiment period.
2.4 Tentacle insertion rate
The tentacle insertion rate of the sea cucumber was measured in the last week of this study. The tentacle insertion rate (TIR) defi ned as the number of tentacles put into the mouth per minute can be used as indicator of food ingestion for C. frondosa (Holtz and MacDonald, 2009). The TIRs of all the C. frondosa maintained in the laboratory were monitored 2 h after the feeding started using the protocols of Singh et al. (1998). The time was suき cient for the individuals to exhibit normal feeding behavior, as it generally takes approximately 30 min for the C. frondosa to begin feeding when they are exposed to food (Holtz and MacDonald, 2009). The TIR of sea cucumbers maintained in the fi eld was not directly observed during this study.
2.5 Physiological properties
2.5.1 Fecal production rate
The fecal production rate (FR, mg/(g∙d)) of the sea cucumbers was measured just after the routine daytime feeding in the last week of this study. All individuals, including the ones used in the fi eld trial, were placed individually into cylinder traps (diameter 15 cm, height 25 cm; covered with 0.6-cm mesh net), and maintained in the rearing tanks in the laboratory overnight (~12 h, no diet was provided during this period). The feces produced by each sea cucumber was suction fi ltered onto pre-treated Whatman GF/C fi lters (see method for measuring TPM below) and rinsed with distilled, deionized water to remove excess salt, and then dried at 60°C for 24 h to get a constant weight. The wet weight of sea cucumbers were measured at the end of the experiment (see below). The FR was calculated using the following equation:

where F is the dry weight of feces (mg); W is the wet weight of sea cucumbers (g); and t is the duration of the experiment (d).
2.5.2 Oxygen consumption and ammonium excretion rates
After the fecal production rate measurement, sea cucumbers were deprived of food for 24 h to completely empty their gut, and then they were carefully placed individually into 3.7-L transparent glass jars, sealed under water to exclude air bubbles. Three jars fi lled with seawater only were prepared concurrently as controls. The jars were incubated under dark condition in the rearing tanks with fl owing water for 3-4 h, and then the oxygen concentration in each jar were measured directly from outside with a fi bre optical oxygen meter (Presens, Precision Sensing GmbH, Regensburg, Germany) using oxygensensitive luminescent sensor foil mounted inside the jars. Oxygen concentrations in the experimental jars were never less than 75% of the controls after incubation. Water samples were then collected for the measurement of ammonium concentration using an improved fl uorometric method (Taylor et al., 2007).
After water sample collection, sea cucumbers were blotted on paper towel for 30 s to get a constant weight, and their body volumes were then measured using the water displacement method (King II, 1993).
The oxygen consumption rate (OCR, μg O2/(g∙h)) and ammonium excretion rate (AER, μmol/(g∙h)) of the sea cucumbers were calculated using the following equations:

where C0is the oxygen concentration (μg/L) or ammonium concentration (μmol/L) after incubation in the control jar; Ceis the oxygen concentration (μg/L) or ammonium concentration (μmol/L) after incubation in the experimental jar; V is the volume of the jar corrected by the volume of sea cucumber (L); W is the wet weight of the sea cucumber (g); and t is the duration of the experiment (h).
The atomic O/N ratio, a useful physiological index for measuring the catabolic balance process, was estimated for each of the tested sea cucumber from the OCR and AER (Yang et al., 2006; Yu et al., 2013).
2.6 Environmental parameters
Water parameters were monitored once every 3 d during the experiment period. Water temperature (°C) and salinity in both laboratory and fi eld were monitored using a CastAway CTD (Sontek, San Diego, CA, USA). Flow speeds in the laboratory and fi eld were monitored using an Argonaut-ADV ultrasonic velocity meter (SonTek, San Diego, CA, USA). The current speeds beside the wharf were measured at the experimental depth during the spring tide.
2.7 Diet characteristics analysis
Triplicate water samples in the fi eld were collected at the depth where the sea cucumbers were held in the morning, noon and afternoon, with a 2.5-L Niskin sampling bottle. Total particulate matter (TPM, mg/L) and particulate organic matter (POM, mg/L) were analyzed by fi ltering 1 L of the seawater samples through 47 mm Whatman GF/C fi lters that previously had been combusted (475°C for 5 h) and weighed. After fi ltration, the fi lters were rinsed with isotonic ammonium formate solution, dried at 60°C for 48 h to a constant weight and weighed to derive the TPM. Filters were then combusted in a muラ e furnace at 475°C for 5 h and re-weighed; the weight difference erence between 60 and 475°C was defi ned as the POM. The organic matter content (OM, %) of seston was computed as POM/TPM×100 (Cranford et al., 2005). Organic carbon (OC, %) and total nitrogen (TN, %) of seston in the water samples were collected by fi ltering 0.5-L seawater samples through precombusted 25-mm Whatman GF/C fi lters, rinsed and dried as described above. The OC and TN of particles collected on fi lters were measured using a CN analyzer (VarioMax, Elementar GmbH, Hanau, Germany), after fuming the fi lters over concentrated HCl in a closed container overnight to remove inorganic carbon. TPM, POM and OM were monitored once per three days; POC and PN were monitored once every six days.
