Efference ects of light quality on growth rates and pigments of Chaetoceros gracilis (Bacillariophyceae)*
LI Yongfu , LI Ruiqian , YI Xiaoyan
1 Key Laboratory of Experimental Marine Biology, Institute of Oceanology, Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266071, China
2 Laboratory for Marine Biology and Biotechnology, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266237, China
3 Nantong Research and Development Center of Marine Science and Technology, Institute of Oceanology, Chinese Academy of Sciences, Nantong 226019, China
4 School of International Afference airs and Public Administration, Ocean University of China, Qingdao 266100, China
5 Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Mengla 666303, China
Received Jun. 28, 2019; accepted in principle Aug. 19, 2019; accepted for publication Sep. 27, 2019 © Chinese Society for Oceanology and Limnology, Science Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Abstract Light is an important parameter in algal culturing. In this work, the efference ects of difference erent light qualities on growth of the marine diatom Chaetoceros gracilis are evaluated. The cells were cultured under light quantum fl ux density of 60 μmol photons/(m 2·s) with nine light qualities: LED red light (LR), LED blue light (LB), LED red plus LED blue light (LR+LB), LED white light (LW), fl uorescent white light (FW), and combinations of LW and FW lights with increased proportions of red or blue light (FW+LR, FW+LB, LW+LR, and LW+LB). Blue light promoted the growth of C. gracilis largely. Three light qualities, FW+LR, LW+LR, and LR, resulted in the lowest growth rate. Both chlorophyll and carotenoids reached the highest level under LB and the lowest level under LR among monochromatic light sources; however, increasing of the proportion of blue or red light in the white light induced an increase of the chlorophyll and carotenoid concentrations. Light absorption ability of microalgae was critical for the growth of these organisms, as we observed a positive correlation between the extinction coeき cient at difference erent wavelengths and the specifi c growth rate. These fi ndings are of importance to improve the culturing of this alga in bioreactors.
Key word: Chaetoceros gracilis; light quality; pigments; wavelength efference ect; mean extinction coeき cients
1 INTRODUCTION
Chaetoceros gracilis (Bacillariophyceae), an important microalgal species from an economic point of view, represents a direct or indirect food source for bivalve mollusks, sea urchins, sea cucumbers, crustaceans and other valuable ocean aquatic animals. Currently, the closed photobioreactor (PBR) has become one of the most used production methods for cultivating C. gracilis (Pérez et al., 2017). One of the critical parameters that dramatically afference ect the growth of microalgae, regardless of the adopted production method, is light. Indoor PBRs require artifi cial light sources. In the past, fl uorescent lamps and metal halide lamps were used (Yeh and Chung, 2009; Gao et al., 2018). However, in order to efference ectively increase the biomass accumulation of microalgae, an economical, durable and eき cient light source that is easy to assemble and automatically controlled is highly desirable (Wagner et al., 2016). LED light is the best option among the existing light sources, meeting all the above-mentioned requirements. Moreover, it provides the possibility of integrating solar power technology, which would further decrease light costs for microalgal culture (Gordon and Polle, 2007). Recently, the use of LED light in the cultivation of microalgae has greatly increased (Yim et al., 2016; Shu et al., 2018). The fl exible chromatic LED strip, in particular, is suitable for PBR as it possesses a good waterproof property and multi-angled bendability (Cui et al., 2016). However, little is so far known about the infl uence and the eき ciency of difference erent monochromatic LED lights on C. gracilis growth. Since light is a crucial factor in the cultivation of this organism, a detailed understanding of how microalgae perform in response to difference erent light sources is needed.
In this work, we analyze the efference ects of light quality on C. gracilis growth. Difference erent light sources (all with the same quantum fl ux density) are compared: LED red right (LR), LED blue light (LB), fl uorescent lamp white light (FW) and LED white light (LW) as well as combinations of white light with enhanced proportions of monochromatic red or blue light, i.e., LW+LB, LW+LR, FW+LB, and FW+LR. A simplifi ed Lambert-Beer law is used to calculate the average extinction coeき cient of microalgae at the specifi c wavelength and to identify its relationship with the specifi c growth rate. The obtained results provide a basis to explore the mechanism by which light infl uences the growth rate of microalgae.
