Behavioral responses to ocean acidifi cation in marine invertebrates: new insights and future directions*
WANG Ting , WANG Youji ,
1 National Demonstration Center for Experimental Fisheries Science Education (Shanghai Ocean University), Shanghai 201306, China
2 International Research Center for Marine Biosciences at Shanghai Ocean University, Ministry of Science and Technology, Shanghai 201306, China
3 Key Laboratory of Exploration and Utilization of Aquatic Genetic Resources (Shanghai Ocean University), Ministry of Education, Shanghai 201306, China
Received Apr. 24, 2019; accepted in principle Jun. 17, 2019; accepted for publication Aug. 26, 2019 © Chinese Society for Oceanology and Limnology, Science Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Abstract Ocean acidifi cation (OA) afference ects marine biodiversity and alters the structure and function of marine populations, communities, and ecosystems. Recently, efference ects of OA on the behavioral responses of marine animals have been given with much attention. While many of previous studies focuses on marine fi sh. Evidence suggests that marine invertebrate behaviors were also be afference ected. In this review, we discussed the efference ects of CO 2-driven OA on the most common behaviors studied in marine invertebrates, including settlement and habitat selection, feeding, anti-predatory, and swimming behaviors, and explored the related mechanisms behind behaviors. This review summarizes how OA afference ects marine invertebrate behavior, and provides new insights and highlights novel areas for future research.
Keyword: carbon dioxide; global climate change; invertebrate behavior; ocean acidifi cation (OA); pH
1 INTRODUCTION
Rapid increase in atmospheric carbon dioxide (CO2) concentration and subsequent ocean acidifi cation (OA) have been reported for having a broad range of biological impacts on marine animals, including efference ects on physiology, growth and development, calcifi cation, and overall survival (Kroeker et al., 2010, 2013 for meta-analytical reviews). More recently, behavioral consequences of OA for marine animals have been of great interest (Brifference a et al., 2012; Clements and Hunt, 2015; Nagelkerken and Munday, 2016). Animal behaviors not only regulate the overall welfare and status of specifi c species and their populations (Sih et al., 2004), but also have a potential evolutionary ability to afference ect ecosystems (Fabry et al., 2008). For example, changes in foraging or feeding behavior have a certain impact on the survival and reproduction of animals, and the prey’s resistance or evasion to predators can afference ect populations and community structures and ultimately ecosystem functions (Persons et al., 2001). It is thus critical to understand the potential efference ects of global change stressors such as ocean acidifi cation on the behavior of marine animals. Here, we provide an updated overview of the behavioral impacts of OA on the most common marine invertebrates, such as settlement and habitat selection, feeding behavior, anti-predator responses, swimming and movement, and explore the potential mechanisms behind behaviors, highlighting new insights and key directions for future research of OA and the behavior.
2 OVERVIEW OF OCEAN ACIDIFICATION ON INVERTEBRATE BEHAVIORS
Experimental evidences suggest that near-future ocean acidifi cation can exert impacts on a number of behavioral processes that are important for growth and survival of marine invertebrates (Table 1).
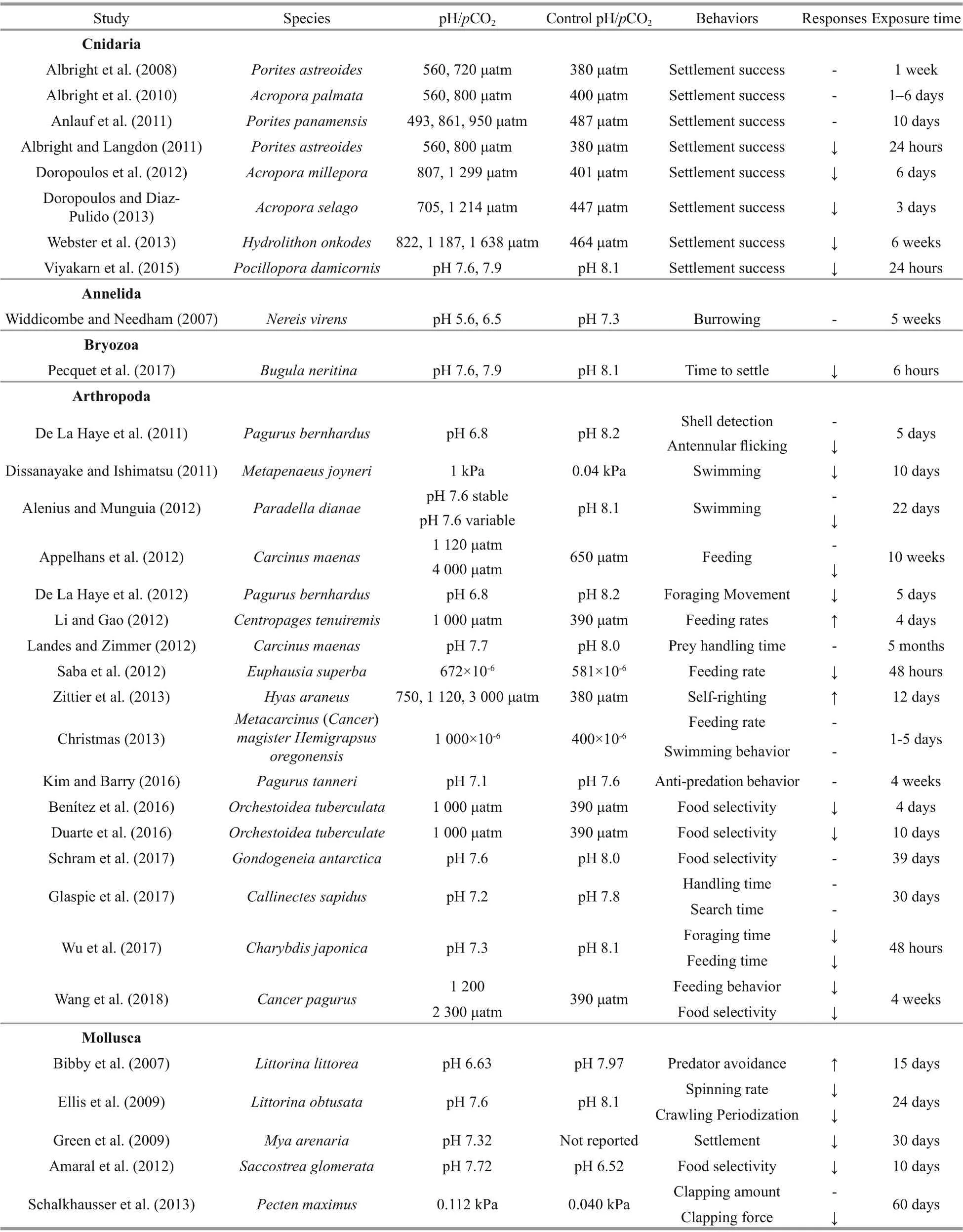
Table 1 A summary of the impacts of ocean acidifi cation on marine invertebrate behaviors
To be continued
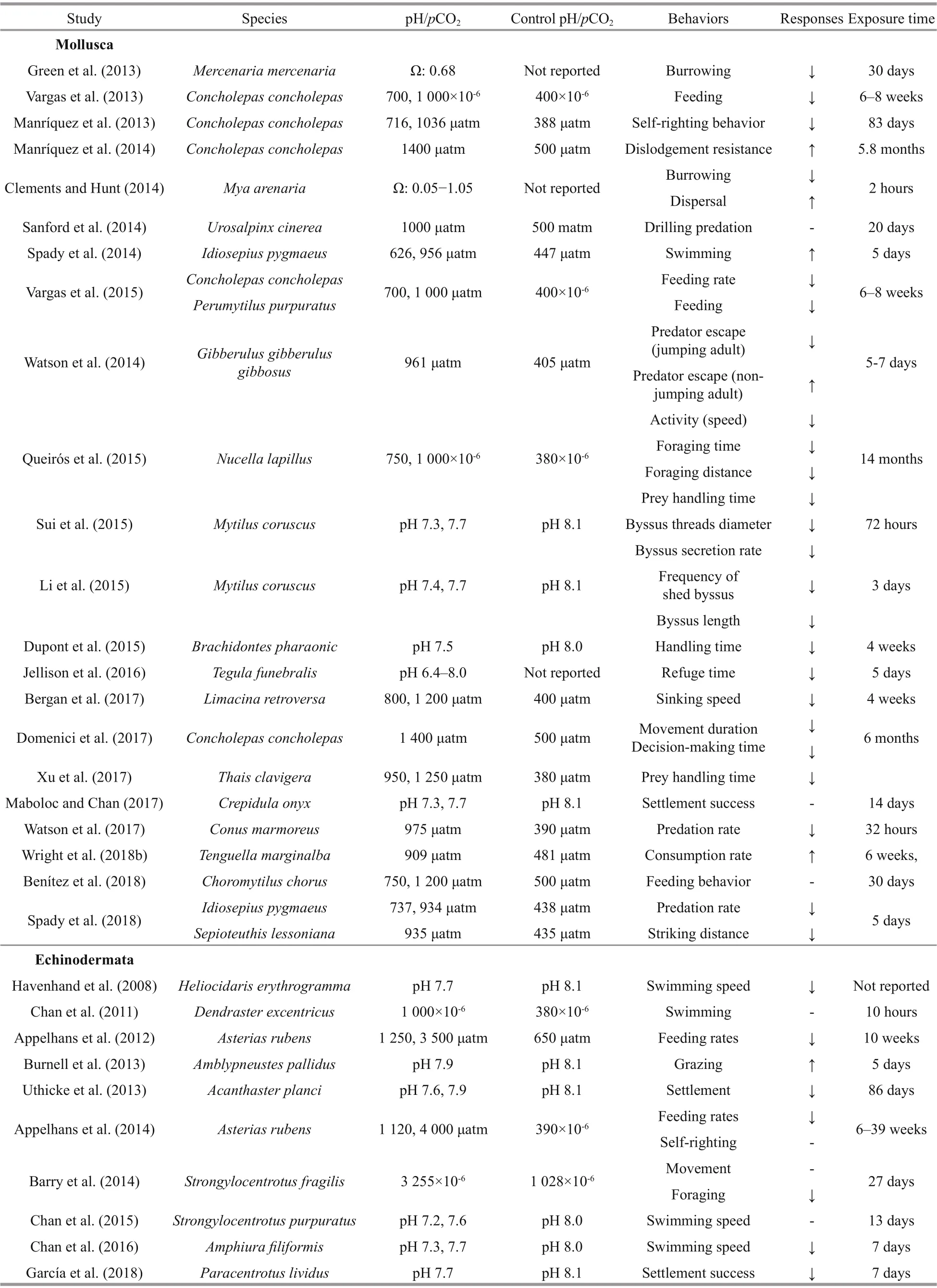
Table 1 Continued
2.1 Settlement and habitat selection
Most marine invertebrates have a planktonic larval stage. When competent, they settle and metamorphose, switching from a pelagic to benthic lifestyle. This transitionary period has a signifi cant impact on the population dynamics and community structure, as successful settlement is necessary for population recruitment (Rodríguez et al., 1993; Caley et al., 1996). Suitable habitat selection and the avoidance of predators are key for at-settlement and post-settlement success (Gosselin and Qian, 1997; Hunt and Scheibling, 1997). Thus, larvae not only need to locate suitable benthic habitats properly, but also must assess the environmental quality in order to choose an ideal habitat (Igulu et al., 2011, 2013). To do this, settling invertebrate larvae make use of olfactory and visual functions to detect environmental cues and select suitable habitats during settlement (Huijbers et al., 2012). However, difference erent sense functions may exhibit difference erent sensitivities to elevated CO2. For example, animals using visual cues for settlement may be signifi cantly afference ected by elevated CO2(Ferrari et al., 2012). Some animals using visual or other perceptual pathways can compensate for diminished olfactory ability under elevated CO2(Devine et al., 2012). Those difference erential sensitivities may be a compensatory mechanism to overcome efference ects of OA on settlement. Moreover, during larval settlement, some species using multiple sensory pathways to detect and choose habitat have been proved less susceptible to OA efference ects than the species using a single sensory pathway.