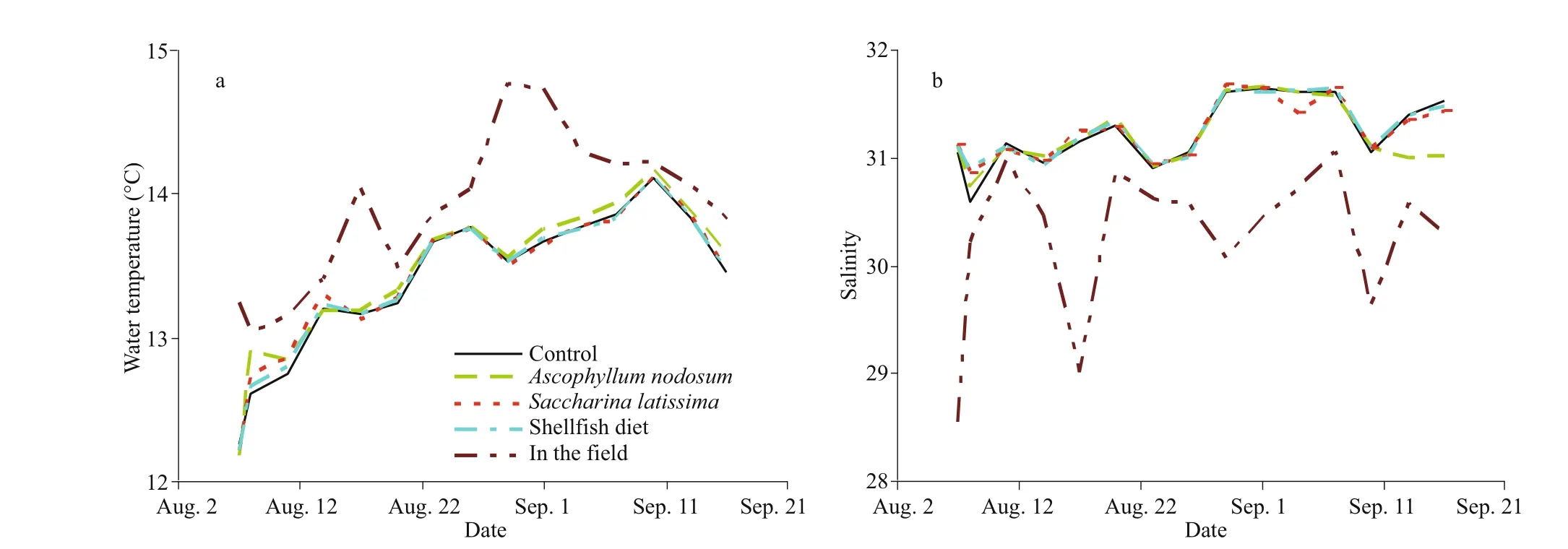
Fig.1 Temporal variation of environmental parameters in the laboratory and fi eld from August to September, 2014
The OM of seaweed powder was analysed using the combustion method (475°C for 5 h), the organic carbon (OC, %) and total nitrogen (TN, %) were measured using a CN analyzer as described above.
The commercial microalgae diet (Shellfi sh Diet 1800) composition was measured by fi ltering a small volume (~0.2 mL) of the concentrated microalgae solution through a pre-treated Whatman GF/C fi lter. OM, OC and TN of particles retained on the fi lters were then treated and analysed as the seston samples.
Diet quantity was measured as TPM in the water column, diet qualities are expressed as OM, OC and TN.
2.8 Statistical analysis
Statistical analysis was performed using SPSS 19.0 for Windows (IBM Corp., Armonk, NY, USA). Data on the feeding behaviour and physiological parameters from difference erent experimental groups were analysed using one-way ANOVA, followed by comparisons of means by the Student-Newman-Keuls (SNK) test. Statistical signifi cance was set at P <0.05.
3 RESULT
3.1 Environmental parameters
The ambient water temperatures in the laboratory ranged between 12.2 and 14.2°C during the study period (Fig.1a). All the rearing tanks had very similar values and water temperatures increased steadily with time until September 10thsampling, after which they began to decline. The water temperatures in the fi eld ranged between 13.0 and 14.8°C during the experiment, which were slightly higher than those in laboratory. The salinity levels in the laboratory were stable (around 31) over the whole experiment (Fig.1b). By contrast, the salinity in the fi eld fl uctuated over time, with comparatively low values (ranging between 28.5 and 31.0) throughout the experiment. The salinities in the laboratory were always higher than these in the fi eld at comparable sampling times. Low salinities in August 7 and 17 were mainly caused by heavy rainfalls. The fl ow speeds in the rearing tanks of the laboratory were all less than 30 cm/s, and the average current speed in the fi eld during the spring tide was only 25 cm/s.
3.2 Diet characteristics
The TPM concentrations of seston in the fi eld were relatively low (<3.5 mg/L) during the fi rst three weeks of this study, and then the mean values increased greatly with higher variability during the September samplings (ranging from 7.38±1.47 to 15.71±6.87 mg/L) (Fig.2). A similar time trend was observed in POM, with levels ranging between 0.97±0.06 to 5.52±1.43 mg/L during the study period. Values of OM were relatively constant, ranging between 31.90±2.76 to 48.86±6.73 in % (average 39.46±4.90 in %, see Table 1) throughout the study period. Values in September were often lower than those in August.