2 MATERIAL AND METHOD
2.1 Microalgal strain and culture conditions
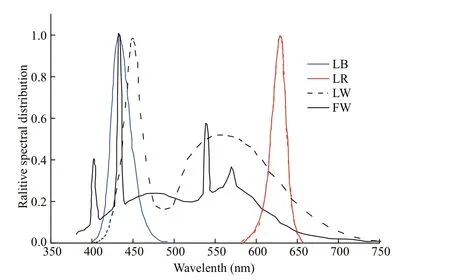
Fig.1 Relative emission spectra of the light qualities used, blue light (LB), red light (LR), white LED light (LW) and white fl uorescent light (FW)
Chaetoceros gracilis was obtained from the Microalgae Culture Center (MACC), Ocean University of China. The algae were placed in a 500-mL conical fl ask with 300 mL of F/2 culture medium with 2 times concentrated silicate (Guillard and Ryther, 1962). Nine light qualities were tested, all at the same light quantum fl ux density (60 μmol photons/(m2·s)), and three biological replicates were performed for each treatment. The light intensity was selected to avoid light saturation of the microalgal cells, so that only the efference ect of the light sources was measured (Chen et al., 1982). LED light sources in our study, i.e. LW, LR, and LB, are all surface mounted light emitting diodes. Each LED lamp bead emits light, and difference erent light quality lamp beads have a specifi c light intensity. The luminous intensity of each LW bead measured by using a portable light quantum meter (3415F type, pulse photoelectric sensor; Spectrum Technologies, Inc., USA) is 1.0 μmol photons/(m2·s), and both LR and LB are 0.5 μmol photons/(m2·s). The incident light intensity of microalgal culture under difference erent light qualities was set by controlling the number of light beads for each specifi c color. We lit 60 white LED beads, 120 red LED beads and 120 blue LED beads, respectively, to create 60 μmol photons/(m2·s) of white, red and blue light. Similarly, 60 red or blue LED beads and 30 white LED beads were used to provide LW+LR or LW+LB. Sixty red beads and 60 blue beads were simultaneously lit to provide LR+LB of 60 μmol photons/(m2·s). As for FW+LR and FW+LB, the 30 μmol photons/(m2·s) fl uorescent white light was fi rst constructed and then 30 μmol photons/(m2·s) LR or LB provided by 60 red or blue beads was added. The algae were continuously cultured under sterile conditions for seven days. The starting inoculum was 5×105cells/mL placed in 500 mL fl asks. Temperature was maintained stable at 35°C, cultures were hand shaken every 8 h throughout the experiments and continuous light was provided. This temperature was chosen for this study to accelerate the growth of this alga. According to a previous study, 35°C was not high enough to induce heat stress of algal cells (Liang et al., 2006). Light qualities, i.e., LW, LB and LR were provided by fl exible LED lamp strips (LXHL, Philips Ltd., Holland). The FW was provided by a fl uorescent lamp tube (F25T8/TL 950, Philips Ltd., Holland). Light spectra were measured with a plant illumination spectroradiometer (PLA-20, EVERFINE’s Quality Measurement Instruments, China) between 350 and 800 nm with 1-nm resolution (Fig.1).
2.2 Analytical procedures
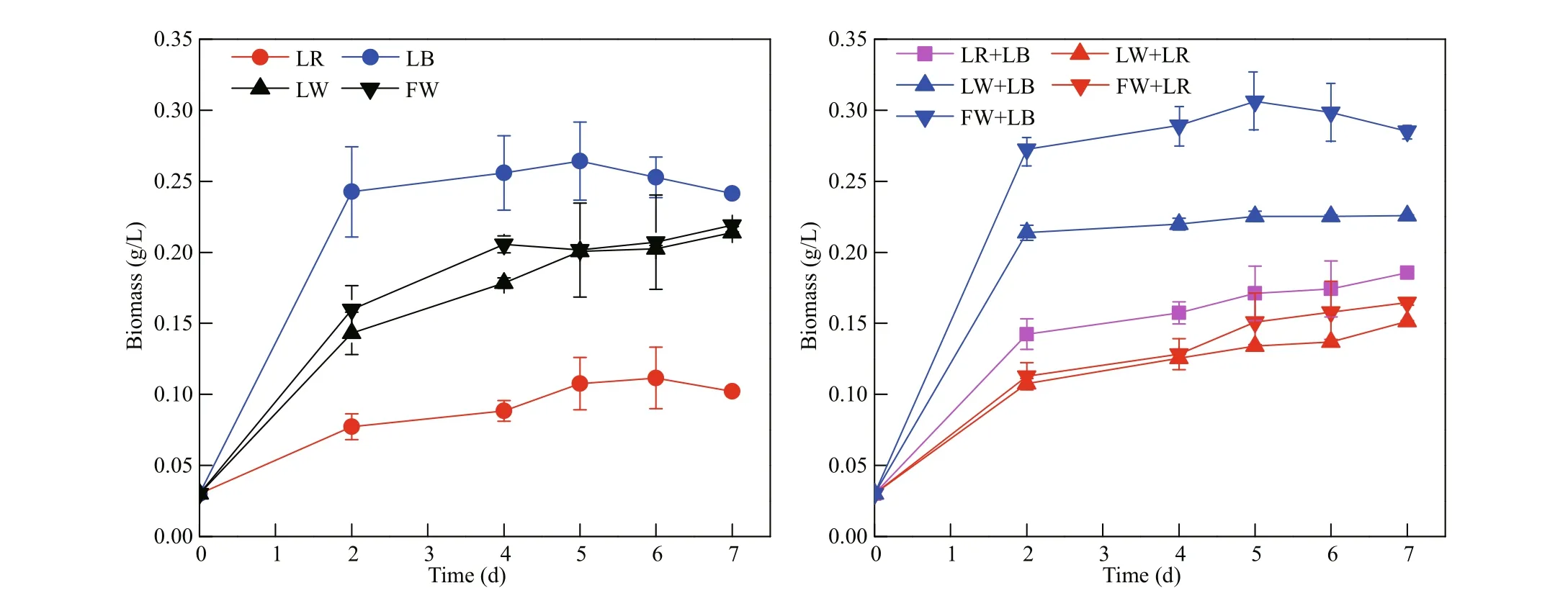
Fig.2 Mean biomass production of C. gracilis under difference erent light qualities ( n=3)