OA can also have indirect efference ects on invertebrate settlement. For example, OA can afference ect metamorphosis and settlement of coral larvae by afference ecting the symbiosis of corals and microorganisms (Webster et al., 2013). Nakamura et al. (2011) reported that metamorphosis in the coral Acropora digitifera was hindered under OA conditions, and the delayed metamorphosis indirectly afference ected coral settlement and recruitment, despite sustained larval survival. In addition to coral settlement, OA has also been reported to alter the settlement behavior of other invertebrates as well, including marine molluscs, echinoderms, foraminifera, nematodes, polychaetes, crustaceans, and chaetognaths (Cigliano et al., 2010; Uthicke et al., 2013; Maboloc and Chan, 2017; Pecquet et al., 2017; García et al., 2018). Additionally, recent evidences suggest that OA can alter biogenic habitat (created by plants and animals), which can afference ect the composition of settlement cues and potentially reduce the availability of suitable settlement habitat (Uthicke et al., 2013; Sunday et al., 2017). However, much more work is needed to determine how the combined direct (e.g. sensory interference) and indirect (e.g. delayed metamorphosis, alteration of biogenic habitat, etc.) efference ects of elevated CO2act to alter settlement behavior in marine invertebrates.
2.2 Feeding behavior
Food provides the necessary energy to carry out functions for life. Experimental evidences suggest that elevated CO2can afference ect the ability of marine invertebrates to feed, although efference ects are variable. For example, Christmas (2013) reported that there was no signifi cant difference erence in feeding rates of either the dungeness crab Metacarcinus magister or the Pacifi c green shore crab Hemigrapsus oregonensis larvae between the CO2treatments. The predation success rate of the predatory sea snail Conus marmoreus on the tropical conch Gibberulus gibberulus gibbosus was reported to be negatively afference ected by elevated CO2, as predation rates decreased by ~50% under acidifi cation (Watson et al., 2017). The predatory muricid gastropods Morula marginalba consumed higher amount of oysters Saccostrea glomerata from elevated CO2estuarine sites than oysters from reference sites (Amaral et al., 2012). In a predator-prey system containing mussels Brachidontes pharaonic (prey) and predatory crabs Eriphia verrucosa, Dupont et al. (2015) showed that handling time of the crabs was signifi cantly reduced by ~27% when feeding on mussels from elevated CO2conditions. Xu et al. (2017) found that the prey handling time of the muricid gastropod Thais clavigera on the mussel Brachidontes variabilis was decreased signifi cantly while the prey consumption rate was independent of p CO2levels, although the prey searching time was increased signifi cantly at elevated p CO2. These fi ndings indicated that the predator-prey interaction between T. clavigera and B. variabilis was altered under OA, which may have a long-term impact on the population dynamics of the interspecifi c interaction. However, Glaspie et al. (2017) found that the handling time and search time for the crab Callinectes sapidus preying on clams was not impacted by the acidifi ed treatment. Recent evidences emphasized that the ecological outcomes of predator-prey interactions are dependent on the efference ects of elevated CO2on both the predator and the prey (Kroeker et al., 2014 for detailed examples). The potential for near-future OA to afference ect such nuanced ecological interactions requires further research.
Other than active foraging, fi lter feeding is a quite typical feeding behavior of many sessile invertebrates such as bivalve mollusks. Recent studies have reported that OA could negatively afference ect the feeding of bivalves. For example, Zhao et al. (2017a) demonstrated that clearance rate of the blood clam Tegillarca granosa was signifi cantly suppressed by OA treatment, indicating the feeding activity of the clam was impaired under OA scenarios. In addition, after exposure to OA, the mussel Mytilus coruscus would reduce the feeding rate (Wang et al., 2015), and the Manila clam Ruditapes philippinarum would decrease the food uptake (Xu et al., 2016). The impaired feeding activity may reduce the energy uptake from food sources, infl uence the growth and reproduction of these organisms, and ultimately have population and community consequences.
Wu et al. (2017) reported that food selectivity of Japanese shore crab Charybdis japonica, was unaltered by elevated CO2, while foraging and feeding time were increased under elevated CO2, and the same situation was also found in the brown crab Cancer pagurus (Wang et al., 2018). The lack of selectivity efference ect may have resulted from that the weaker periwinkle shell under OA ofference sets the weaker crab claw on crushing prey (Landes and Zimmer, 2012). Wright et al. (2018a) demonstrated that the endemic whelk Tenguella marginalba showed no preference between native preys (the Sydney rock oyster Saccostrea glomerate and the mussel Trichomya hirsuta) and invasive Pacifi c oyster Crassostrea gigas. However, when both oysters and whelks were kept under elevated p CO2, the whelk T. marginalba consumed more oysters C. gigas than S. glomerate (Wright et al., 2018b). Elevated p CO2may increase the energy requirements of the predatory whelks to maintain homoeostasis, and thus the prey consumption by the predator increased (Wright et al., 2018b). Under acidifi cation conditions, the nutritional quality of the brown alga Durvillea antarctica was decreased, and the amphipod Orchestoidea tuberculate showed lower preference for these algae compared with normal D. antarctica (Duarte et al., 2016). However, this amphipod grazed more OA exposed algae compared with normal D. antarctica under no-food choice conditions (Duarte et al., 2016). In a later study, both juvenile and adult O. tuberculate consumed more algae cultured under normal pH than algae cultured under low pH when there was a food choice (Benítez et al., 2016). However, when they were fed with only one type algae (i.e. no food choice), juveniles consumed signifi cantly more algae exposed to low pH compared with algae cultured under normal pH, while adults consumed more algae maintained at normal pH level (Benítez et al., 2016). These results highlight the efference ects of OA on algae and subsequently the ontogenetic variability (i.e. juvenile and adult) in the feeding behavior of amphipods.