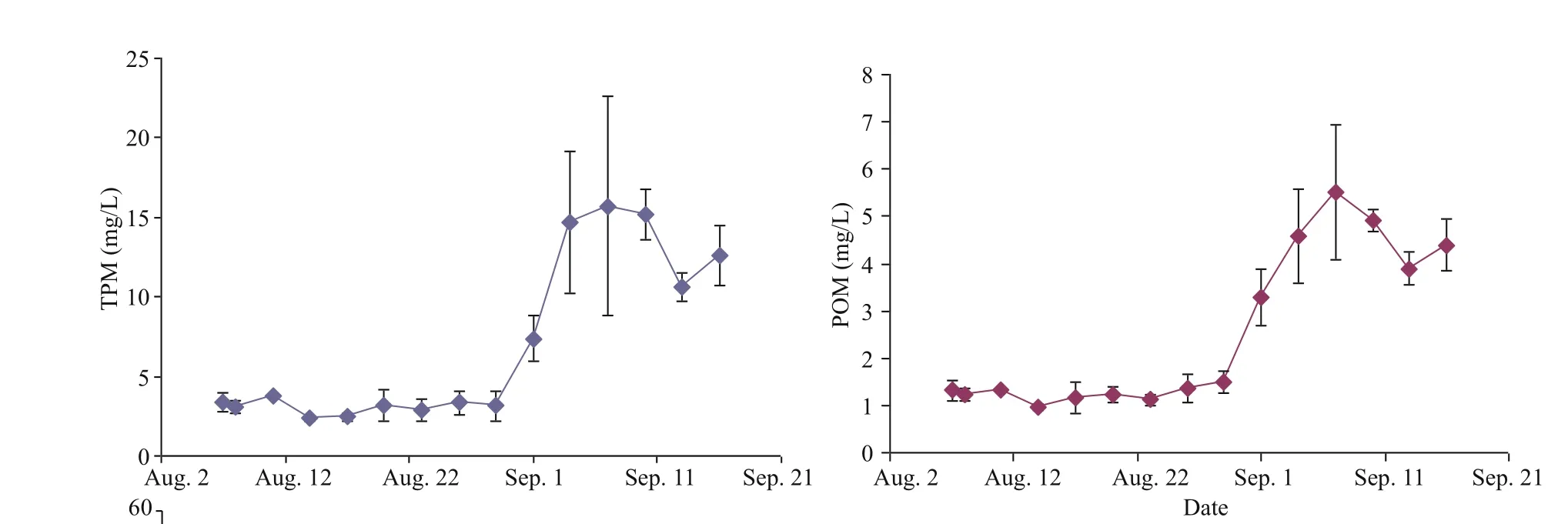
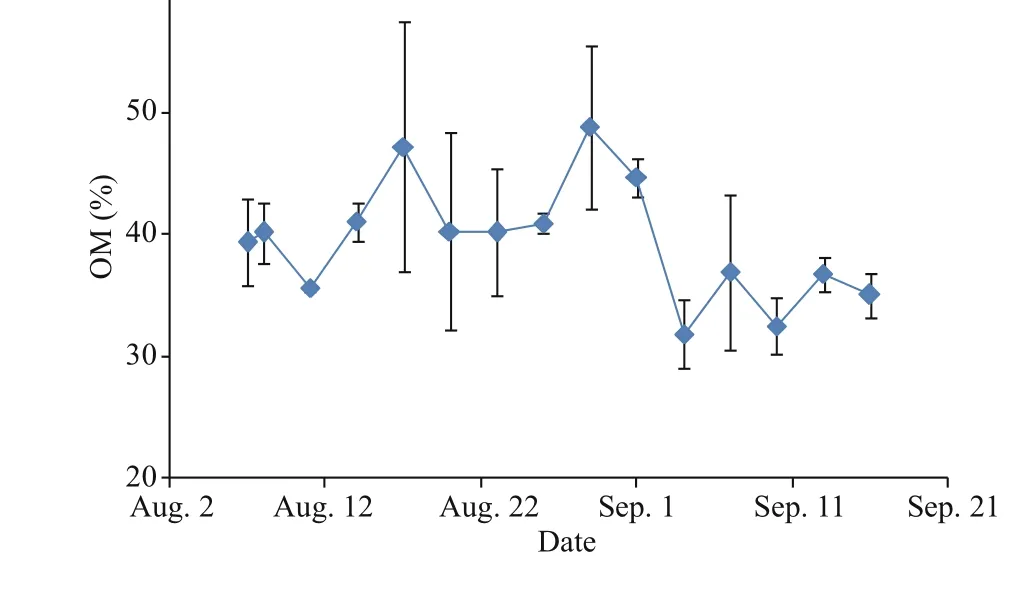
Fig.2 Temporal variation of total particulate matter (TPM), particulate organic matter (POM) and organic matter content (OM) of the seston in the field in mean±SD (n=3)
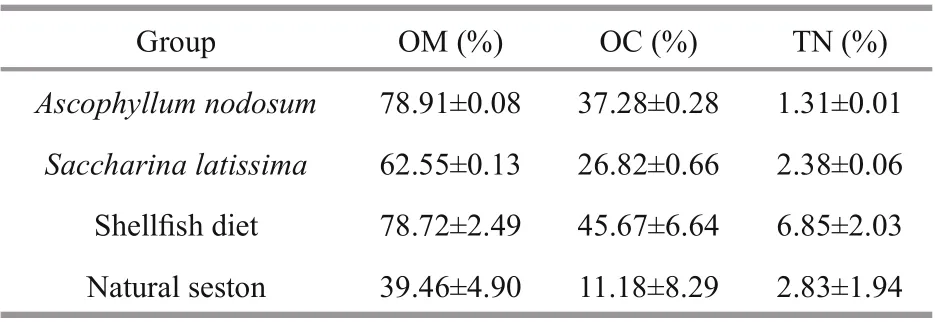
Table 1 Characteristics of the experimental diets used in the current study
The quality of the diets varied as the average OM of natural seston (39.46±4.90 in %) was only half that of shellfi sh diet (78.72±2.49 in %) and A. nodosum (78.91±0.08 in %) (Table 1). The OM of S. latissima (62.55±0.13 in %) was lower than that of shellfi sh diet and A. nodosum, but much higher than that of the natural seston. Among the four diets, the natural seston had the lowest OC value (11.18±8.29 in %), while the maximum OC value was found in the shellfi sh diet (45.67±6.64 in %). The minimum TN value was found in the A. nodosum (1.31±0.01 in %), which was only 20% of the maximum value found in the shellfi sh diet (6.85±2.03 in %), and half that of S. latissima (2.38±0.06 in %) and natural seston (2.83±1.94 in %).
