Marine bacterial surfactin CS30-2 induced necrosis-like cell death in Huh7.5 liver cancer cells*
ZHOU Shengnan , LIU Ge , , WU Shimei ,
1 College of Life Sciences, Qingdao University, Qingdao 266071, China
2 Key Laboratory of Experimental Marine Biology, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China
3 Laboratory for Marine Biology and Biotechnology, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266071, China
Received May 15, 2019; accepted in principle Aug. 19, 2019; accepted for publication Aug. 26, 2019 © Chinese Society for Oceanology and Limnology, Science Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Abstract Marine bacterial strain Bacillus sp. CS30 exhibited high anticancer activity against Huh7.5 human liver cancer. We purifi ed the corresponding anticancer agent by sequential acidic precipitation, methanol extraction, Sephadex LH-20 chromatography, and reversed phase high-performance liquid chromatography (RP-HPLC), then analyzed it in mass spectrometry. Based on the results of purifi cation and mass spectrometry, we deduced that the anticancer agent was the same component as our previously purifi ed antifungal agent surfactin CS30-2. However, to the best of our knowledge, this is the fi rst report on the surfactin possessing both antifungal and anticancer activities. Surfactin CS30-2 was demonstrated to exhibit high anticancer activity in a dose-dependent manner against Huh7.5 liver cancer cells. Further investigation showed that surfactin CS30-2 induced the increased generation of reactive oxygen species (ROS) and severe disruption of cell membrane, thus leading to cell death. However, unlike previously reported surfactins, surfactin CS30-2 caused cancer cell death via necrosis instead of apoptosis.
Keyword: Bacillus; surfactin; anticancer activity; reactive oxygen species (ROS)
1 INTRODUCTION
Cancer is characterized by uncontrolled cell growth and proliferation, and causes more deaths than all coronary heart disease or all stroke according to WHO estimates for 2011 (Ferlay et al., 2015; Torre et al., 2017). The deaths caused by cancer are approximately 8.2 million in 2012 and could potentially rise to 17 million by 2020 (Obtel et al., 2015). Among the most commonly diagnosed cancers, liver cancer (hepatocellular carcinoma, HCC) is one of the seriously deadliest diseases, being the second highest cause of cancer mortality in the world (Sia et al., 2017; Liu et al., 2018). Because HCC is often diagnosed at an advanced stage, many patients with HCC are not eligible for liver transplantation or surgical resection, and the 5-year survival rate is around only 30%-40% (Zhong et al., 2017; Fei et al., 2019). Therefore, searching for more efference ective anticancer drugs, especially for liver cancer drugs, is urgently needed.
Natural products have long been an important source of anticancer drugs. About 60% anticancer drugs are isolated from natural origins, such as plants, microorganisms, vertebrates and invertebrates (Demain and Sanchez, 2009; Hermawan and Putri, 2018). Among various sources for anticancer drugs, microorganisms are of high interest for their ease in production manipulation (Wu et al., 2017; Liu et al., 2018), and microbial lipopeptides have attracted much attention due to its multiple bioactivities, such as anti-bacterial, antifungal, antiviral and anticancer activities (Iwasaki et al., 2015; Zhao et al., 2017). Surfactin is an important lipopeptide produced by various strains of Bacillus genus, which contains a cyclic lactone ring of a C13-C16β-hydroxy fatty acid and a heptapeptide (Yang et al., 2015). It has been reported that surfactin can induce cytotoxic efference ect against many types of cancer, such as Ehrlich ascites, breast cancer, colon cancers, cervical cancer and leukemia (Park et al., 2013; Gudiña et al., 2016).
The marine environment is greatly difference erent from that of land, thus conferring marine microorganisms unique metabolic and physiological abilities. Hence, marine microorganisms hold great promise to explore novel bioactive agents for drug development (Schinke et al., 2017; Barzkar et al., 2019). In our present study, we found the supernatant of marine bacterial strain Bacillus sp. CS30 exhibited high anticancer activity, and then we purifi ed and characterized the corresponding anticancer agent, and tried to disclose the underlying anticancer mechanism.
2 MATERIAL AND METHOD
2.1 Strain, medium and growth condition
The marine bacterial strain Bacillus sp. CS30 was cultured in Luria Bertani (LB) medium (10 g/L peptone, 5 g/L yeast extract, 10 g/L NaCl, pH adjusted to 7.0) and incubated at 28°C. The Huh7.5 liver cancer cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS) and incubated at 37°C in a humidifi ed atmosphere of 5% CO2and 95% air.
2.2 Isolation and purifi cation of the anticancer agent
In order to get the bioactive anticancer agent, the procedure was carried out as our previously described methods with little modifi cation (Wu et al., 2019). Briefl y, the strain Bacillus sp. CS30 was cultured at 28°C in a 250-mL glass fl ask fi lled with 100 mL LB medium on a shaker incubator at 160 r/min. After being incubated for 2 d, the supernatant was collected by centrifugation at 8 000× g for 10 min, and then adjusted to pH about 2.5 using HCl solution. The corresponding precipitate from the supernatant was collected by centrifugation and washed with phosphate-bufference ered saline (PBS). The bioactive anticancer agent was extracted with methanol, then loaded onto a Sephadex LH-20 column and eluted with methanol as the mobile phase. Each eluted fraction was detected for their anticancer activity by the MTT method as described in the following part of activity assay of the anticancer agent. Further purifi cation of the anticancer agent was achieved via reversed phase high-performance liquid chromatography (RP-HPLC) (Agilent 1260 Infi nity, USA) on an Eclipse XDB-C18 column (5 μm, 9.4 by 250 mm) (Agilent, USA). The column was eluted with a linear gradient of 80% to 100% methanol over 45 min at a fl ow rate of 2.0 mL/min, then eluted with 100% methanol until 60 min. The elution was monitored using a UV detector set at 230 nm. Fractions of each eluted peak were tested for their anticancer activity against Huh7.5 liver cancer cells.