Altered feeding behaviors have been investigated, and some potential mechanisms need to be assessed. For example, neurological function has been linked to ciliary beating in marine bivalves, which is an important behavior in bivalve feeding. More specifi cally, serotonin and dopamine have been linked to ciliary beating in the gills of bivalve molluscs and GABA plays a major inhibitory role in regulating the actions of serotonin on ciliary beating (e.g., Catapane et al., 1978, 1979, 2016; Carroll et al., 2007). The elevated CO2is known to infl uence neurological function (GABAAreceptor functioning and associated behaviors) in marine invertebrates (Watson et al., 2014; Clements et al., 2017), neurological efference ects of elevated CO2may afference ect ciliary beating and thus alter fi lter feeding behavior. Given the likelihood that numerous mechanistic drivers may act to alter invertebrate feeding behavior under elevated CO2, future studies would benefi t from teasing out the respective contributions of mechanistic CO2efference ects to better understand how elevated CO2afference ects difference erent modes of feeding at multiple life history stages in a wide range of invertebrate taxa.
2.3 Anti-predatory behavior
Anti-predatory defenses are the reactions that prey produced when they detected the predation risk (Smee and Weissburg, 2016). These responses are very individual for difference erent species. Of the current review, 53% of the relevant anti-predator behaviors showed negative responses to OA. Anti-predator escape responses of the gastropod Gibberulus gibberulus gibbosus, which has a strong foot to escape predators by jumping, were impaired under elevated p CO2as the number of leaping individuals halved and the jumping latency increased (Watson et al., 2014). Under elevated CO2, self-righting (i.e., re-orientation after dislodgement) time was doubled in the Chilean abalone Concholepas concholepas in the presence of the predatory crab Acanthocyclus hassleri (Manríquez et al., 2013). The ability to escape from predators in this abalone had also been reported to decrease under elevated CO2(Manríquez et al., 2014). Defensive behaviors in response to visual predator cues in the squid Idiosepius pygmaeus can be impacted by low pH, as activity levels increased by 19%-25%, movement (number of line crosses) increased threefold, and the possibility of activating inkjet defense strategy had a two-fold increase under elevated CO2conditions (Spady et al., 2014). Byssus thread, a proteinous material, is secreted by the byssal gland at the base of the foot of mussels for anchorage on hard substratum. Enhanced byssus production can reduce the probabilities of being dislodged from the substratum and consumed by their predators. It serves as anti-predatory responses when mussels are exposed to predators. The number of byssus (attachment threads) produced by the mussel Mytilus coruscus was found to decrease under low pH (Sui et al., 2015), it may be explained by the decreased byssusassociated proteins (Sui et al., 2017). Both mechanical performance (such as strength and extensibility) and the numbers of byssal threads produced by M. coruscus were signifi cantly reduced by OA (Zhao et al., 2017b). However, the presence of predators Charybdis japonica resulted in an increase in byssus production for M. corsucus, indicating that enhanced anti-predation ability occurred under elevated CO2when there is a threat of predation (Li et al., 2015). Ocean acidifi cation is also reported to negatively infl uence morphological defenses in the form of shell thickness. The intertidal gastropod Littorina littorea can produce thicker shells in response to predation of crab, but this response was limited at low pH (Bibby et al., 2007). At the same time, L. littorea also increase avoidance behavior (percentage of time a snail spent above or at the water surface in trials) to response to the stress (Bibby et al., 2007). A recent study has found elevated p CO2did not afference ect the ability of whelks Tenguella marginalba to detect a predator, although there were signifi cant efference ects on their antipredatory defense including a reduction in growth and the time spent in refuge (Jellison et al., 2016). In the case of adult deep-sea hermit crab Pagurus tanneri, the time taken to re-emerge from shells after a simulated predatory attack was not infl uenced by OA (Kim and Barry, 2016). Self-righting in adult toad crab Hyas araneus was unafference ected by elevated CO2conditions (Zittier et al., 2013). Similar results were also found in juvenile sea star Asterias rubens (Appelhans et al., 2014). These above results suggest that ontogeny may play a key role in behavioral efference ects, whereby adults and juveniles are likely more tolerant to elevated CO2conditions than larvae.
2.4 Movement and swimming behavior
The population dynamics of marine invertebrate species is largely infl uenced by dispersion, movement and pre-and-post settlement (the periods before and after initial settlement through to adulthood, Pilditch et al. 2015), and the behaviors enumerated above have been reported to be afference ected by elevated CO2. For example, Domenici et al. (2017) found that the keystone gastropod Concholepas concholepas would increase the movement duration and decision-making time under elevated p CO2conditions, while Manríquez et al. (2016) demonstrated this kind of gastropod was nearly still under high p CO2conditions, possibly by reducing metabolic activity to meet the high energy requirements associated with attachment.
Larval swimming speed in the two crabs, Metacarcinus magister and Hemigrapsus oregonensis, appeared to increase under elevated CO2(Christmas, 2013). Under elevated CO2, the swimming speed of brittle star Amphiura fi liformis larvae was reduced (Chan et al., 2016), but the purple sea urchin Strongylocentrotus purpuratus larvae appeared to be unafference ected (Chan et al., 2015). The observed difference erences between species may be related to preexposure in their natural habitats, as A. fi liformis naturally resides in stable environmental pH conditions, while S. purpuratus tend to live in the upwelling region (Droebak, Norway) where low pH may occur (Chan et al., 2015). Although the efference ects of near-future OA on the swimming behavior of larval invertebrates have been documented, few studies have taken into account such efference ects on larval dispersal (but see Chan et al., 2015). Coupled with larval development rates, the direction and speed of larval movements can infl uence where a given larva ends up after it is released into the water column. It is diき cult to measure directly larval dispersal models incorporating larval development and mortality rates, active larval movements, and local hydrodynamics can provide a useful tool to estimate the dispersal potential of larvae (Quinn, 2014). Given that elevated CO2can have impacts on the movement behavior of marine invertebrates, as well as larval mortality and development (Dupont et al., 2008; Brennand et al., 2010), studies assessing the ecological efference ects of acidifi cation would benefi t from incorporating these efference ects into larval drift/dispersal models to better understand the efference ects of near-future acidifi cation on dispersal potential and population connectivity. Furthermore, such studies should take local adaptation into account (Vargas et al., 2017), as the environmental conditions at a given source can determine the impact of climate change stressors on larval development and subsequent dispersal (Quinn and Rochette, 2015).
Given that sperm can actively swim and seek out an egg, they can be considered to be engaging in an active behavior (Elgeti et al., 2015). In the context of OA, a number of studies have reported that the swimming behavior of marine invertebrate spermatozoa can be altered by elevated CO2. Schlegel et al. (2015) observed the sea urchin Centrosteanus rodgersii sperm motility was slightly altered by OA. Sperm motility and velocity in the crown-of-thorns starfi sh Acanthaster planci has also been reported to decrease under elevated CO2, and the subsequent fertilization rate was decreased (Uthicke et al., 2013). OA also negatively infl uenced the sperm motility of the blood clam Tegillarca granosa (Shi et al., 2017a, b). The percentage of active spermatozoa and swimming speed of sea urchin Heliocidaris erythrogramma was decreased signifi cantly at pH 7.80, and the fertility success rate was 24% lower than the normal pH 8.10 (Havenhand et al., 2008). However, it has been reported that the sperm motility rate of mussel Mytilus galloprovincialis signifi cantly increased under elevated CO2(Eads et al., 2016). Also no signifi cant difference erence in sperm motility and viability of Crassostrea gigas was observed between OA (pH 7.80) and control (pH 8.15) conditions (Havenhand and Schlegel, 2009). Sinking is used by pteropod for predator evasion, and altered speeds and increased visibility could increase the susceptibility of pteropods to predation. Bergan et al. (2017) found that sinking speeds were signifi cantly slower for the pteropod Limacina retroversa exposed to OA in comparison to the ambient pH.