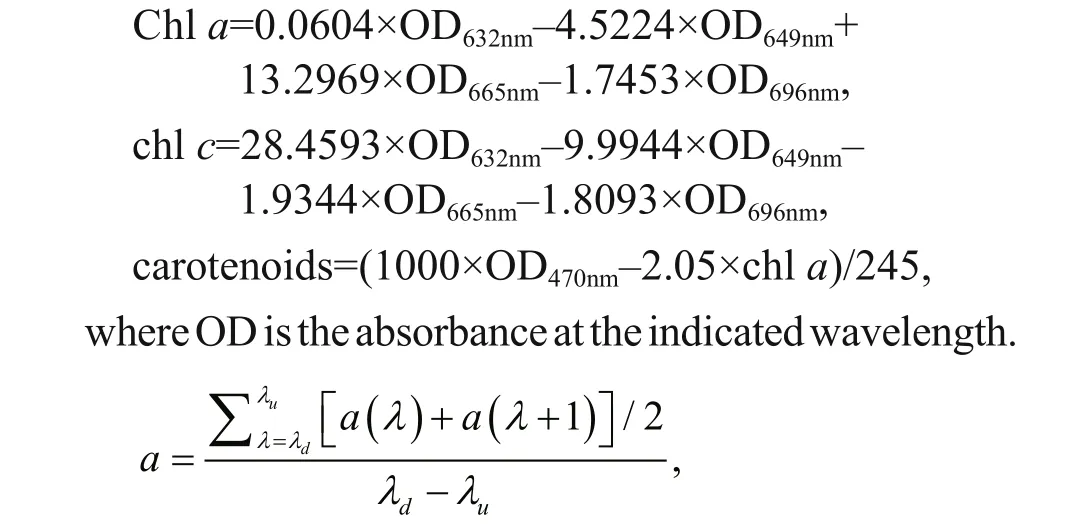
For dry weight (DW) measurements, 10 mL of cultures were fi ltered on pre-treated GF/C Whatman fi lter (Whatman, Maidstone, UK). GF/C fi lters were washed by distilled water and then dried at 50°C. The pre-treated GF/C fi lters were weighted before collecting cells. DWs were calculated in g/L after the fi ltrates were re-dried in an oven at 80°C overnight. The specifi c growth rate ( μ (/d)) during the exponential growth period was obtained according to the method described in Cui et al. (2017). Total chlorophyll (chlorophyll a and c) and carotenoids were extracted as described in (Li et al., 2016) and were quantifi ed using a UV visible spectrophotometer, according to protocols from Ritchie (2008) and Jensen (1978). The method of Li et al. (2016) and a simplifi ed Lambert-Beer law were used to calculate the average extinction coeき cient ( α) of C. gracilis suspension under difference erent wavelengths. where N0and Ntrepresent the cell densities (cells/mL) at the beginning and the end of the exponential phase, and t is the time of the exponential phase in days. In this study, the exponential growth of algae cells fi nished within two days. Cell numbers in the fi rst two days were used to calculate specifi c growth rates.
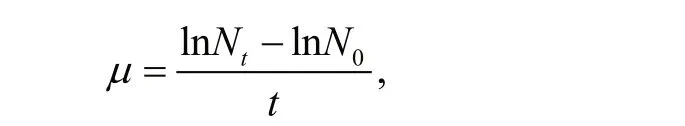
where a represents the average absorbance. λdand λuare the lower-limit wavelength (400 nm for LW and LB, and 570 nm for LR) and the upper-limit wavelength (700 nm for LW, 500 nm for LB, and 680 nm for RL), respectively. a( λ) means the monochromatic light absorbance of each wavelength. The specifi c extinction coeき cient of mixed light quality was expressed by the average value of each monochrome light quality.
SPSS 17.0 was used for the implementation of statistical analyses. Data in the fi gures represent the mean±SD ( n=3) and were subjected to one-way ANOVA and Tukey tests.
3 RESULT AND DISCUSSION
3.1 Infl uence of light quality on C. gracilis growth
After being pre-cultured under fl uorescent white light, C. gracilis cells were transferred and cultured using nine light qualities. For every light treatment, at the initial stage (within 2 days after starting of the culture), C. gracilis grew rapidly, and then the biomass accumulation rate decreased until it reached a plateau stage (Fig.2). In the samples treated with the monochromatic light, the growth rates of C. gracilis under LB and LR were 1.24 and 0.59/d, respectively (Fig.3). In the case of combined light qualities, the growth rate (μ) related to LED white light was LW+LB>LW≈LW+LR, while in the case of fl uorescent white light the μ was FW+LB>FW>FW+LR. Under the exposure of LR+LB, μ value was 0.96/d, ranging between LB and LR. Notably, the μ values under LB (1.24/d), FW+LB (1.31/d) and LW+LB (1.18/d) showed no signifi cant difference erence ( P >0.05), indicating that the blue light played a dominant role among FW+LB and LW+LB.