2.3 Activity assay of the anticancer agent
To evaluate the anticancer activity of the bioactive agent produced by Bacillus sp. CS30, Huh7.5 human liver cancer cells were treated with difference erent concentrations of bioactive agent and detected by MTT method as described by Liu et al. (2018) with little modifi cation. Briefl y, logarithmically growing Huh7.5 cells were pre-seeded on a 96-well plate at 37°C for 24 h, and then treated with difference erent concentrations of bioactive agent for 24 h. MTT solution (5 mg/mL, 20 μL/well) was added and incubated for another 4 h. To dissolve purple crystals of formazan, DMSO was added to each well with gentle shaking for 10 min. The death of cancer cells was determined spectrophotometrically at OD490by a multi-detection microplate reader (Infi nite M1000 Pro, TECAN). Relative cell viability of Huh7.5 human liver cancer cells that were treated with the purifi ed bioactive agent was normalized to that of the cells in the control group treated with equal amounts of methanol. All experiments were performed in triplicate.
2.4 ESI-MS analysis of the anticancer agent
To obtain the molecular weight of the purifi ed anticancer agent, the experiment was conducted with a Bruker maXis mass spectrometer (Bruker, Berlin, Germany) and analyzed by an electrospray ionization and quadrupole time-of-fl ight mass spectrometry (ESI-QTOF-MS) according to previously described method (Xiu et al., 2017). Data from ESI-QTOF-MS were acquired in positive ion mode under the following conditions: 3200-V capillary voltage, 4.0 L/min dry gas, and 200°C dry gas temperature.
2.5 Electron microscopy assay
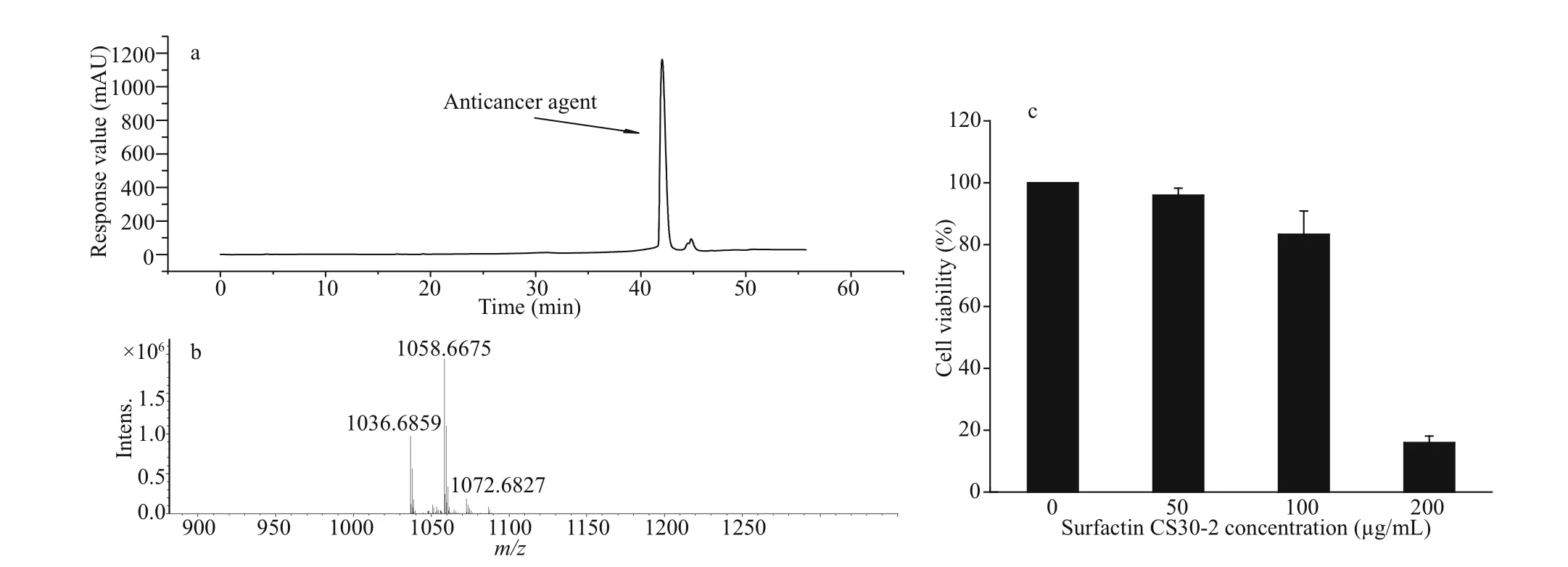
Fig.1 Purif ication and characterization of the anticancer agent produced by Bacillus sp. CS30
To observe the ultrastructural changes of Huh7.5 human liver cancer cells caused by the purifi ed anticancer agent, logarithmically growing Huh7.5 cells were pre-seeded on a 6-well plate at 37°C for 24 h, then treated with 150 μg/mL or 300 μg/mL purifi ed anticancer agent for 24 h. The following experiments were carried out according to previously described method with minor modifi cation (Liu et al., 2019). Briefl y, the cells were collected and prefi xed with 2.5% glutaraldehyde. For the control group, the cells were treated with equal amounts of methanol for 24 h, then collected and prefi xed with 2.5% glutaraldehyde. For scanning electron microscopy (SEM) observation, the samples were observed at 5 kV with SEM (S-3400N; Hitachi, Tokyo, Japan). For the transmission electron microscopy (TEM) observations, samples were embedded in Epon 812, and ultrathin sections were collected and observed at 120 kV with TEM (HT7700; Hitachi).