Migratory behaviors in many marine animals are often driven by sensory perception (Lohmann et al. 2008; Charpentier and Cohen, 2016). While longdistance migrations are less common in marine invertebrates, invertebrate species can exhibit regular and predictable migratory behaviors. For example, larval invertebrates often exhibit diel vertical migrations in the water column, which can serve to avoid predation while maintaining metabolic function (Ohman et al., 1983). Alongside vertical migrations, some benthic invertebrates undergo larger-scale migrations as well. These processes are diき cult to quantify, and emerging technologies are making them possible to be tested regarding larger-scale invertebrate migrations under elevated CO2conditions. For example, satellite tags can document large-scale movements in situ (González-Gurriarán et al., 2002; Morse and Rochette, 2016). The direct efference ect of acidifi cation on an individual’s ability to perceive cues could be tested by rearing animals under elevated CO2conditions in the lab, releasing them into the fi eld, and tracking their movements over time. Acoustic telemetry can also be used to understand other behaviors of juvenile and adult invertebrates, such as the sheltering behavior, diel movement and activity patterns (e.g., Morse and Rochette, 2016).
3 THE MECHANISMS BEHIND BEHAVIORS
Sensory impairment under elevated CO2has been observed in an array of marine invertebrates, and has been identifi ed as a vulnerable biological attribute to near-future OA (Brifference a et al., 2012; Clements and Hunt, 2015; Ashur et al., 2017). Indeed, many of the behavioral efference ects of elevated CO2on marine invertebrates described above are a direct consequence of sensory impairment. Sensory systems are critical for animals to perceive their external environment. For example, chemoreception (organismal response to chemical stimuli; e.g., taste, smell) relies on both an animal’s ability to receive and interpret stimuli, as well as the particular chemical composition of a given cue. In this sense, elevated CO2can exert morphological or chemical efference ects on the sensory organs of a given organism (Tierney and Atema, 1988). Additionally, elevated CO2may afference ect some aspect of the chemical structure of a cue. While the former remains undescribed, the latter has been reported in a single study for signaling molecules associated with chemosensory behavior in the crab Carcinus maenas, whereby elevated CO2altered the charge, electrostatic properties, and physical composition of three peptide signaling molecules, and that a higher cue concentration was subsequently required to elicit a behavioral response from crabs (Roggatz et al., 2016). Interestingly, physiological alterations under elevated CO2play less of a role than cue alterations in driving crab behavior. Ultimately, more work is required to understand the independent and combined efference ects of CO2on sensory organs and production and transmission of chemical cues.
Although alterations to sensory organs and chemical cues may afference ect marine invertebrate capacity to engage in sensory perception under elevated CO2condition, neurological (particularly GABAAneuroreceptor interference) has been given far more attention. GABA is the primary inhibitory neurotransmitter found in the nervous systems of vertebrates (central and peripheral) and some invertebrates (peripheral) (Jessen et al., 1979; Lunt, 1991), and the GABAAneuroreceptor has a specifi c conductance for chloride (Cl-) and bicarbonate (HCO3ˉ) ions. During periods of environmental stress that can invoke acidosis, GABAAneuroreceptors have reversing ability to maintain internal acid-base balance and thus proper cellular and physiological functioning (Boron, 1987; Widdicombe and Spicer, 2008). When GABA binds to the GABAAreceptor under optimal conditions, the ionic gradient at the receptor is such that Cl-and HCO3ˉ ions fl ow into the cell, thus preventing depolarization and resulting in a negative membrane potential and reduced neural activity (Nilsson et al., 2012). Under elevated CO2, however, HCO3ˉ are accumulated and Cl-are pumped out of the cell in order to maintain acid-base balance and avoid acidosis, ultimately resulting in an outfl ow (rather than an infl ow) of ions (Heuer and Grosell, 2014). This reversed ionic gradient can potentially lead to membrane depolarization, neural pathway excitation, and altered behavior.
Treating animals with gabazine provides a simple and elegant method to determine whether or not GABAAinterference acts as the mechanism behind CO2-associated behavioral impairments. The gabazine method has been used in a number of marine fi shes (Nilsson et al., 2012; Hamilton et al., 2013; Chivers et al., 2014; Chung et al., 2014; Lai et al., 2015; Ou et al., 2015); however the role of GABAAinterference in driving invertebrate behavioral responses to elevated CO2is less well known. In a pioneering study using an epifaunal snail, Watson et al. (2013) reported that gabazine-treated individuals reared under elevated CO2conditions exhibited predator avoidance behaviors on par with those of individuals reared under control CO2conditions. Clements and Hunt (2017) reported that gabazine-treated clams Mya arenaria burrowed into acidifi ed sediments in proportions similar to control sediments, while gabazine-untreated clams showed reduced burrowing proportions in acidifi ed sediments. Similar results also appeared in the razor clam Sinonovacula constricta (Peng et al., 2017). Ren et al. (2018) showed GABA expression of the larval crab Portunus trituberculatus was upregulated after OA exposure. Be accompanied, are anxiety-like behaviors, such as the increased average speed, preference for dark environment and fast exploration (Ren et al., 2018). Charpentier and Cohen (2016) indicated that for Asian shore crab Hemigrapsus sanguineus larvae, GABA receptor might not dominate pH efference ects to sensation and behavior. The above results indicate that GABA might be involved in the interactions of GABA receptors and elevated-CO2in seawater, but further studies on GABA acting mechanism in marine animals are needed for clarifi cation.
Alternatively, CO2may have negatively afference ected sensory processes through other pathways than the GABAergic. Alongside sensory impairment, alterations to morphological structures associated with elevated CO2can afference ect marine invertebrate behaviors. As mentioned previously, the efference ects of elevated CO2on the feeding structures of predators (e.g. crab claws, snail radulas) and the defence structures of prey (e.g. shell thickness, foot musculature) can interact in complex ways to determine the outcome of predator-prey interactions (Landes and Zimmer, 2012; Sanford et al., 2014). Such morphological changes can also afference ect other behaviors that have gone largely ignored in the OA literature, such as inter- and intra-specifi c contests/confl icts. Shell deformation in larval bivalves may also hinder proper feeding under elevated CO2(Talmage and Gobler, 2010; Gray et al., 2017). Although efference ects of CO2stress on cellular function may also drive difference erences in feeding rates, some behavioral changes can be adaptive. For example, sea urchin larvae Strongylocentrotus droebachiensis exposed to elevated CO2expressed a high level of morphological plasticity associated with changes in swimming behavior (Chan et al., 2015), implying changes in swimming biomechanics despite a delay in development due to increased physiological costs. The above observations highlight a strong evolutionary pressure to maintain swimming in a varying environment.
4 MOVING FORWARD
The existing studies have largely confi rmed the efference ects of acidifi cation on marine invertebrate behaviors, although signifi cant knowledge gaps remain. Based on recent advances, we suggest that future studies streamline efference orts toward a number of key questions including (i) multiple sensory pathways (e.g., the efference ects on GABAA, chemical composition of cues, and sensory organs and structures); (ii) the ecological outcomes of behaviors such as feeding (i.e., predator-prey interactions and other feeder-food systems) in which both the feeder and the food are reared under elevated CO2; (iii) a range of difference erent feeding behaviors (e.g. involving ciliary action); (iv) larval dispersal and invertebrate movements. As highlighted in previous reviews, our current understanding of the OA efference ects on invertebrate behavior relies heavily on laboratory experiments, and constant acidifi cation was usually chosen to conduct the environment. However, in the fi eld, seawater pH tends to be fl uctuated; current research method may not refl ect the actual environment appropriately. Technologies such as electronic tagging, telemetry, and hall efference ect sensors can enable a shift from observing behavior in the lab to the fi eld. Finally, studies incorporating multiple stressors, natural variability, and potential evolutionary efference ects (i.e., transgenerational and local acclimation and adaptation) will substantially increase predictive power with respect to understanding marine invertebrate behavior and associated ecological functioning under projected future ocean conditions.
5 DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
6 ACKNOWLEDGMENT
We are grateful to Dr. Jefference Clements, Dr. Sam Dupont, and two anonymous reviewers for their valuable comments and revisions for this review.
References
Albright R, Langdon C. 2011. Ocean acidifi cation impacts multiple early life history processes of the Caribbean coral Porites astreoides. Global Change Biol., 17(7): 2 478-2 487, https://doi.org/10.1111/j.1365-2486.2011.02404.x.
Albright R, Mason B, Langdon C J. 2008. Efference ect of aragonite saturation state on settlement and post-settlement growth of Porites astreoides larvae. Coral Reefs, 27(3): 485-490, https://doi.org/10.1007/s00338-008-0392-5.