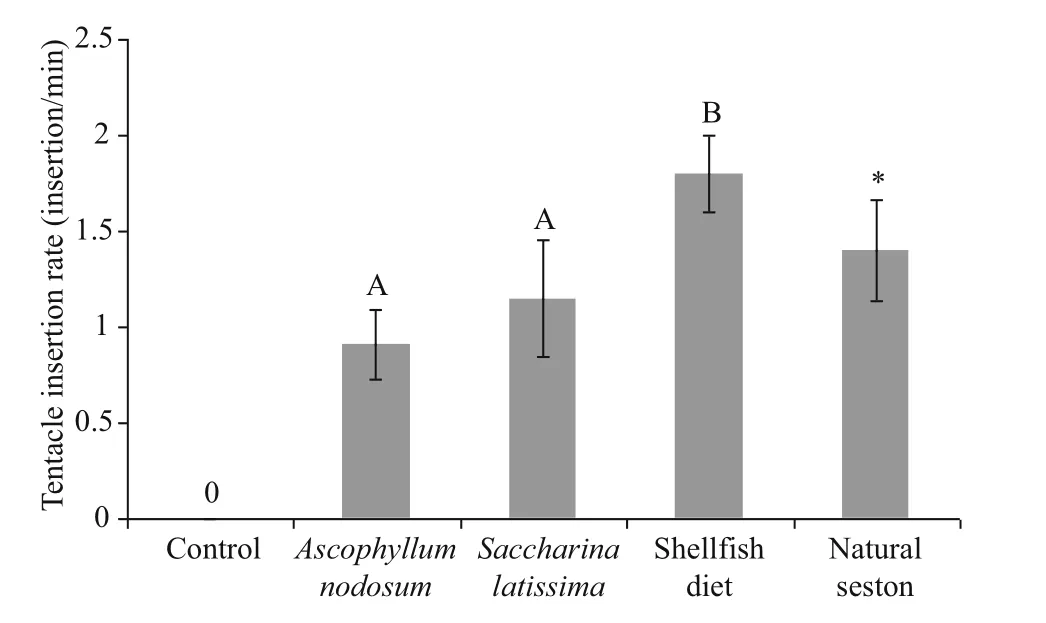
Fig.3 Tentacle insertion rate in Cucumaria frondosa for difference erent diet treatments in mean±SD ( n=5)
3.3 Tentacle insertion rate
The TIR of C. frondosa difference ered signifi cantly among the three feeding groups in the laboratory (one-way ANOVA, F=31.326, df=2, 14, P <0.001) (Fig.3), with the highest value observed in the shellfi sh diet group (1.80±0.20 insertion/min), and there was no signifi cant difference erent between the two groups fed by seaweed powders. No feeding behaviour was observed in the control group. The TIR of C. frondosa from Passamaquoddy Bay in the literature was 1.40±0.26 insertion/min when the individuals were exposed to relatively low current velocities (2-33 cm/s) (Holtz and MacDonald, 2009) and are only shown here for reference. In this study, the current speed around the wharf was very low, therefore we take the aforementioned value to represent the TIR of C. frondosa maintained in the fi eld; it was lower than that of individuals fed with shellfi sh diet, but higher than these fed with seaweed powders.