2.6 Reactive oxygen species (ROS) detection
To detect the ROS generation in Huh7.5 human liver cancer cells after cells were treated with the purifi ed anticancer agent, logarithmically growing Huh7.5 cells were pre-seeded on a 6-well plate at 37°C for 24 h, then treated with 50 μg/mL of purifi ed anticancer agent and incubated for another 3 h. The cells were collected and washed in 10 mmol/L PBS bufference er, then stained with DCFH2-DA at 37°C in the dark as described previously (Kuang et al., 2017). After stained for 20 min, the cells were analyzed by fl ow cytometry (FACS Aria II, BD, San Jose, California, USA).
2.7 Cell status detection
In order to investigate the cancer cell status after treated with the purifi ed anticancer agent, logarithmically growing Huh7.5 cells were preseeded on a 6-well plate at 37°C for 24 h, then treated with difference erent concentrations of purifi ed anticancer agent and incubated for another 24 h. The cells were collected and stained with Annexin V-FITC/propidium iodide double staining kit, then analyzed by fl ow cytometry. For the control group, cells were treated with an equal volume of methanol, and stained as the same way as described for the purifi ed anticancer agent.
3 RESULT
3.1 Isolation and purifi cation of the anticancer agent
To elucidate the bioactive agent inhibiting growth of Huh7.5 liver cancer cells, the supernatant of Bacillus sp. CS30 was precipitated by HCl, extracted with methanol, and then sequentially purifi ed in Sephadex LH-20 chromatography and RP-HPLC. To further purify the anticancer agent, the anticancer fraction from RP-HPLC was concentrated and reinjected onto the column of RP-HPLC, then eluted with the same condition. As shown in Fig.1a, a sharp single peak with high anticancer activity was observed. Actually, the eluted time of the anticancer agent at 42 min was very similar to that of our previously purifi ed antifungal agent surfactin CS30-2 (Wu et al., 2019), indicating that perhaps the anticancer agent is surfactin CS30-2.
3.2 ESI-MS analysis of the anticancer agent
To investigate whether the purifi ed anticancer agent was surfactin CS30-2 or not, the purifi ed fraction was analyzed by electrospray ionization mass spectrometry (ESI-MS). As shown in Fig.1b, two peaks appeared at m/ z values of 1 036.685 9 and 1 058.667 5 corresponding to the single protonated agent [M+H]+and its sodium-cationized ion [M+Na]+, which is very close to those of surfactin CS30-2 (Wu et al., 2019). Along with the similar elution time of surfactin CS30-2 as shown in the RP-HPLC analysis, we deduced that the anticancer agent in Bacillus sp. CS30 is surfactin CS30-2.
3.3 Anticancer activity assay of surfactin CS30-2
To measure the growth-inhibitory activity of surfactin CS30-2 against the Huh7.5 liver cancer cells, cell viability was measured by the MTT assay. As shown in Fig.1c, surfactin CS30-2 could gradually reduce the viability of Huh7.5 human liver cancer cells as the concentration of surfactin CS30-2 increased. The viability remained more than 80% when surfactin CS30-2 was used less than 100 μg/mL. However, the cell viability was greatly reduced to lower than 20% when the concentration of surfactin CS30-2 increased to 200 μg/mL, indicating that the anticancer activity of surfactin CS30-2 was in a dosedependent manner.
3.4 Ultrastructural changes of Huh7.5 liver cancer cells caused by surfactin CS30-2
Since surfactin CS30-2 exhibited strong anticancer activity, the efference ects of surfactin CS30-2 on ultrastructure of Huh7.5 human liver cancer cells were investigated in SEM and TEM. The results of SEM show that cancer cells in the control group were multangular with long and multiple fi liform structures (Fig.2A, panels a and d), while the numbers of the fi liform structures were signifi cantly dropped when cells were treated with 150 μg/mL surfactin CS30-2 (Fig.2A, panels b and e), and the cancer cells lost almost the fi liform structures and changed to a round shape when 300 μg/mL surfactin CS30-2 was present (Fig.2A, panels c and f). To investigate what happened in cancer cells after the surfactin CS30-2 treatment, cells were observed under TEM. As shown in Fig.2B (panels a and d), in absence of surfactin CS30-2, the cell membrane were intact and the cytoplasm distributed evenly, while the cell membrane was disrupted and cytoplasm leaked out when cells were treated with 150 μg/mL surfactin CS30-2 (Fig.2B, panels b and e). The Huh7.5 liver cancer cells were completely fragmented with invisible cell membrane when cells were treated with 300 μg/mL surfactin CS30-2, indicating that the cell membrane of cancer cell was completely damaged by surfactin CS30-2 (Fig.2B, panels c and f).