Albright R, Mason B, Miller M, Langdon C. 2010. Ocean acidifi cation compromises recruitment success of the threatened Caribbean coral Acropora palmata. Proc. Natl. Acad. Sci. USA, 107(47): 20 400-20 404, https://doi.org/10.1073/pnas.1007273107.
Alenius B, Munguia P. 2012. Efference ects of pH variability on the intertidal isopod, Paradella dianae. Mar. Freshw. Behav. Physiol., 45(4): 245-259, https://doi.org/10.1080/102362 44.2012.727235.
Amaral V, Cabral H N, Bishop M J. 2012. Efference ects of estuarine acidifi cation on predator-prey interactions. Mar. Ecol. Prog. Ser., 445: 117-127, https://doi.org/10.3354/meps09487.
Anlauf H, D’Croz L, O’Dea A. 2011. A corrosive concoction: the combined efference ects of ocean warming and acidifi cation on the early growth of a stony coral are multiplicative. J. Exp. Mar. Biol. Ecol., 397(1): 13-20, https://doi.org/10.1016/j.jembe.2010.11.009.
Appelhans Y S, Thomsen J, Opitz S, Pansch C, Melzner F, Wahl M. 2014. Juvenile sea stars exposed to acidifi cation decrease feeding and growth with no acclimation potential. Mar. Ecol. Prog. Ser., 509: 227-239, https://doi.org/10.3354/meps10884 .
Appelhans Y S, Thomsen J, Pansch C, Melzner F, Wahl M. 2012. Sour times: seawater acidifi cation efference ects on growth, feeding behaviour and acid-base status of Asterias rubens and Carcinus maenas. Mar. Ecol. Prog. Ser., 459: 85-98, https://doi.org/10.3354/meps09697.
Ashur M M, Johnston N K, Dixson D L. 2017. Impacts of ocean acidifi cation on sensory function in marine organisms. Integr. Comp. Biol., 57(1): 63-80, https://doi.org/10.1093/icb/icx010.
Barry J P, Lovera C, Buck K R, Peltzer E T, Taylor J R, Walz P, Whaling P J, Brewer P G. 2014. Use of a free ocean CO2enrichment (FOCE) system to evaluate the efference ects of ocean acidifi cation on the foraging behavior of a deep-sea urchin. Environ. Sci. Technol., 48(16): 9 890-9 897, https://doi.org/10.1021/es501603r.
Benítez S, Duarte C, López J, Manríquez P H, Navarro J M, Bonta C C, Torres R, Quijón P A. 2016. Ontogenetic variability in the feeding behavior of a marine amphipod in response to ocean acidifi cation. Mar. Pollut. Bull., 112(1-2): 375-379, https://doi.org/10.1016/j.marpolbul.2016.07.016.
Benítez S, Lagos N A, Osores S, Opitz T, Duarte C, Navarro J M, Lardies M A. 2018. High p CO2levels afference ect metabolic rate, but not feeding behavior and fi tness, of farmed giant mussel Choromytilus chorus. Aquac. Environ. Interact., 10: 267-278, https://doi.org/10.3354/aei00271.
Bergan A J, Lawson G L, Maas A E, Wang Z A. 2017. The efference ect of elevated carbon dioxide on the sinking and swimming of the shelled pteropod Limacina retroversa. ICES J. Mar. Sci., 74(7): 1 893-1 905, https://doi.org/10. 1093/icesjms/fsx008.
Bibby R, Cleall-Harding P, Rundle S, Widdicombe S, Spicer J. 2007. Ocean acidifi cation disrupts induced defences in the intertidal gastropod Littorina littorea. Biol. Lett., 3(6): 699-701, https://doi.org/10.1098/rsbl.2007.0457.
Boron W F. 1987. Intracellular pH regulation. In: Andreoli T E, Hofference man J F, Fanestil D D, Schultz S G eds. Membrane Transport Processes in Organized Systems. Springer, Boston, MA. p.39-51, https://doi.org/10.1007/978-1-4684-5404-8_3.
Brennand H S, Soars N, Dworjanyn S A, Davis A R, Byrne M. 2010. Impact of ocean warming and ocean acidifi cation on larval development and calcifi cation in the sea urchin Tripneustes gratilla. PLoS One, 5(6): e11372, https://doi.org/10.1371/journal.pone.0011372.
Brifference a M, De La Haye K, Munday P L. 2012. High CO2and marine animal behaviour: potential mechanisms and ecological consequences. Mar. Pollut. Bull., 64(8): 1 519-1 528, https://doi.org/10.1016/j.marpolbul.2012.05.032.
Burnell O W, Russell B D, Irving A D, Connell S D. 2013. Eutrophication ofference sets increased sea urchin grazing on seagrass caused by ocean warming and acidifi cation. Mar. Ecol. Prog. Ser., 485: 37-46, https://doi.org/10.3354/meps10323.
Caley M J, Carr M H, Hixon M A, Hughes T P, Jones G P, Menge B A. 1996. Recruitment and the local dynamics of open marine populations. Annu. Rev. Ecol. Syst., 27: 477-500, https://doi.org/10.1146/annurev.ecolsys.27.1.477.
Carroll M A, Catapane E J, Molecular. 2007. The nervous system control of lateral ciliary activity of the gill of the bivalve mollusc, Crassostrea virginica. Comp. Biochem. Physiol. A: Mol. Integr. Physiol., 148(2): 445-450, https://doi.org/10.1016/j.cbpa.2007.06.003.
Catapane E J, Nelson M, Adams T, Carroll M A. 2016. Innervation of gill lateral cells in the bivalve mollusc Crassostrea virginica afference ects cellular membrane potential and cilia activity. J. Pharmacol. Rep., 1(2): 109.
Catapane E J, Stefano G B, Aiello E. 1978. Pharmacological study of the reciprocal dual innervation of the lateral ciliated gill epithelium by the CNS of Mytilus edulis (Bivalvia). J. Exp. Biol., 74(1): 101-113. .
Catapane E J, Stefano G B, Aiello E. 1979. Neurophysiological correlates of the dopaminergic cilio-inhibitory mechanism of Mytilus edulis. J. Exp. Biol., 83: 315-323.
Chan K Y K, García E, Dupont S. 2015. Acidifi cation reduced growth rate but not swimming speed of larval sea urchins. Sci. Rep., 5: 9 764, https://doi.org/10.1038/srep09764.
Chan K Y K, Grünbaum D, Arnberg M, Dupont S. 2016. Impacts of ocean acidifi cation on survival, growth, and swimming behaviours difference er between larval urchins and brittlestars. ICES J. Mar. Sci., 73(3): 951-961, https://doi.org/10.1093/icesjms/fsv073.
Chan K Y K, Grünbaum D, O’Donnell M J. 2011. Efference ects of ocean-acidifi cation-induced morphological changes on larval swimming and feeding. J. Exp. Biol., 214(22): 3 857-3 867, https://doi.org/10.1242/jeb.054809.
Charpentier C L, Cohen J H. 2016. Acidifi cation and γaminobutyric acid independently alter kairomone-induced behaviour. R. Soc. Open Sci., 3(9): 160 311, https://doi.org/10.1098/rsos.160311.
Chivers D P, McCormick M I, Nilsson G E, Munday P L, Watson S A, Meekan M G, Mitchell M D, Corkill K C, Ferrari M C O. 2014. Impaired learning of predators and lower prey survival under elevated CO2: a consequence of neurotransmitter interference. Global Change Biol., 20(2): 515-522, https://doi.org/10.1111/gcb.12291.
Christmas A M F. 2013. Efference ects of Ocean Acidifi cation on Dispersal Behavior in the Larval Stage of the Dungeness Crab and the Pacifi c Green Shore Crab. Western Washington University, Bellingham.
Chung W S, Marshall N J, Watson S A, Munday P L, Nilsson G E. 2014. Ocean acidifi cation slows retinal function in a damselfi sh through interference with GABAAreceptors. J. Exp. Biol., 217(3): 323-326, https://doi.org/10.1242/jeb.092478.
Cigliano M, Gambi M C, Rodolfo-Metalpa R, Patti F P, Hall-Spencer J M. 2010. Efference ects of ocean acidifi cation on invertebrate settlement at volcanic CO2vents. Mar. Biol., 157(11): 2 489-2 502, https://doi.org/10.1007/s00227-010-1513-6.
Clements J C, Bishop M M, Hunt H L. 2017. Elevated temperature has adverse efference ects on GABA-mediated avoidance behaviour to sediment acidifi cation in a wideranging marine bivalve. Mar. Biol., 164(3): 56, https://doi.org/10.1007/s00227-017-3085-1.