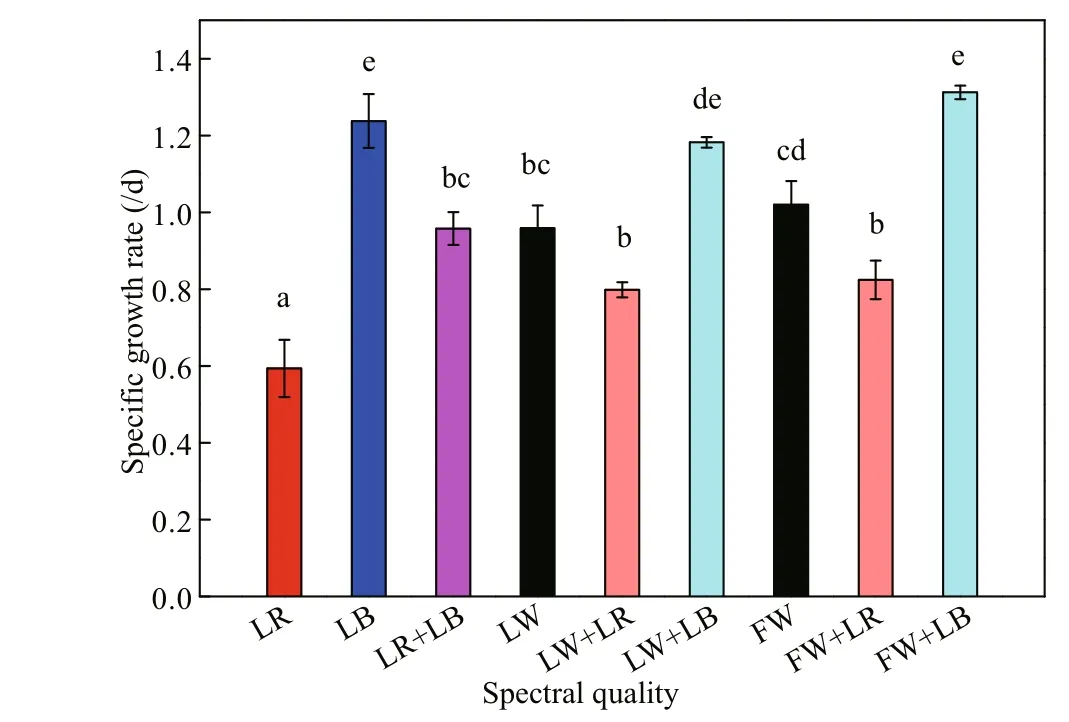
Fig.3 Specifi c growth rates of C. gracilis cultivated for 2 d under difference erent light qualities ( n=3)
The efference ect of light quality on the growth of microalgae has been reported in many studies. It was previously shown that the maximum biomass accumulation of Spirulina platensis was reached at 3 000 μmol photons/(m2·s) under LR radiation; however, this alga showed no obvious difference erences below the light intensity of 300 μmol photons/(m2·s) under red, white, yellow, green and blue lights of LED lamp (Wang et al., 2007). Tang et al. (2011) demonstrated that red LED light and white fl uorescent light were all suitable for the growth of Dunaliella tertiolecta. Conversely, the optimal light condition for Chlorella vulgaris growth was shown to be red light (Yan et al., 2013); in addition, germplasm diversities of microalgae are important parameters to consider when determining the most suitable light quality (Aidar et al., 1994). For another marine diatom, Haslea ostrearia, low blue light favored growth (Mouget et al., 2005). Yoshioka et al. (2012) also reported the efference ects of three light qualities, i.e. white, blue, and red provided by LED panels, on the growth and fatty acid composition of Isochrysis galbana and showed that the highest biomass production and lipid content was obtained under blue light. Marchetti et al. (2013) further compared the efference ects of blue light and white light derived from fl uorescent tubes, on the biochemical composition and photosynthetic rate of Isochrysis sp. (T-iso) CCAP 927/14. Cell concentration and productivity did not vary signifi cantly under the difference erent light conditions at a steady state, whereas the metabolism of carbohydrates and proteins were especially regulated by blue light. For C. gracilis, our study illustrates that blue light facilitates biomass accumulation when the light intensity is 60 μmol photons/(m2·s) and regardless of the combination with other light spectra (FW+LB or LW+LB). In view of a practical application, LB seems to be the most suitable light source to be used in a closed photobioreactor.