3.5 ROS detection in Huh7.5 cells induced by surfactin CS30-2
To detect whether there is ROS generation in Huh7.5 human liver cancer cells after the treatment of the purifi ed surfactin CS30-2, the cells were treated with difference erent concentrations of surfactin CS30-2 for 3 h, then detected by a fl uorescent probe DCFH2-DA and analyzed by fl ow cytometry. As shown in Fig.3, the intracellular ROS was detected after cells were treated with surfactin CS30-2 for only 3 h, and the intensity of ROS was gradually increased as the concentration of surfactin CS30-2 increased. The ROS intensity in cells that were treated with up to 200 μg/mL surfactin CS30-2 is more than two times of that in the control group.
3.6 Cell status detection
Reactive oxygen species (ROS) has been reported to induce various biological processes in human cancer cells, including apoptosis (Valko et al., 2007). In order to investigate whether the increased generation of ROS is accompanied by apoptosis upon the treatment of surfactin CS30-2, Annexin V-FITC/ propidium iodide (PI) double staining kit was used to detect the cell status. As shown in Fig.4, after treated with surfactin CS30-2, the necrosis cells were increased from 7.4% to 87.5% as the concentration of surfactin CS30-2 was increased from 50 μg/mL to 300 μg/mL. However, the cancer cells in early apoptotic or late apoptotic status hardly observed no matter what concentration of surfactin CS30-2 was used, indicating that the cell death caused by surfactin CS30-2 is mainly by inducing necrosis.
4 DISCUSSION
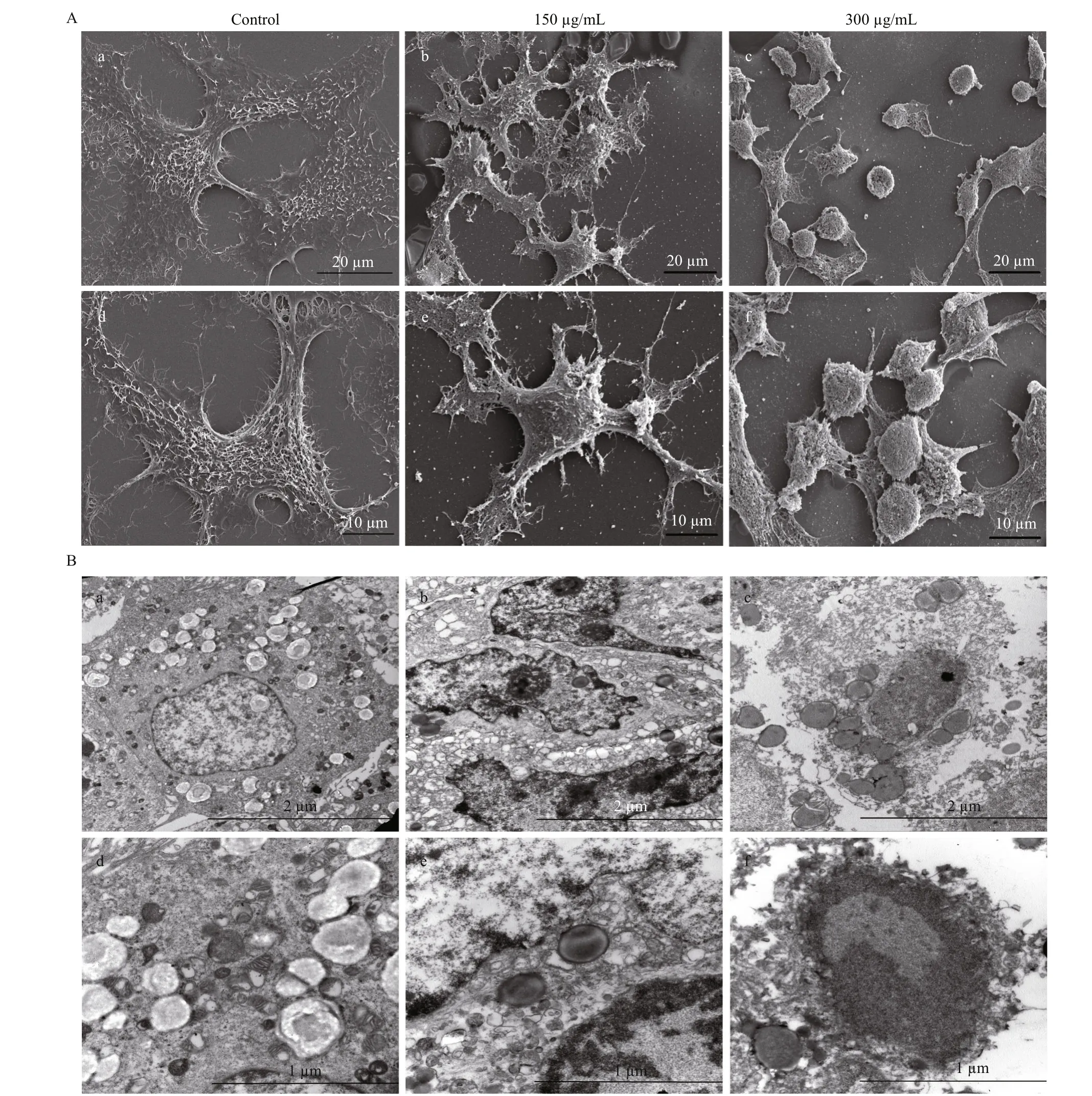
Fig.2 Efference ects of surfactin CS30-2 on the ultrastructure of Huh7.5 human liver cancer cells observed by SEM (A) and TEM (B)
Apoptosis is an important process in regulation of tissue development and homeostasis, and inappropriate apoptosis is a leading cause in many types of cancer (Elmore, 2007; Ouyang et al., 2012). Surfactin was reported to cause cell death mainly by inducing apoptosis (Cao et al., 2010; Wang et al., 2013). However, in our present study, we found that the cell death caused by surfactin CS30-2 was mainly by inducing necrosis other than apoptosis. In order to further confi rm our results, we checked previous reports about the phenomenon of necrosis, and noticed that cells swelled rapidly and lost the integrity of plasma membrane when cell death was caused by inducing necrosis (Zong et al., 2004; Ouyang et al., 2012), which were consistent with our observation in our TEM experiments. In our study, the cancer cell membrane was disrupted in a dose-dependent style, and the cell membrane was completely damaged when cells were treated with high concentration of surfactin CS30-2. Taken together, we proposed that surfactin CS30-2 caused cell death by inducing necrosis through destroying the function of membrane. Our results are difference erent from other reports about surfactins causing cell death via inducing apoptosis (Wang et al., 2013). In order to solidify our results, further experiments still need to be carried out in the future to elucidate the detailed pathway of surfactin CS30-2 killing cancer cells.