Clements J C, Hunt H L. 2014. Infl uence of sediment acidifi cation and water fl ow on sediment acceptance and dispersal of juvenile soft-shell clams ( Mya arenaria L.). J. Exp. Mar. Biol. Ecol., 453: 62-69, https://doi.org/10. 1016/j.jembe.2014.01.002.
Clements J C, Hunt H L. 2015. Marine animal behaviour in a high CO2ocean. Mar. Ecol. Prog. Ser., 536: 259-279, https://doi.org/10.3354/meps11426.
Clements J C, Hunt H L. 2017. Efference ects of CO2-driven sediment acidifi cation on infaunal marine bivalves: a synthesis. Mar. Pollut. Bull., 117(1-2): 6-16, https://doi.org/10.1016/ j.marpolbul.2017.01.053.
De La Haye K L, Spicer J I, Widdicombe S, Brifference a M. 2011. Reduced sea water pH disrupts resource assessment and decision making in the hermit crab Pagurus bernhardus. Anim. Behav., 82(3): 495-501, https://doi.org/10.1016/j.anbehav.2011.05.030.
De La Haye K L, Spicer J I, Widdicombe S, Brifference a M. 2012. Reduced pH sea water disrupts chemo-responsive behaviour in an intertidal crustacean. J. Exp. Mar. Biol. Ecol., 412: 134-140, https://doi.org/10.1016/j.jembe.2011. 11.013.
Devine B M, Munday P L, Jones G P. 2012. Rising CO2concentrations afference ect settlement behaviour of larval damselfi shes. Coral Reefs, 31(1): 229-238, https://doi.org/10.1007/s00338-011-0837-0.
Dissanayake A, Ishimatsu A. 2011. Synergistic efference ects of elevated CO2and temperature on the metabolic scope and activity in a shallow-water coastal decapod ( Metapenaeus joyneri; Crustacea: Penaeidae). ICES J. Mar. Sci., 68(6): 1 147-1 154, https://doi.org/10.1093/icesjms/fsq188.
Domenici P, Torres R, Manriquez P H. 2017. Efference ects of elevated carbon dioxide and temperature on locomotion and the repeatability of lateralization in a keystone marine mollusc. J. Exp. Biol., 220(4): 667-676, https://doi.org/10.1242/jeb.151779.
Doropoulos C, Diaz-Pulido G. 2013. High CO2reduces the settlement of a spawning coral on three common species of crustose coralline algae. Mar. Ecol. Prog. Ser., 475: 93-99, https://doi.org/10.3354/meps10096.
Doropoulos C, Ward S, Diaz-Pulido G, Hoegh-Guldberg O, Mumby P J. 2012. Ocean acidifi cation reduces coral recruitment by disrupting intimate larval-algal settlement interactions. Ecol. Lett., 15(4): 338-346, https://doi.org/10.1111/j.1461-0248.2012.01743.x.
Duarte C, López J, Benítez S, Manríquez P H, Navarro J M, Bonta C C, Torres R, Quijón P. 2016. Ocean acidifi cation induces changes in algal palatability and herbivore feeding behavior and performance. Oecologia, 180(2): 453-462, https://doi.org/10.1007/s00442-015-3459-3.
Dupont S T, Mercurio M, Giacoletti A, Rinaldi A, Mirto S, D’Acquisto L, Sabatino M A, Sara G. 2015. Functional consequences of prey acclimation to ocean acidifi cation for the prey and its predator. PeerJ PrePr., 3: e1438v1.
Dupont S, Havenhand J, Thorndyke W, Peck L S, Thorndyke M. 2008. Near-future level of CO2-driven ocean acidifi cation radically afference ects larval survival and development in the brittlestar Ophiothrix fragilis. Mar. Ecol. Prog. Ser., 373: 285-294.
Eads A R, Kennington W J, Evans J P. 2016. Interactive efference ects of ocean warming and acidifi cation on sperm motility and fertilization in the mussel Mytilus galloprovincialis. Mar. Ecol. Prog. Ser., 562: 101-111, https://doi.org/10.3354/meps11944.
Elgeti J, Winkler R G, Gompper G. 2015. Physics of microswimmers—single particle motion and collective behavior: a review. Rep. Prog. Phys., 78(5): 056601, https://doi.org/10.1088/0034-4885/78/5/056601.
Ellis R P, Bersey J, Rundle S D, Hall-Spencer J M, Spicer J I. 2009. Subtle but signifi cant efference ects of CO2acidifi ed seawater on embryos of the intertidal snail, Littorina obtusata. Aquat. Biol., 5(1): 41-48, https://doi.org/10. 3354/ab00118.
Fabry V J, Seibel B A, Feely R A, Orr J C. 2008. Impacts of ocean acidifi cation on marine fauna and ecosystem processes. ICES J. Mar. Sci., 65(3): 414-432, https://doi.org/10.1093/icesjms/fsn048.
Ferrari M C O, McCormick M I, Munday P L, Meekan M G, Dixson D L, Lönnstedt O, Chivers D P. 2012. Efference ects of ocean acidifi cation on visual risk assessment in coral reef fi shes. Funct Ecol, 26(3): 553-558, https://doi.org/10.1111/ j.1365-2435.2011.01951.x.
García E, Clemente S, Carlos Hernández J. 2018. Efference ects of natural current pH variability on the sea urchin Paracentrotus lividus larvae development and settlement. Mar. Environ. Res., 139: 11-18, https://doi.org/10.1016/j.marenvres.2018.04.012.
Glaspie C N, Longmire K, Seitz R D. 2017. Acidifi cation alters predator-prey interactions of blue crab Callinectes sapidus and soft-shell clam Mya arenaria. J. Exp. Mar. Biol. Ecol., 489: 58-65, https://doi.org/10.1016/j.jembe.2016. 11.010.
González-Gurriarán E, Freire J, Bernárdez C. 2002. Migratory patterns of female spider crabs Maja squinado detected using electronic tags and telemetry. J. Crustacean Biol., 22(1): 91-97, https://doi.org/10.1163/20021975-99990212.
Gosselin L A, Qian P Y. 1997. Juvenile mortality in benthic marine invertebrates. Mar. Ecol. Prog. Ser., 146: 265-282, https://doi.org/10.3354/meps146265.
Gray M W, Langdon C J, Waldbusser G G, Hales B, Kramer S. 2017. Mechanistic understanding of ocean acidifi cation impacts on larval feeding physiology and energy budgets of the mussel Mytilus californianus. Mar. Ecol. Prog. Ser., 563: 81-94, https://doi.org/10.3354/meps11977.
Green M A, Waldbusser G G, Hubazc L, Cathcart E, Hall J. 2013. Carbonate mineral saturation state as the recruitment cue for settling bivalves in marine muds. Estuar. Coasts, 36(1): 18-27, https://doi.org/10.1007/s12237-012-9549-0.
Green M A, Waldbusser G G, Reilly S L, Emerson K, O’Donnell S. 2009. Death by dissolution: sediment saturation state as a mortality factor for juvenile bivalves. Limnol. Oceanogr., 54(4): 1 037-1 047, https://doi.org/10. 4319/lo.2009.54.4.1037.
Hamilton T J, Holcombe A, Tresguerres M. 2013. CO2-induced ocean acidifi cation increases anxiety in rockfi sh via alteration of GABAAreceptor functioning. Proc. Biol. Sci., 281(1775): 20132509, https://doi.org/10.1098/rspb.2013.2509.
Havenhand J N, Buttler F R, Thorndyke M C, Williamson J E. 2008. Near-future levels of ocean acidifi cation reduce fertilization success in a sea urchin. Curr. Biol., 18(15): R651-R652, https://doi.org/10.1016/j.cub.2008.06.015.
Havenhand J N, Schlegel P. 2009. Near-future levels of ocean acidifi cation do not afference ect sperm motility and fertilization kinetics in the oyster Crassostrea gigas. Biogeosciences, 6(12): 3 009-3 015, https://doi.org/10.5194/bg-6-3009-2009.
Heuer R M, Grosell M. 2014. Physiological impacts of elevated carbon dioxide and ocean acidifi cation on fi sh. Am. J. Physiol. - Regul Integr. Comp. Physiol., 307(9): R1 061-R1 084, https://doi.org/10.1152/ajpregu.00064. 2014.
Huijbers C M, Nagelkerken I, Lössbroek P A C, Schulten I E, Siegenthaler A, Holderied M W, Simpson S D. 2012. A test of the senses: fi sh select novel habitats by responding to multiple cues. Ecology, 93(1): 46-55.