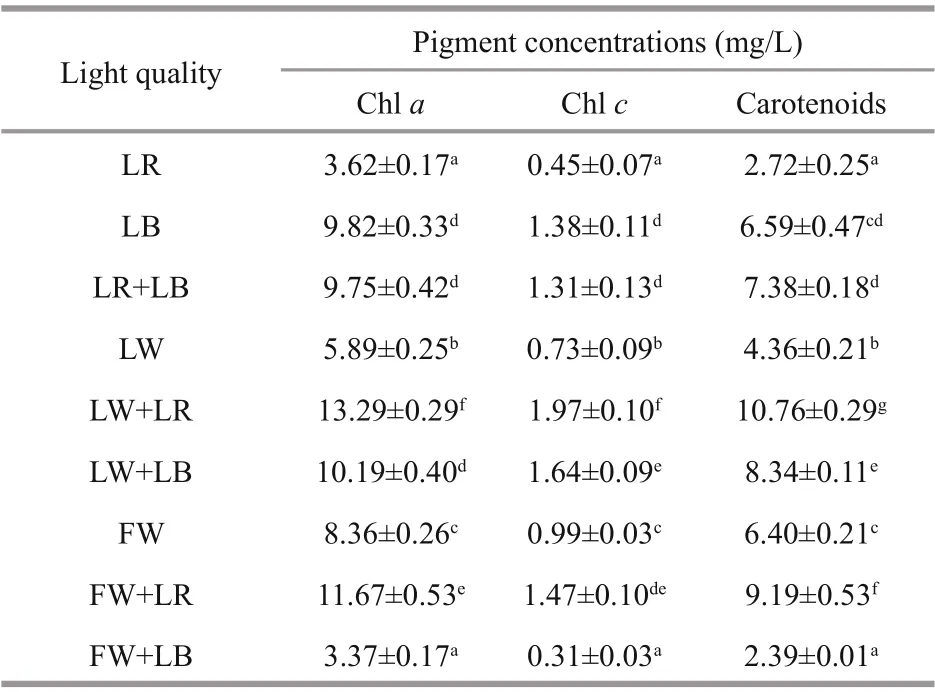
Table 1 Pigment concentrations of C. gracilis under various light qualities with a constant light intensity of 60 μmol photons/(m 2·s)
Although chl a is commonly used as a measure of pigment content, other pigments such as chl c and carotenoids can contribute signifi cantly to light absorption (Macintyre et al., 2002). These accessory pigments may also contribute to light harvesting or to the dissipation of excitation energy. When the incident light spectrum changes, microalgae are able to adjust photosynthetic organs in order to obtain the maximum light use eき ciency and the concentration of additional photosynthetic pigments is normally observed (MacIntyre et al., 2002). It can be observed that LBcultured C. gracilis showed the highest concentrations of chl a, chl c, and carotenoids (9.82, 1.38, 6.59 mg/L, respectively), under monochromatic light and white light (LW, FW). However, LW+LR and FW+LR induced the highest concentration of carotenoids for all light qualities, either monochromatic or trichromic light. Except for FW+LB, the increase of the proportion of monochromatic light quality (both LR and LB) in the white light induced an increase in the pigment concentration (Table 1). This work is not yet able to explain this point. However, it has been reported that green light yielded the best chlorophyll-a production in Gloeothece membranacea, compared to violet, orange, and red light (Mohsenpour et al., 2012). Green light, an important component of white light, may also play an important role in regulating the accumulation of pigments in C. gracilis, although this efference ect may not be as obvious as LB or LR. This may be the reason why increasing the proportion of LR or LB in white light can signifi cantly increase the content of pigments.
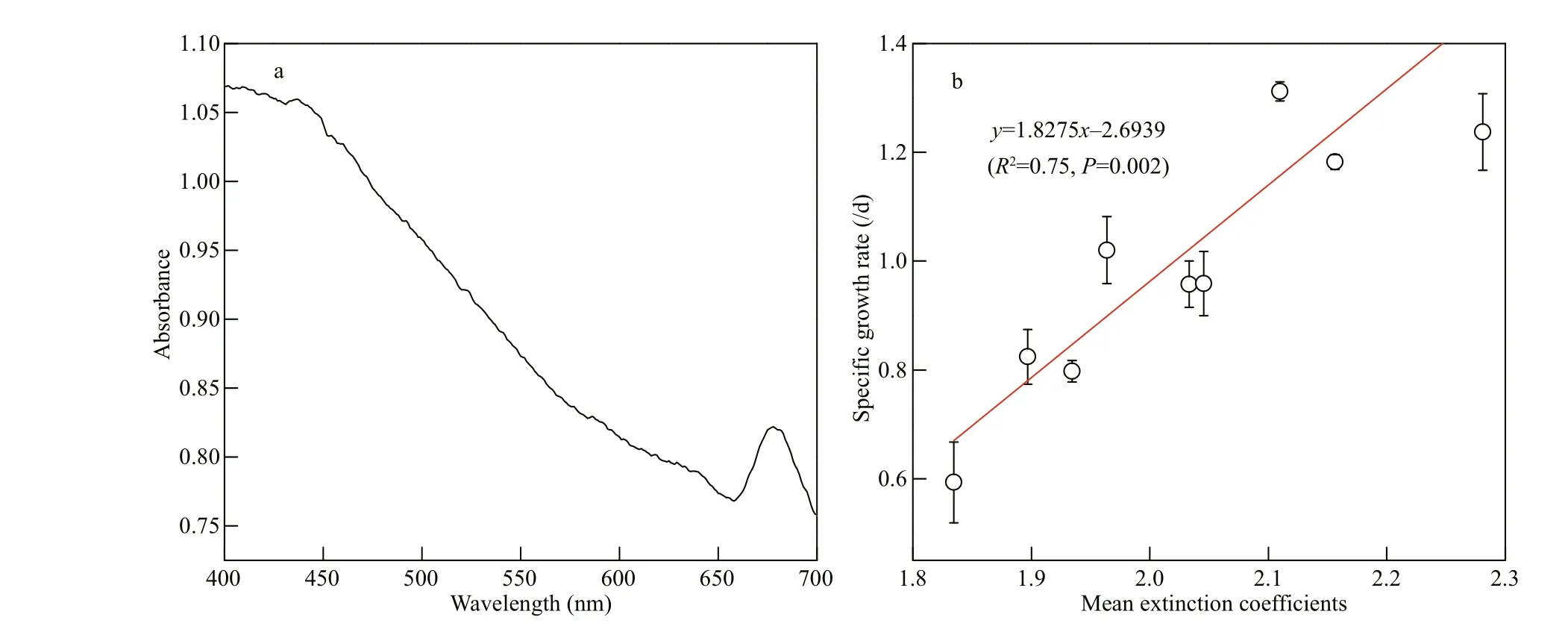
Fig.4 Absorption spectrum (a) and the relation between the specifi c growth rates and mean extinction coeき cients (b) of C. gracilis under photosynthetically active radiation
3.2 Absorption spectra under photosynthetically active radiation
Under photosynthetically active radiation (PAR, 400-700 nm), C. gracilis suspension was scanned to obtain the absorption spectrogram (Fig.4a). Two absorption peaks of chlorophyll at 433 nm and 675 nm were observed without any other visible peaks. Our results were consistent with the observation of Moberg et al. (2002) and indicated that algal cells can absorb more blue light photons under the same photosynthetic quantum fl ux.
Although large chromatic efference ects do not occur in some microalgae under light-limited conditions, some studies found that light quality actually regulates physiological and biochemical processes of microalgae (i.e. growth, photosynthetic pigment synthesis and photosynthesis parameter) (Mouget et al., 2004, 2005). Since such difference erences cannot be simply explained by germplasm difference erences of microalgae, underlying mechanisms, that remain unknown, are likely to be involved.