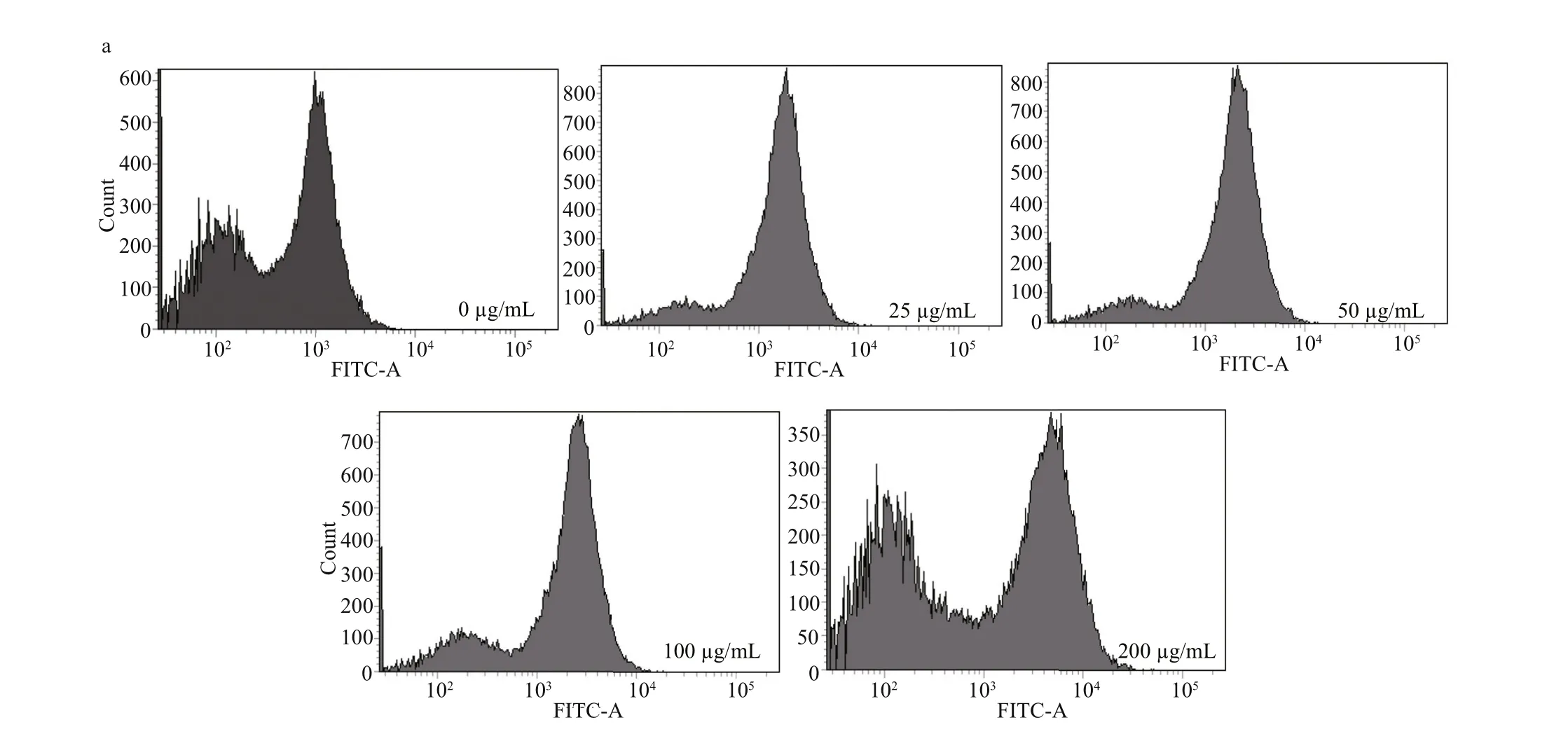
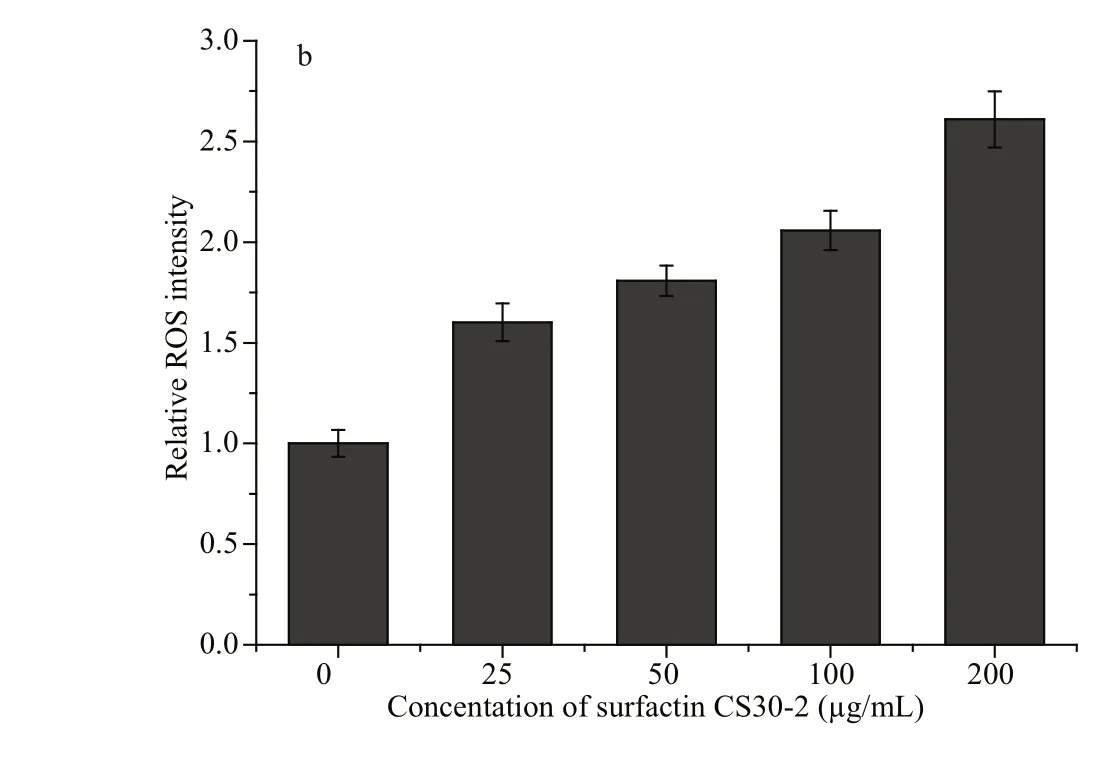
Fig.3 ROS accumulation in Huh7.5 human liver cancer cells caused by surfactin CS30-2
High concentrations of ROS are harmful to cells and fi nally lead to cell death of cancer cells (Cadenas and Davies, 2000). Lipopeptides have been reported to induce death of cancer cells by increasing ROS generation (Hajare et al., 2013). In our study, increased generation of ROS was also observed when Huh7.5 human liver cancer cells were treated with surfactin CS30-2. Furthermore, the ratio of cancer cells in necrosis increased as the concentration of surfactin CS30-2 increased. Therefore, the excessive ROS caused by surfactin CS30-2 is an important factor to induce necrosis of cancer cells, which is in coincidence with previous reports (Ni et al., 2014; Zhang et al., 2017). However, the exact mechanisms of surfactin CS30-2 causing cancer cell death are still needed to be further investigated.
5 CONCLUSION
Surfactin CS30-2 produced by marine bacterium strain Bacillus sp. CS30, can not only greatly inhibit the cell growth of plant pathogen M. grisea as our previous report, but also can dramatically cause cell death of live cancer cells by necrosis through increasing ROS generation, indicating that surfactin CS30-2 has great potentials in the future. Furthermore, to the best of our knowledge, this is the fi rst report about a lipopeptide surfactin that possesses both antifungal and anticancer activities.
6 DATA AVAILABILITY STATEMENT
The data that support the fi ndings of this study are available from the corresponding author upon reasonable request.