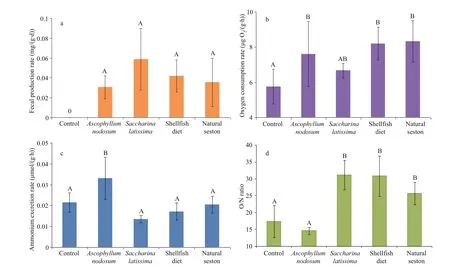
Fig.4 Physiological properties in Cucumaria frondosa for difference erent diet treatments in mean±SD ( n=5)
3.4 Physiological properties
3.4.1 Fecal production rate
The fecal production rates of sea cucumbers fed with A. nodosum, S. latissima, shellfi sh diet and natural seston were 0.03±0.01, 0.06±0.03, 0.04±0.02 and 0.04±0.02 mg/(g∙d), respectively. There was no signifi cant difference erence among these four feeding groups (one-way ANOVA, F=1.235, df=3, 19, P=0.340) (Fig.4a). No feces were produced by the sea cucumbers in the control group.
3.4.2 Oxygen consumption and ammonium excretion rates
The oxygen consumption rate of C. frondosa varied signifi cantly in response to diet treatments (one-way ANOVA, F=3.518, df=4, 24, P=0.032) (Fig.4b). The minimum value was observed with the control group (5.76±0.99 μg O2/(g∙h)), which was signifi cantly lower than those individuals fed with A. nodosum, S. latissima, shellfi sh diet and natural seston (7.61±1.85, 6.67±0.41, 8.21±0.93 and 8.34±1.17μg O2/(g∙h), respectively). No statistically signifi cant difference erence was observed among the four feeding groups.
The AER (ammonium excretion rate) of C. frondosa varied signifi cantly in response to diet treatments (one-way ANOVA, F=6.985, df=4, 24, P=0.002) (Fig.4c). The maximum value recorded in the group fed with A. nodosum (0.03±0.01 μmol/(g.h)), was signifi cantly higher than the other groups (0.02±0.00, 0.01±0.00, 0.02±0.00 and 0.02±0.00 μmol/(g∙h), respectively). There was no signifi cant difference erence for AERs among the other four groups.
For the atomic O/N ratios calculated on OCR and AER, there were signifi cant difference erences among groups with difference erent diet treatments (one-way ANOVA, F=13.125, df=4, 24, P <0.001) (Fig.4d). The minimum O/N ratio of the group fed with A. nodosum (14.57±1.04) was signifi cantly lower than that of group fed with S. latissima, shellfi sh diet and natural seston (31.13±4.33, 30.86±5.97 and 25.73±3.31, respectively). There was no signifi cant difference erence between the values of control and A. nodosum groups.
4 DISCUSSION
4.1 Tentacle insertion rate
The TIR is a useful indicator of food intake for C. frondosa (Singh et al., 1998; Holtz and MacDonald, 2009). Previous studies indicated that the TIR of C. frondosa could be infl uenced by the water velocity, however it was independent of fl ow with water velocities <40 cm/s (Holtz and MacDonald, 2009). In the current study, the fl ow velocities both in laboratory and fi eld were all <30 cm/s, therefore, it can be concluded that the fl ow velocity was not the key factor infl uencing the TIR of the four feeding groups. The feeding response of C. frondosa may be initiated by chemical and/or physical stimuli, and food concentration was found to be a key factor infl uencing the TIR (Hamel and Mercier, 1998; Singh et al., 1998). In this study, although the food concentrations were similar among the feeding groups in the laboratory (10-15 mg/L), the C. frondosa fed with the shellfi sh diet exhibited a signifi cantly higher TIR than those fed with seaweed powders, which indicated that the microalgae diet could give a stronger stimulus for the feeding response than the seaweed powders.
4.2 Physiological properties
4.2.1 Fecal production rate
The fecal production rate of sea cucumbers is well associated with the food ingestion rate (Yuan et al., 2006; Liu et al., 2009; Maxwell et al., 2009; Shi et al., 2013). In this study, the FR of C. frondosa did not vary signifi cantly among the three feeding groups in laboratory; therefore we concluded that individuals might have similar food ingestion rates, since the TIR of the sea cucumbers fed with shellfi sh diet was higher, the amount of food captured per tentacle insertion might have been lower than other groups. The high TIR, but comparatively low FR for C. frondosa fed with shellfi sh diet, indicated that the individuals spent more energy capturing food particles than those fed with seaweed powders, and this may not be economical and eき cient for the aquaculture of this sea cucumber.
4.2.2 Oxygen consumption and ammonium excretion rates
The respiration and excretion rates are used as important metabolic indexes for bioenergetics measurement of sea cucumbers and therefore, these parameters have been well studied for several depositfeeding species, e.g., A. japonicus, Australostichopus mollis and Holothuria leucospilota (Dong et al., 2006; Yang et al., 2006; Yuan et al., 2007; Zamora and Jefference s, 2012; Yu et al., 2013), and suspension-feeding species, e.g., Eupentacta quinquesemita (Sabourin and Stickle 1981). This is the fi rst data available on the sea cucumber C. frondosa. The OCR can be used as an indication of energy production of sea cucumbers, since the uptake of oxygen from ambient water is correlated well with the tissue oxygen demand of echinoderms and is positively correlated with metabolism (Yuan et al., 2007; Yu et al., 2013). The low OCR in the control group indicated low level of energy production in the sea cucumbers without feeding, and is a common mechanism for marine animals (e.g., Calanus plumchrus, Strongylocentrotus droebachiensis and H. leucospilota) to conserve energy under starvation and salinity stress (Mayzaud, 1976; Ikeda, 1977; Sabourin and Stickle, 1981; Yu et al., 2013).