Generally, light afference ects microalgae through three pathways: (i) photon energy; (ii) distribution of light energy on the surface of microalgae and the penetration inside cells; (iii) capture and use of light energy. In the fi rst case, when microalgae use PAR for photosynthesis, the light energy is absorbed by quantization. The photon energy is directly proportional to electromagnetic radiation frequency ( υ) and inversely proportional to wavelength ( λ). Under the same photon fl ux density, the photon in the short wavelength area (approaching the visible bluepurple band) has more energy for photosynthesis than the one in the long wavelength area (approaching the red band) (Prézelin et al., 1991). The second process is connected to the penetrability of light in water and the apparent absorption of photons by microalgae. When the color spectrum with a narrow wavelength band overlaps with the absorption spectrum, the light utilization rate shows a rising trend (Chen et al., 2011). In the third pathway, the absorption of light energy in the cell depends on the protein complex of photosynthetic and light-harvesting pigments. The light energy is transferred between pigment molecules toward the two light reaction centers (PSI and PSII on the eukaryotic thylakoid membrane) to complete the fi nal photosynthesis process. Extinction coeき cient refl ects the absorption magnitude of light energy from the spectrum. Our results showed a signifi cant positive correlation calculated with the Pearson’s correlation test between the specifi c growth rate and each light quality (Fig.4b). Thus, the light absorption property of microalgal cells played, in part, an important role in regulating cell growth. It is known that the quantum energy of photons at 680 nm is needed for the photolysis of water. Additional energy of lower wavelength photons, e.g. blue, is dissipated as heat (Barber, 2009). This brings forward the question why blue light is the optimum light quality to promote the growth of algae cells. It can be speculated that the constituents of the cells, e.g., proteins, lipids, pigments, and carbohydrates, have their own optical properties that reduce the energy of photons and decrease the light energy reaching the PSII reaction center (Lehmuskero et al., 2018). Future studies are needed to investigate this topic in more detail. Our results also indicated that C. gracilis grown under blue light had higher chl a, chl c, and carotenoid concentrations than that under red light. This phenomenon suggested that LB enhanced the light absorption capacity by increasing the pigment levels, and ultimately improved biomass accumulation. Correspondingly, blue light induces a higher pigmentation content, resulting in more eき cient energy absorption and utilization when the equivalent photons, far below the saturated light intensity, are provided. It has been reported that in the diatom H. ostrearia, the most obvious efference ect of blue light is an enhancement of marennine production, which could refl ect the involvement of nitrogen metabolism in the marennine synthesis pathway (Mouget et al., 2005). Interestingly, it has been confi rmed that blue light stimulated the carotenogenesis pathway in some fungi by regulating the gene expression, e.g., Monascus (Wang et al., 2012), Fusarium (Avalos et al., 2017) and Neurospora (Polaino et al., 2017). Information about the response of the genes encoding the pigment biosynthesis in the microalgae is still lacking. It is a very interesting subject needing to be investigated further.