Hunt H L, Scheibling R E. 1997. Role of early post-settlement mortality in recruitment of benthic marine invertebrates. Mar. Ecol. Prog. Ser., 155: 269-301, https://doi.org/10.3354/meps155269.
Igulu M M, Nagelkerken, I, Beek, M V D, Schippers, M, Eck, R.V, Mgaya, Y D. 2013. Orientation from open water to settlement habitats by coral reef fi sh: behavioral fl exibility in the use of multiple reliable cues. Mar. Ecol. Prog. Ser., 493: 243-257, https://doi.org/10.3354/meps 10542
Igulu, M M, Nagelkerken, I, Fraaije, R, Hintum, R V, Ligtenberg, H, Mgaya, Y.D. 2011. The potential role of visual cues for microhabitat selection during the early life phase of a coral reef fi sh ( Lutjanus fulvifl amma). J. Exp. Mar. Biol. Ecol., 401: 118-125, https://doi.org/10.1016/j.jembe.2011.01.022
Jellison B M, Ninokawa A T, Hill T M, Sanford E, Gaylord B. 2016. Ocean acidifi cation alters the response of intertidal snails to a key sea star predator. Proc. Biol. Sci., 283(1833): 20160890, https://doi.org/10.1098/rspb.2016.0890.
Jessen K R, Mirsky R, Dennison M E, Burnstock G. 1979. GABA may be a neurotransmitter in the vertebrate peripheral nervous system. Nature, 281(5726): 71-74, https://doi.org/10.1038/281071a0.
Kim T W, Barry J P. 2016. Boldness in a deep sea hermit crab to simulated tactile predator attacks is unafference ected by ocean acidifi cation. Ocean Sci. J., 51(3): 381-386, https://doi.org/10.1007/s12601-016-0034-8.
Kroeker K J, Kordas R L, Crim R N, Singh G G. 2010. Metaanalysis reveals negative yet variable efference ects of ocean acidifi cation on marine organisms. Ecol. Lett., 13(11): 1 419-1 434, https://doi.org/10.1111/j.1461-0248.2010. 01518.x.
Kroeker K J, Kordas R L, Crim R, Hendriks I E, Ramajo L, Singh G S, Duarte C M, Gattuso J P. 2013. Impacts of ocean acidifi cation on marine organisms: quantifying sensitivities and interaction with warming. Global Change Biol., 19(6): 1 884-1 896, https://doi.org/10.1111/gcb.12179.
Kroeker K J, Sanford E, Jellison B M, Gaylord B. 2014. Predicting the efference ects of ocean acidifi cation on predatorprey interactions: a conceptual framework based on coastal molluscs. Biol. Bull., 226(3): 211-222, https://doi.org/10.1086/BBLv226n3p211.
Lai F, Jutfelt F, Nilsson G E. 2015. Altered neurotransmitter function in CO2-exposed stickleback ( Gasterosteus aculeatus): a temperate model species for ocean acidifi cation research. Conserv. Physiol., 3(1): cov018, https://doi.org/10.1093/conphys/cov018.
Landes A, Zimmer M. 2012. Acidifi cation and warming afference ect both a calcifying predator and prey, but not their interaction. Mar. Ecol. Prog. Ser., 450: 1-10, https://doi.org/10.3354/meps09666.
Li L S, Lu W Q, Sui Y M, Wang Y J, Gul Y, Dupont S. 2015. Confl icting efference ects of predator cue and ocean acidifi cation on the mussel Mytilus coruscus byssus production. J. Shellfi sh Res., 34(2): 393-400, https://doi.org/10.2983/035. 034.0222.
Li W, Gao K. 2012. A marine secondary producer respires and feeds more in a high CO2ocean. Mar. Pollut. Bull., 64(4): 699-703, https://doi.org/10.1016/j.marpolbul.2012.01.033
Lohmann K J, Lohmann C M F, Endres C S. 2008. The sensory ecology of ocean navigation. J. Exp. Biol., 211(11): 1 719-1 728, https://doi.org/10.1242/jeb.015792.
Lunt G G. 1991. GABA and GABA receptors in invertebrates. Semin. Neurosci., 3(3): 251-258, https://doi.org/10.1016/ 1044-5765(91)90022-G.
Maboloc E A, Chan K Y K. 2017. Resilience of the larval slipper limpet Crepidula onyx to direct and indirect-diet efference ects of ocean acidifi cation. Sci. Rep., 7(1): 12 062, https://doi.org/10.1038/s41598-017-12253-2.
Manríquez P H, Jara M E, Mardones M L, Navarro J M, Torres R, Lardies M A, Vargas C A, Duarte C, Widdicombe S, Salisbury J, Lagos N A. 2013. Ocean acidifi cation disrupts prey responses to predator cues but not net prey shell growth in Concholepas concholepas (loco). PLoS One, 8(7): e68643.
Manríquez P H, Jara M E, Mardones M L, Torres R, Navarro J M, Lardies M A, Vargas C A, Duarte C, Lagos N A. 2014. Ocean acidifi cation afference ects predator avoidance behaviour but not prey detection in the early ontogeny of a keystone species. Mar. Ecol. Prog. Ser., 502: 157-167, https://doi.org/10.3354/meps10703.
Manríquez P H, Jara M E, Seguel M E, Torres R, Alarcon E, Lee M R. 2016. Ocean acidifi cation and increased temperature have both positive and negative efference ects on early ontogenetic traits of a rocky shore keystone predator species. PLoS One, 11(3): e0151920, https://doi.org/10.1371/journal.pone.0151920.
Morse B, Rochette R. 2016. Movements and activity levels of juvenile American lobsters Homarus americanus in nature quantifi ed using ultrasonic telemetry. Mar. Ecol. Prog. Ser., 551: 155-170, https://doi.org/10.3354/meps11721.
Nagelkerken I, Munday P L. 2016. Animal Behaviour shapes the ecological efference ects of ocean acidifi cation and warming: moving from individual to community-level responses. Global Change Biol., 22(3): 974-989, https://doi.org/10.1111/gcb.13167.
Nakamura M, Ohki S, Suzuki A, Sakai K. 2011. Coral larvae under ocean acidifi cation: survival, metabolism, and metamorphosis. PLoS One, 6(1): e14521, https://doi.org/10.1371/journal.pone.0014521.
Nilsson G E, Dixson D L, Domenici P, McCormick M I, Sørensen C, Watson S A, Munday P L. 2012. Near-future carbon dioxide levels alter fi sh behaviour by interfering with neurotransmitter function. Nat. Clim. Change, 2(3): 201-204, https://doi.org/10.1038/nclimate1352.
Ohman M D, Frost B W, Cohen E B. 1983. Reverse diel vertical migration: an escape from invertebrate predators. Science, 220(4604): 1 404-1 407, https://doi.org/10.1126/science.220.4604.1404.
Ou M, Hamilton T J, Eom J, Lyall E M, Gallup J, Jiang A, Lee J, Close D A, Yun S S, Brauner C J. 2015. Responses of pink salmon to CO2-induced aquatic acidifi cation. Nat. Clim. Change, 5(10): 950-955, https://doi.org/10.1038/nclimate2694.
Pecquet A, Dorey N, Chan K Y K. 2017. Ocean acidifi cation increases larval swimming speed and has limited efference ects on spawning and settlement of a robust fouling bryozoan, Bugula neritina. Mar. Pollut. Bull., 124(2): 903-910, https://doi.org/10.1016/j.marpolbul.2017.02.057.
Peng C, Zhao X G, Liu S X, Shi W, Han Y, Guo C, Peng X, Chai X L, Liu G X. 2017. Ocean acidifi cation alters the burrowing behaviour, Ca2+/Mg2+-ATPase activity, metabolism, and gene expression of a bivalve species, Sinonovacula constricta. Mar. Ecol. Prog. Ser., 575: 107-117, https://doi.org/10.3354/meps12224.
Persons M H, Walker S E, Rypstra A L, Marshall S D. 2001. Wolf spider predator avoidance tactics and survival in the presence of diet-associated predator cues (Araneae: Lycosidae). Anim. Behav., 61(1): 43-51, https://doi.org/10.1006/anbe.2000.1594.
Pilditch C A, Valanko S, Norkko J, Norkko A. 2015. Postsettlement dispersal: the neglected link in maintenance of soft-sediment biodiversity. Biol. Lett., 11(2): 20140795, https://doi.org/10.1098/rsbl.2014.0795.