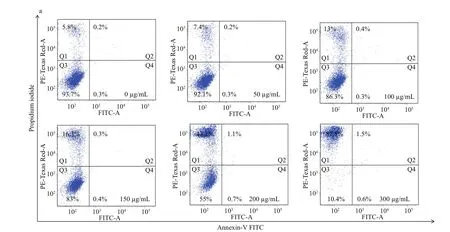
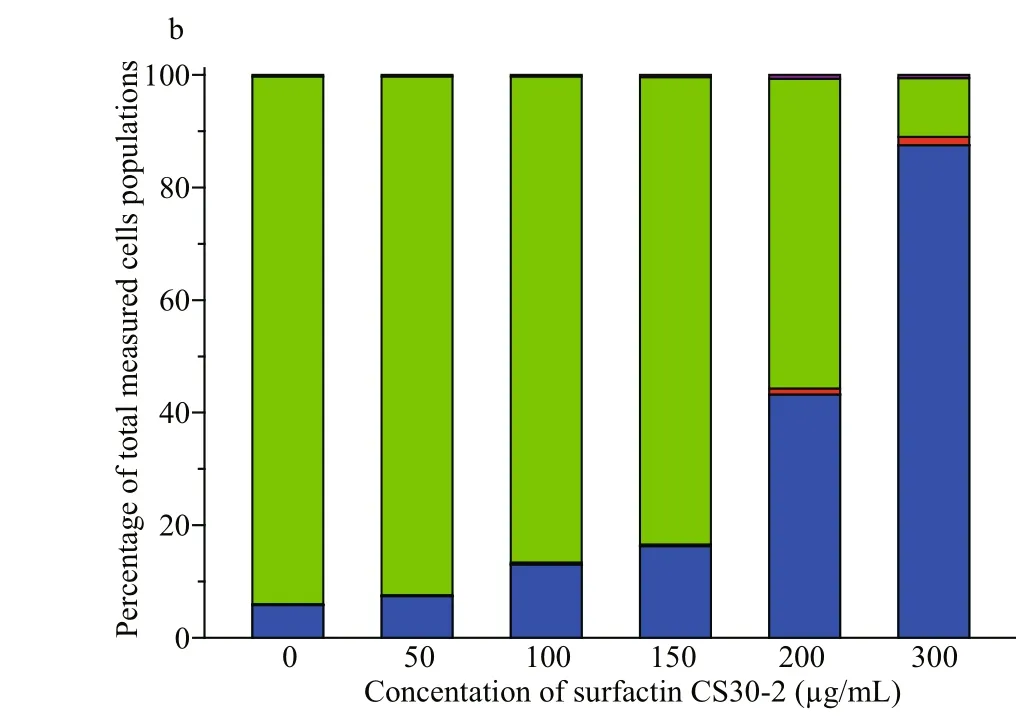
Fig.4 Flow cytometric analysis of Huh7.5 human liver cancer cells after treated with different concentrations of surfactin CS30-2 for 12 h (a); graphical presentation showing percentage of cells in different phases (b)
References
Barzkar N, Tamadoni Jahromi S, Poorsaheli H B, Vianello F. 2019. Metabolites from marine microorganisms, micro, and macroalgae: immense scope for pharmacology. Mar. Drugs, 17(8): 464.
Cadenas E, Davies K J A. 2000. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic. Bio l. Med., 29(3-4): 222-230.
Cao X H, Wang A H, Wang C L, Mao D Z, Lu M F, Cui Y Q, Jiao R Z. 2010. Surfactin induces apoptosis in human breast cancer MCF-7 cells through a ROS/JNK-mediated mitochondrial/caspase pathway. Chem- Biol. Interact., 183(3): 357-362.
Demain A L, Sanchez S. 2009. Microbial drug discovery: 80 years of progress. J. Antibiot., 62(1): 5-16.
Elmore S. 2007. Apoptosis: a review of programmed cell death. Toxicol. Pathol., 35(4): 495-516.
Fei F R, Hu R Y, Gong W W, Pan J, Wang M. 2019. Analysis of mortality and survival rate of liver cancer in Zhejiang Province in China: a general population-based study. Can. J. Gastroenterol. Hepatol., 2 0 19: 1074286.
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin D M, Forman D, Bray F. 2015. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer, 136(5): E359-86.
Gudiña E J, Teixeira J A, Rodrigues L R. 2016. Biosurfactants produced by marine microorganisms with therapeutic applications. Mar. Drugs, 14(2): E38.
Hajare S N, Subramanian M, Gautam S, Sharma A. 2013. Induction of apoptosis in human cancer cells by a Bacillus lipopeptide bacillomycin D. Biochimie, 95(9): 1 722-1 731.
Hermawan A, Putri H. 2018. Current report of natural product development against breast cancer stem cells. Int. J. Biochem . Cell Biol., 104: 114-132.
Iwasaki A, Ohno O, Katsuyama S, Morita M, Sasazawa Y, Dan S, Simizu S, Yamori T, Suenaga K. 2015. Identifi cation of a molecular target of kurahyne, an apoptosis-inducing lipopeptide from marine cyanobacterial assemblages. Bioorg. Med. Chem. Lett., 25(22): 5 295-5 298, https://doi.org/10.1016/j.bmcl.2015.09.044.
Kuang S, Liu G, Cao R B, Zhang L L, Yu Q, Sun C M. 2017. Mansouramycin C kills cancer cells through reactive oxygen species production mediated by opening of mitochondrial permeability transition pore. Oncotarget, 8(61): 104 057-104 071.
Liu G, Kuang S, Cao R B, Wang J, Peng Q C, Sun C M. 2019. Sorafenib kills liver cancer cells by disrupting SCD1-mediated synthesis of monounsaturated fatty acids via the ATP-AMPK-mTOR-SREBP1 signaling pathway. FASEB J., 33(9): 10 089-10 103.
Liu G, Wang K, Kuang S, Cao R B, Bao L, Liu R, Liu H W, Sun C M. 2018. The natural compound GL22, isolated from Ganoderma mushrooms, suppresses tumor growth by altering lipid metabolism and triggering cell death. Cell Death Dis., 9(6): 689, https://doi.org/10.1038/s41419-018-0731-6.