Ammonium is the main metabolic nitrogenous waste product in echinoderms, and the ammonium excretion correlates well with protein and amino acid catabolism of echinoderms (Talbot and Lawrence, 2002; Zamora and Jefference s, 2012; Yu et al., 2013). Some studies have indicated that the AER of echinoderms (e.g., Psammechinus miliaris and Stichopus mollis) is small, and does not vary signifi cantly as a function of diet (Otero-Villanueva et al., 2004; Maxwell et al., 2009). However, the AER of sea cucumber A. japonicus varied with the diet composition (Yuan et al., 2006; Shi et al., 2013). In this study, the high AER of the A. nodosum group indicated the positive efference ect of this diet on the nitrogen catabolism of C. frondosa. The AER can refl ect the nutritional status of echinoderms, e.g., in most cases, the excretion of nitrogen by A. japonicus was positively correlated with its growth (Yuan et al., 2006; Liu et al., 2009); a positive correlation was found between the nutritional condition and AER of H. leucospilota (Yu et al., 2013). Therefore, it is possible that C. frondosa fed with A. nodosum was in better nutritional condition than other groups; however, this requires further follow-up study.
The O/N ratio is considered as a useful physiological index, which can indicate catabolic balance between the uses of protein versus carbonbased substrates. Generally, high values (e.g., >30) indicate a carbohydrate- and/or lipid-dominated catabolism, and low values (e.g., ≤10) indicate a protein-dominated catabolism (Sabourin and Stickle, 1981; Yang et al., 2006; Zamora and Jefference s, 2012; Yu et al., 2013). In this study, C. frondosa fed with S. latissima, shellfi sh diet and natural seston all had high O/N ratios (>25), suggesting that they depended on carbon-based substrates as an energy source. Meanwhile, low values of the control and A. nodosum groups suggested these individuals utilised a high proportion of protein as the primary energy source rather than carbohydrate and/or lipid. In some cases, both a long-term infl uence of starvation and a high-quality diet could induce decreases in O/N ratio (Otero-Villanueva et al., 2004). In this study, the C. frondosa in the control and A. nodosum groups had similarly low O/N ratios. The low O/N ratio of the control group resulted from the reduction in OCR, which was an energy-saving mechanism for the sea cucumber under starvation. Meanwhile, despite the better nutritional status, the increment in AER (which derived from high protein catabolism) induced a decrease in O/N ratio in the sea cucumbers fed with A. nodosum.
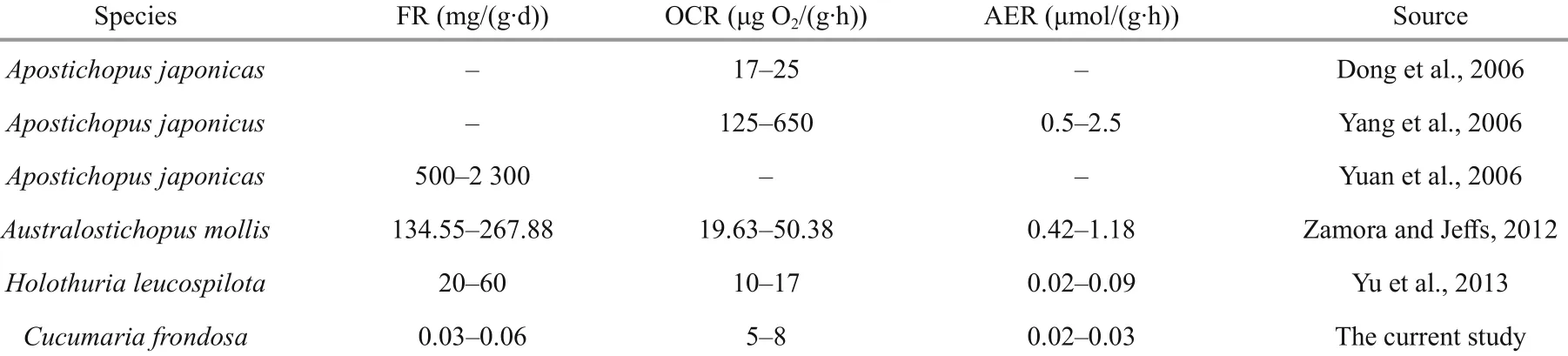
Table 2 Physiological properties of some deposit-feeding sea cucumbers compared to the suspension feeding cucumber Cucumaria frondosa in this study
In this study, all the FR, OCR and AER of C. frondosa were much lower than those of the depositfeeding sea cucumbers (Dong et al., 2006; Yang et al., 2006; Yuan et al., 2006; Zamora and Jefference s, 2012; Yu et al., 2013) (Table 2), which may be mainly due to the sedentary and inactive nature of this species.
5 CONCLUSION
Results of this study show that diets greatly afference ected the feeding, respiration, and excretion rates of C. frondosa. However, no signifi cant efference ect was found on the fecal production rate in the four feeding groups. Sea cucumbers fed with A. nodosum exhibited a higher AER, but lower O/N ratio, which might imply that the A. nodosum had a higher nutritional value than other diets for C. frondosa. It was not that surprising to fi nd seaweed powder could be used as potential diet of C. frondosa, since the long intestine of this species is particularly suitable for digesting refractory plant material.