4 CONCLUSION
A blue light LED strip was a suitable light source for cultivating C haetoceros gracilis. Chlorophyll and carotenoid contents under blue light was signifi cantly higher than those under red light. Increasing of the proportion of blue or red light in the white light induced an increase of the chl a, chl c, and carotenoid concentrations. The correlation between the extinction coeき cient at difference erent wavelengths and the specifi c growth rate was positive, suggesting that the light absorption property of microalgal cells played an important role in regulating cell growth.
5 DATA AVAILABILITY STATEMENT
Full information developed from this study is available from the corresponding author upon reasonable request.
6 ACKNOWLEDGMENT
The authors thank Dr. MENG Fanping of the Ocean University of China for providing LED light sources. We thank Dr. LI Xian, Key Laboratory of Experimental Marine Biology, Chinese Academy of Sciences, Institute of Oceanology, for her kind help in measuring the light spectra. The authors also thank Dr. John van der Meer of the Pan-American Marine Biotechnology Association for his assistance with proofreading.
References
Aidar E, Gianesella-Galvão S M F, Sigaud T C S, Asano C S, Liang T H, Rezende K R V, Oishi M K, Aranha F J, Milani G M, Sandes M A L. 1994. Efference ects of light quality on growth, biochemical composition and photo synthetic production in Cyclotella caspia Grunow and Tetraselmis gracilis (Kylin) Butcher. Journal of Experimental Marine Biology and Ecology, 180(2): 175-187.
Avalos J, Pardo-Medina J, Parra-Rivero O, Ruger-Herreros M, Rodríguez-Ortiz R, Hornero-Méndez D, Limón M C. 2017. Carotenoid biosynthesis in F usarium. Journal of Fungi, 3(3): 39.
Barber J. 2009. Photosynthetic energy conversion: Natural and artifi cial. Chemical Society Reviews, 38(1): 185-196.
Chen C Y, Yeh K L, Aisyah R, Lee D J, Chang J S. 2011. Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: a critical review. Bioresource Technology, 102(1): 71-81.
Chen S F, Tan G Y, Pan Y Y. 1982. The efference ect of temperature, light intensity and photoperiod on the growth of Chaetoceros sp. Marine Sciences, (2): 38-40. (in Chinese with English abstract)
Cui H W, Meng F P, Li Y F, Wang Y J, Duan W Y. 2016. Efference ects of artifi cial light source and light quality on the growth of two species of microalgae. Science Discovery, 4(2): 129-136.
Cui H W, Meng F P, Li F, Wang Y J. 2017. Application of sodium erythorbate to promote the growth of Chlorella vulgaris. Journal of Applied Phycology, 29(3): 1 135-1 144.
Gao X B, Kong B, Vigil R D. 2018. Simulation of algal photobioreactors: recent developments and challenges. Biotechnology Letters, 40(9-10): 1 311-1 327.
Gordon J M, Polle J E W. 2007. Ultrahigh bioproductivity from algae. Applied Microbiology and Biotechnology, 76(5): 969-975.
Guillard R R L, Ryther J H. 1962. Studies of marine planktonic diatoms: I. Cyclotella nana hustedt, and Detonula confervacea (Cleve) Gran. Canadian Journal of Microbiology, 8(2): 229-239.
Jensen A. 1978. Chlorophylls and carotenoids. In: Hellebust J, Craigie J S, eds. Handbook of Phycological Methods; Physiological and Biochemical Methods. Cambridge University Press, Cambridge, UK. p.61-64.
Lehmuskero A, Chauton M S, Boström T. 2018. Light and photosynthetic microalgae: a review of cellular- and molecular-scale optical processes. Progress in Oceanography, 168: 43-56.
Li Y F, Liu J G, Zhang L T, Pang T. 2016. Changes of photosynthetic performances in mature thalli of the red alga Gelidium amansii (gelidiaceae) exposed to difference erent salinities. Marine Biology Research, 12(6): 631-639.
Liang Y, Liang L X, Yin C L, Cao C H. 2006. Efference ects of high temperature stress on the chlorophyll fl uorescence kinetics of Phaeodactylum tricornutum and Chaetoceros gracilis. Periodical of Ocean University of China, 36(3): 427-433. (in Chinese with English abstract)
MacIntyre H L, Kana T M, Anning T, Geider R J. 2002. Photoacclimation of photosynthesis irradiance response curves and photosynthetic pigments in microalgae and cyanobacteria. Journal of Phycology, 38(1): 17-38.
Marchetti J, Bougaran G, Jaufference rais T, Lefebvre S, Rouxel C, Saint-Jean B, Lukomska E, Robert R, Cadoret J P. 2013. Efference ects of blue light on the biochemical composition and photosynthetic activity of Isochrysis sp. (T-iso). Journal of Applied Phycology, 25(1): 109-119.