Ni C H, Yu C S, Lu H F, Yang J S, Huang H Y, Chen P Y, Wu S H, Ip S W, Chiang S Y, Lin J G, Chung J G. 2014. Chrysophanol-induced cell death (Necrosis) in human lung cancer A549 cells is mediated through increasing reactive oxygen species and decreasing the level of mitochondrial membrane potential. Environ. Toxicol., 29(7): 740-749.
Obtel M, Lyoussi B, Tachfouti N, Pelissier S M, Nejjari C. 2015. Using surveillance data to understand cancer trends: an overview in Morocco. Arch. Public Health, 73: 45.
Ouyang L, Shi Z, Zhao S, Wang F T, Zhou T T, Liu B, Bao J K. 2012. Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis. Cell Prolif., 45(6): 487-498.
Park S Y, Kim J H, Lee Y J, Lee S J, Kim Y. 2013. Surfactin suppresses TPA-induced breast cancer cell invasion through the inhibition of MMP-9 expression. Int. J. Oncol., 42(1): 287-296.
Schinke C, Martins T, Queiroz S C N, Melo I S, Reyes F G R. 2017. Antibacterial compounds from marine bacteria, 2010-2015. J. Nat. Prod., 80(4): 1 215-1 228.
Sia D, Villanueva A, Friedman S L, Llovet J M. 2017. Liver cancer cell of origin, molecular class, and efference ects on patient prognosis. Gastroenterology, 152(4): 745-761.
Torre L A, Islami F, Siegel R L, Ward E M, Jemal A. 2017. Global cancer in women: burden and trends. Cancer Epidemiol. Biomarkers Prev., 26(4): 444-457.
Valko M, Leibfritz D, Moncol J, Cronin M T D, Mazur M, Telser J. 2007. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Bio l., 39(1): 44-84.
Wang C L, Liu C, Niu L L, Wang L R, Hou L H, Cao X H. 2013. Surfactin-induced apoptosis through ROS-ERSCa2+-ERK pathways in HepG2 cells. Cell Biochem. Biophys., 67(3): 1 433-1 439.
Wu S M, Liu G, Zhou S N, Sha Z X, Sun C M. 2019. Characterization of antifungal lipopeptide biosurfactants produced by marine bacterium Bacillus sp. CS30. Mar. Drugs, 17(4): 199, https://doi.org/10.3390/md17040199.
Wu Y S, Ngai S C, Goh B H, Chan K G, Lee L H, Chuah L H. 2017. Anticancer activities of surfactin and potential application of nanotechnology assisted surfactin delivery. Front. Pharmacol., 8: 761, https://doi.org/10.3389/fphar.2017.00761.
Xiu P Y, Liu R, Zhang D C, Sun C M. 2017. Pumilacidin-like lipopeptides derived from marine bacterium Bacillus sp. Strain 176 suppress the motility of Vibrio alginolyticus. Appl. Environ. Microbiol., 83(12): e00450-17, https://doi.org/10.1128/AEM.00450-17.
Yang H, Li X, Li X, Yu H M, Shen Z Y. 2015. Identifi cation of lipopeptide isoforms by MALDI-TOF-MS/MS based on the simultaneous purifi cation of iturin, fengycin, and surfactin by RP-HPLC. Anal. Bioanal. Chem., 407(9): 2 529-2 542.
Zhang Y X, Yu P F, Gao Z M, Yuan J, Zhang Z. 2017. Cafference eic acid n-butyl ester-triggered necrosis-like cell death in lung cancer cell line A549 is prompted by ROS mediated alterations in mitochondrial membrane potential. Eur. Rev. Med. Pharmaco l. Sci., 21(7): 1 665-1 671.
Zhao H B, Shao D Y, Jiang C M, Shi J L, Li Q, Huang Q S, Rajoka M S R, Yang H, Jin M L. 2017. Biological activity of lipopeptides from Bacillus. Appl. Microbiol. Biot e chnol., 101(15): 5 951-5 960, https://doi.org/10.1007/s00253-017-8396-0.
Zhong H Q, Xiao M Q, Zarkovic K, Zhu M J, Sa R N, Lu J H, Tao Y Z, Chen Q, Xia L, Cheng S Q, Waeg G, Zarkovic N, Yin H Y. 2017. Mitochondrial control of apoptosis through modulation of cardiolipin oxidation in hepatocellular carcinoma: a novel link between oxidative stress and cancer. Free Radical Bio l. Med., 102: 67-76, https://doi.org/10.1016/j.freeradbiomed.2016.10.494.
Zong W X, Ditsworth D, Bauer D E, Wang Z Q, Thompson C B. 2004. Alkylating DNA damage stimulates a regulated form of necrotic cell death. Genes Dev., 18(11): 1 272-1 282.
 Journal of Oceanology and Limnology2020年3期
Journal of Oceanology and Limnology2020年3期
- Journal of Oceanology and Limnology的其它文章
- List of the Most Outstanding Papers Published by CJOL/JOL in 2017-2018
- Who is the “murderer” of the bloom in coastal waters of Fujian, China, in 2019?*
- The investigation of internal solitary waves over a continental shelf-slope*
- Efference ect of diets on the feeding behavior and physiological properties of suspension-feeding sea cucumber Cucumaria frondosa*
- Efference ects of light quality on growth rates and pigments of Chaetoceros gracilis (Bacillariophyceae)*
- PI3K/Akt pathway is involved in the activation of RAW 264.7 cells induced by hydroxypropyltrimethyl ammonium chloride chitosan*