Seaweeds such as A. nodosum and S. latissima are widely distributed along the coastal area of the North Atlantic Ocean, and it is an economical and promising approach to utilize these local seaweeds as diets for the intensive aquaculture of C. frondosa. However, there is still further research required for aquaculture of C. frondosa, such as diet preparation methods, cultivation condition optimization and an energy budget calculation should be conducted to improve the production eき ciency of this sea cucumber.
6 DATA AVAILABILITY STATEMENT
The datasets in the current study are not publicly available due to author request. It can be available from the corresponding author on reasonable request.
References
Al Shemaili J, Mensah-Brown E, Parekh K, Thomas S A, Attoub S, Hellman B, Nyberg F, Nyberg A, Collin P, Adrian T E. 2014. Frondoside A enhances the antiproliferative efference ects of gemcitabine in pancreatic cancer. European Journal of Cancer, 50(7): 1 391-1 398.
Bruckner A W. 2005. The recent status of sea cucumber fi sheries in the continental United States of America. SPC Beche- de- mer Information Bulletin, 22: 39-46.
Cranford P J, Armsworthy S L, Mikkelsen O A, Milligan T G. 2005. Food acquisition responses of the suspensionfeeding bivalve Placopecten magellanicus to the fl occulation and settlement of a phytoplankton bloom. Journal of Experimental Marine Biology and Ecology, 326(2): 128-143.
Dong Y W, Dong S L, Tian X L, Wang F F, Zhang M Z. 2006. Efference ects of diel temperature fl uctuations on growth, oxygen consumption and proximate body composition in the sea cucumber Apostichopus japonicus Selenka. Aquaculture, 255(1-4): 514-521.
Eriksson H, Robinson G, Slater M J, Troell M. 2012. Sea cucumber aquaculture in the Western Indian Ocean: challenges for sustainable livelihood and stock improvement. Ambio, 41(2): 109-121.
Gianasi B L, Parrish C C, Hamel J F, Mercier A. 2016. Infl uence of diet on growth, reproduction and lipid and fatty acid composition in the sea cucumber cucumaria frondosa. Aquac ulture Res earch, 48(7): 3 413-3 432.
Hamel J F, Conand C, Pawson D L, Mercier A. 2001. The sea cucumber Holothuria scabra (Holothuroidea: Echinodermata): Its biology and exploitation as Bechede-mer. Advances in Marine Biology, 41: 129-223.
Hamel J F, Mercier A. 1996a. Early development, settlement, growth, and spatial distribution of the sea cucumber Cucumaria frondosa (Echinodermata: Holothuroidea). Canadian Journal of Fisheries and Aquatic Sciences, 53(2): 253-271.
Hamel J F, Mercier A. 1996b. Gamete dispersion and fertilisation success of the sea cucumber Cucumaria frondosa. SPC Beche- de- mer Information Bulletin, 8: 34-40.
Hamel J F, Mercier A. 1998. Diet and feeding behaviour of the sea cucumber Cucumaria frondosa in the St. Lawrence estuary, eastern Canada. Canadian Journal of Zoology, 76(6): 1 194-1 198.
Hamel J F, Mercier A. 2008. Precautionary management of Cucumaria frondosa in Newfoundland and Labrador, Canada. In: Toral-Granda V, Lovatelli A, Vasconcellos M eds. Sea Cucumbers. A Global Review of Fisheries and Trade. FAO, Rome. p.293-306.
Holtz E H, MacDonald B A. 2009. Feeding behaviour of the sea cucumber Cucumaria frondosa (Echinodermata: Holothuroidea) in the laboratory and the fi eld: relationships between tentacle insertion rate, fl ow speed, and ingestion. Marine Biology, 156(7): 1 389-1 398.
Hu S W, Xu L L, Shi D, Wang J F, Wang Y M, Lou Q M, Xue C H. 2014. Eicosapentaenoic acid-enriched phosphatidylcholine isolated from Cucumaria frondosa exhibits anti-hyperglycemic efference ects via activating phosphoinositide 3-kinase/protein kinase B signal pathway. Journal of Bioscience and Bioengineering, 117(4): 457-463.
Ikeda T. 1977. The efference ect of laboratory conditions on the extrapolation of experimental measurements to the ecology of marine zooplankton. IV. Changes in respiration and excretion rates of boreal zooplankton species maintained under fed and starved conditions. Marine Biology, 41(3): 241-252.
King II T I. 1993. The efference ect of water temperature on hand volume during volumetric measurement using the water displacement method. Journal of Hand Therapy, 6(3): 202-204.
Liu Y, Dong S L, Tian X L, Wang F, Gao Q F. 2009. Efference ects of dietary sea mud and yellow soil on growth and energy budget of the sea cucumber Apostichopus japonicus (Selenka). Aquaculture, 286(3-4): 266-270.
Maxwell K H, Gardner J P A, Heath P L. 2009. The efference ect of diet on the energy budget of the brown sea cucumber, Stichopus mollis (Hutton). Journal of the World Aquaculture Society, 40(2): 157-170.
Mayzaud P. 1976. Respiration and nitrogen excretion of zooplankton. IV. The infl uence of starvation on the metabolism and the biochemical composition of some species. Marine Biology, 37(1): 47-58.