Moberg L, Karlberg B, Sørensen K, Källqvist T. 2002. Assessment of phytoplankton class abundance using absorption spectra and chemometrics. Talanta, 56(1): 153-160.
Mohsenpour S F, Richards B, Willoughby N. 2012. Spectral conversion of light for enhanced microalgae growth rates and photosynthetic pigment production. Bioresource Technology, 125: 75-81.
Mouget J L, Rosa P, Tremblin G. 2004. Acclimation of Haslea ostrearia to light of difference erent spectral qualitiesconfi rmation of ‘chromatic adaptation’ in diatoms. Journal of Photochemistry and Photobiology B: Biology, 75(1-2): 1-11.
Mouget J L, Rosa P, Vachoux C, Tremblin G. 2005. Enhancement of marennine production by blue light in the diatom H aslea ostrearia. Journal of Applied Phycology, 17(5): 437-445.
Pérez L, Salgueiro J L, González J, Parralejo A I, Maceiras R, Cancela Á. 2017. Scaled up from indoor to outdoor cultures of Chaetoceros gracilis and Skeletonema costatum microalgae for biomass and oil production. Biochemical Engineering Journal, 127: 180-187.
Polaino S, Villalobos-Escobedo J M, Shakya V P S, Miralles-Durán A, Chaudhary S, Sanz C, Shahriari M, Luque E M, Eslava A P, Corrochano L M, Herrera-Estrella A, Idnurm A. 2017. A Ras GTPase associated protein is involved in the phototropic and circadian photobiology responses in fungi. Scientifi c Reports, 7: 44790.
Prézelin B B, Tilzer M M, Schofi eld O, Haese C. 1991. The control of the production process of phytoplankton by the physical structure of the aquatic environment with special reference to its optical properties. Aquatic Sciences, 53(2-3): 136-186.
Ritchie R J. 2008. Universal chlorophyll equations for estimating chlorophylls a, b, c, and d and total chlorophylls in natural assemblages of photosynthetic organisms using acetone, methanol, or ethanol solvents. Photosynthetica, 46(1): 115-126.
Shu C H, Tseng K, Jaiswal R. 2018. Efference ects of light intensity and wavelength on the production of lactobionic acid from whey by Pseudomonas taetrolens in batch cultures. Journal of Chemical Technology & Biotechnology, 93(6): 1 595-1 600.
Tang H, Abunasser N, Garcia M E D, Chen M, Ng K Y S, Salley S O. 2011. Potential of microalgae oil from Dunaliella tertiolecta as a feedstock for biodiesel. Applied Energy, 88(10): 3 324-3 330.
Wagner I, Steinweg C, Posten C. 2016. Mono- and dichromatic LED illumination leads to enhanced growth and energy conversion for high-eき ciency cultivation of microalgae for application in space. Biotechnology Journal, 11(8): 1 060-1 071.
Wang C L, Yang H, Chen M H, Wang Y R, Li F J, Luo C, Zhao S Y, He D. 2012. Real-time quantitative analysis of the infl uence of blue light on citrinin biosynthetic gene cluster expression in Monascus. Biotechnology Letters, 34(9): 1 745-1 748.
Wang C Y, Fu C C, Liu Y C. 2007. Efference ects of using lightemitting diodes on the cultivation of Spirulina platensis. Biochemical Engineering Journal, 37(1): 21-25.
Yan C, Luo X Z, Zheng Z. 2013. Efference ects of various LED light qualities and light intensity supply strategies on purifi cation of slurry from anaerobic digestion process by Chlorella vulgaris. International Biodeterioration & Biodegradation, 79(2): 81-87.
Yeh N, Chung J P. 2009. High-brightness LEDs-Energy eき cient lighting sources and their potential in indoor plant cultivation. Renewable and Sustainable Energy Reviews, 13(8): 2 175-2 180.
Yim S K, Ki D W, Doo H S, Kim H, Kwon T H. 2016. Internally illuminated photobioreactor using a novel type of lightemitting diode (LED) bar for cultivation of Arthrospira platensis. Biotechnology and Bioprocess Engineering, 21(6): 767-776.
Yoshioka M, Yago T, Yoshie-Stark Y, Arakawa H, Morinaga T. 2012. Efference ect of high frequency of intermittent light on the growth and fatty acid profi le of Isochrysis galbana. Aquaculture, 338-341: 111-117.
 Journal of Oceanology and Limnology2020年3期
Journal of Oceanology and Limnology2020年3期
- Journal of Oceanology and Limnology的其它文章
- List of the Most Outstanding Papers Published by CJOL/JOL in 2017-2018
- Who is the “murderer” of the bloom in coastal waters of Fujian, China, in 2019?*
- The investigation of internal solitary waves over a continental shelf-slope*
- Efference ect of diets on the feeding behavior and physiological properties of suspension-feeding sea cucumber Cucumaria frondosa*
- Marine bacterial surfactin CS30-2 induced necrosis-like cell death in Huh7.5 liver cancer cells*
- PI3K/Akt pathway is involved in the activation of RAW 264.7 cells induced by hydroxypropyltrimethyl ammonium chloride chitosan*