Nelson E J, MacDonald B A, Robinson S M C. 2012a. A review of the northern sea cucumber Cucumaria frondosa (Gunnerus, 1767) as a potential aquaculture species. Reviews in Fisheries Science, 20(4): 212-219.
Nelson E J, MacDonald B A, Robinson S M C. 2012b. The absorption eき ciency of the suspension-feeding sea cucumber, Cucumaria frondosa, and its potential as an extractive integrated multi-trophic aquaculture (IMTA) species. Aquaculture, 370-371: 19-25.
Otero-Villanueva M D M, Kelly M S, Burnell G. 2004. How diet infl uences energy partitioning in the regular echinoid Psammechinus miliaris; constructing an energy budget. Journal of Experimental Marine Biology and Ecology, 304(2): 159-181.
Purcell S W, Samyn Y, Conand C. 2012. Commercially Important Sea Cucumbers of the World. FAO Species Catalogue for Fishery Purposes. No. 6. FAO, Rome.
Sabourin T D, Stickle W B. 1981. Efference ects of salinity on respiration and nitrogen excretion in two species of echinoderms. Marine Biology, 65(1): 91-99.
Shi C S, Dong S L, Wang F, Gao Q F, Tian X L. 2013. Efference ects of four fresh microalgae in diet on growth and energy budget of juvenile sea cucumber Apostichopus japonicus (Selenka). Aquaculture, 416-417: 296-301.
Singh R, MacDonald B A, Lawton P, Thomas M L H. 1998. Feeding response of the dendrochirote sea cucumber Cucumaria frondosa (Echinodermata: Holothuroidea) to changing food concentrations in the laboratory. Canadian Journal of Zoology, 76(10): 1 842-1 849.
Singh R, MacDonald B A, Lawton P, Thomas M L H. 2001. The reproductive biology of the dendrochirote sea cucumber Cucumaria frondosa (Echinodermata: Holothuriodea) using new quantitative methods. Invertebrate Reproduction & Development, 40(2-3): 125-141.
Talbot T D, Lawrence J M. 2002. The efference ect of salinity on respiration, excretion, regeneration and production in Ophiophragmus fi lograneus (Echinodermata: Ophiuroidea). Journal of Experimental Marine Biology and Ecology, 275(1): 1-14.
Taylor B W, Keep C F, Hall R O, Koch B J, Tronstad L M, Flecker A S, Ulseth A J. 2007. Improving the fl uorometric ammonium method: matrix efference ects, background fl uorescence, and standard additions. Journal of the North American Benthological Society, 26(2): 167-177.
Therkildsen N O, Petersen C W. 2006. A review of the emerging fi shery for the sea cucumber Cucumaria frondosa: Biology, policy, and future prospects. S PC Beche- de- mer Information Bulletin, 23: 16-25.
Uthicke S, Conand C. 2005. Local examples of beche-de-mer overfi shing: An initial summary and request for information. SPC Beche- de- mer Information Bulletin, 21: 9-14.
Yang H S, Zhou Y, Zhang T, Yuan X T, Li X X, Liu Y, Zhang F S. 2006. Metabolic characteristics of sea cucumber Apostichopus japonicus (Selenka) during aestivation. Journal of Experimental Marine Biology and Ecology, 330(2): 505-510.
Yu Z H, Qi Z H, Hu C Q, Liu W G, Huang H H. 2013. Efference ects of salinity on ingestion, oxygen consumption and ammonium excretion rates of the sea cucumber Holothuria leucospilota. Aquaculture Research, 44(11): 1 760-1 767.
Yuan X T, Yang H S, Wang L L, Zhou Y, Zhang T, Liu Y. 2007. Efference ects of aestivation on the energy budget of sea cucumber Apostichopus japonicus (Selenka) (Echinodermata: Holothuroidea). Acta Ecologica Sinica, 27(8): 3 155-3 161.
Yuan X T, Yang H S, Zhou Y, Mao Y Z, Zhang T, Liu Y. 2006. The infl uence of diets containing dried bivalve feces and/or powdered algae on growth and energy distribution in sea cucumber Apostichopus japonicus (Selenka) (Echinodermata: Holothuroidea). Aquaculture, 256(1-4): 457-467.
Zamora L N, Jefference s A G. 2012. Feeding, metabolism and growth in response to temperature in juveniles of the Australasian sea cucumber, Australostichopus mollis. Aquaculture, 358-359: 92-97.
 Journal of Oceanology and Limnology2020年3期
Journal of Oceanology and Limnology2020年3期
- Journal of Oceanology and Limnology的其它文章
- List of the Most Outstanding Papers Published by CJOL/JOL in 2017-2018
- Who is the “murderer” of the bloom in coastal waters of Fujian, China, in 2019?*
- The investigation of internal solitary waves over a continental shelf-slope*
- Efference ects of light quality on growth rates and pigments of Chaetoceros gracilis (Bacillariophyceae)*
- Marine bacterial surfactin CS30-2 induced necrosis-like cell death in Huh7.5 liver cancer cells*
- PI3K/Akt pathway is involved in the activation of RAW 264.7 cells induced by hydroxypropyltrimethyl ammonium chloride chitosan*