Queirós A M, Fernandes J A, Faulwetter S, Nunes J, Rastrick S P S, Mieszkowska N, Artioli Y, Yool A, Calosi P, Arvanitidis C, Findlay H S, Barange M, Cheung W W L, Widdicombe S. 2015. Scaling up experimental ocean acidifi cation and warming research: from individuals to the ecosystem. Global Change Biol., 21(1): 130-143, https://doi.org/10.1111/gcb.12675.
Quinn B K, Rochette R. 2015. Potential efference ect of variation in water temperature on development time of American lobster larvae. ICES J. Mar. Sci., 72(S1): i79-i90, https://doi.org/10.1093/icesjms/fsv010.
Quinn B. 2014. Assessing Potential Infl uence of Larval Development Time and Drift on Large-scale Spatial Connectivity of American Lobster ( Homarus americanus). University of New Brunswick, Fredericton and Saint John, NB.
Ren Z, Mu C, Li R, Song W, Wang C. 2018. Characterization of a γ-aminobutyrate type A receptor-associated protein gene, which is involved in the response of Portunus trituberculatus to CO2-induced ocean acidifi cation. Aquat. Res., 49(7): 2 393-2 403, https://doi.org/10.1111/are. 13699.
Rodríguez S R, Ojeda F P, Inestrosa N C. 1993. Settlement of benthic marine invertebrates. Mar. Ecol. Prog. Ser., 97: 193-207, https://doi.org/10.3354/meps097193.
Roggatz C C, Lorch M, Hardege J D, Benoit D M. 2016. Ocean acidifi cation afference ects marine chemical communication by changing structure and function of peptide signalling molecules. Global Change Biol., 22(12): 3 914-3 926, https://doi.org/10.1111/gcb.13354.
Saba G K, Schofi eld O, Torres J J, Ombres E H, Steinberg D K. 2012. Increased feeding and nutrient excretion of adult Antarctic krill, Euphausia superba, exposed to enhanced carbon dioxide (CO2). PLoS One, 7(12): e52224, https://doi.org/10.1371/journal.pone.0052224.
Sanford E, Gaylord B, Hettinger A, Lenz E A, Meyer K, Hill T M. 2014. Ocean acidifi cation increases the vulnerability of native oysters to predation by invasive snails. Proc. Biol. Sci., 281(1778): 20132681, https://doi.org/10.1098/rspb.2013.2681.
Schalkhausser B, Bock C, Stemmer K, Brey T, Pörtner H O, Lannig G B. 2013. Impact of ocean acidifi cation on escape performance of the king scallop, Pecten maximus, from Norway. Mar. Biol., 160(8): 1 995-2 006, https://doi.org/10.1007/s00227-012-2057-8.
Schlegel P, Binet M T, Havenhand J N, Doyle C J, Williamson J E. 2015. Ocean acidifi cation impacts on sperm mitochondrial membrane potential bring sperm swimming behaviour near its tipping point. J. Exp. Biol., 218(7): 1 084-1 090, https://doi.org/10.1242/jeb.114900.
Schram J B, Schoenrock K M, McClintock J B, Amsler C D, Angus R A. 2017. Ocean warming and acidifi cation alter Antarctic macroalgal biochemical composition but not amphipod grazer feeding preferences. Mar. Ecol. Prog. Ser., 581: 45-56, https://doi.org/10.3354/meps12308.
Shi W, Han Y, Guo C, Zhao X G, Liu S X, Su W H, Wang Y C, Zha S J, Chai X L, Liu G X. 2017a. Ocean acidifi cation hampers sperm-egg collisions, gamete fusion, and generation of Ca2+oscillations of a broadcast spawning bivalve, Tegillarca granosa. Mar. Environ. Res., 130: 106-112, https://doi.org/10.1016/j.marenvres.2017.07.016.
Shi W, Zhao X G, Han Y, Guo C, Liu S X, Su S H, Wang Y C, Zha S J, Chai X L, Fu W D, Yang H C, Liu G X. 2017b. Efference ects of reduced pH and elevated p CO2 on sperm motility and fertilisation success in blood clam, Tegillarca granosa. N. Z. J. Mar. Freshwater Res., 51(4): 543-554, https://doi.org/10.1080/00288330.2017.1296006.
Sih A, Bell A, Johnson J C. 2004. Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol. Evol., 19(7): 372-378, https://doi.org/10.1016/j.tree.2004.04.009.
Smee D L, Weissburg M J. 2006. Hard clams ( Mercenaria mercenaria) evaluate predation risk using chemical signals from predators and injured conspecifi cs. J. Chem. Ecol., 32(3): 605-619, https://doi.org/10.1007/s10886-005-9021-8.
Spady B L, Munday P L, Watson S A. 2018. Predatory strategies and behaviours in cephalopods are altered by elevated CO2. Global Change Biol., 24(6): 2 585-2 596, https://doi.org/10.1111/gcb.14098.
Spady B L, Watson S A, Chase T J, Munday P L. 2014. Projected near-future CO2levels increase activity and alter defensive behaviours in the tropical squid Idiosepius pygmaeus. Biol. Open, 3(11): 1 063-1 070, https://doi.org/ 10.1242/bio.20149894.
Sui Y M, Hu M H, Huang X Z, Wang Y J, Lu W Q. 2015. Antipredatory responses of the thick shell mussel Mytilus coruscus exposed to seawater acidifi cation and hypoxia. Mar. Environ. Res., 109: 159-167, https://doi.org/10. 1016/j.marenvres.2015.07.008.
Sui Y M, Liu Y M, Zhao X, Dupont S, Hu M H, Wu F L, Huang X Z, Li J L, Lu W Q, Wang Y J. 2017. Defense responses to short-term hypoxia and seawater acidifi cation in the thick shell mussel Mytilus coruscus. Front. Physiol., 8: 145, https://doi.org/10.3389/fphys.2017.00145.
Sunday J M, Fabricius K E, Kroeker K J, Anderson K M, Brown N E, Barry J P, Connell S D, Dupont S, Gaylord B, Hall-Spencer J M, Klinger T, Milazzo M, Munday P L, Russell B D, Sanford E, Thiyagarajan V, Vaughan M L H, Widdicombe S, Harley C D G. 2017. Ocean acidifi cation can mediate biodiversity shifts by changing biogenic habitat. Nat. Clim. Change, 7(1): 81-85, https://doi.org/10.1038/NCLIMATE3161.
Talmage S C, Gobler C J. 2010. Efference ects of past, present, and future ocean carbon dioxide concentrations on the growth and survival of larval shellfi sh. Proc. Natl. Acad. Sci. USA, 107(40): 17 246-17 251, https://doi.org/10.1073/pnas.0913804107.
Tierney A J, Atema T. 1988. Amino acid chemoreception: efference ects of pH on receptors and stimuli. J. Chem. Ecol., 14(1): 135-141, https://doi.org/10.1007/BF01022537.
Uthicke S, Pecorino D, Albright R, Negri A P, Cantin N, Liddy M, Dworjanyn S, Kamya P, Byrne M, Lamare M. 2013. Impacts of ocean acidifi cation on early life-history stages and settlement of the coral-eating sea star Acanthaster planci. PLoS One, 8(12): e82938, https://doi.org/10.1371/journal.pone.0082938.
Vargas C A, Aguilera V M, Martín V S, Manríquez P H, Navarro J M, Duarte C, Torres R, Lardies M A, Lagos N A. 2015. CO2-driven ocean acidifi cation disrupts the fi lter feeding behavior in Chilean gastropod and bivalve species from difference erent geographic localities. Estuar. Coasts, 38(4): 1 163-1 177.
Vargas C A, De La Hoz M, Aguilera V, Martín V S, Manríquez P H, Navarro J M, Torres R, Lardies M A, Lagos N A. 2013. CO2-driven ocean acidifi cation reduces larval feeding eき ciency and changes food selectivity in the mollusk Concholepas concholepas. J. Plankton Res., 35(5): 1 059-1 068, https://doi.org/10.1093/plankt/fbt045.
Vargas C A, Lagos N A, Lardies M A, Duarte C, Manríquez P H, Aguilera V M, Broitman B, Widdicombe S, Dupont S. 2017. Species-specifi c responses to ocean acidifi cation should account for local adaptation and adaptive plasticity. Nat. Ecol. Evol., 1(4): 84, https://doi.org/10.1038/s41559-017-0084.
Viyakarn V, Lalitpattarakit W, Chinfak N, Jandang S, Kuanui P, Khokiattiwong S, Chavanich S. 2015. Efference ect of lower pH on settlement and development of coral, Pocillopora damicornis (Linnaeus, 1758). Ocean Sci. J. 50(2): 475-480.
Wang Y J, Hu M H, Wu F L, Storch D, Pörtner H O. 2018. Elevated p CO2afference ects feeding behavior and acute physiological response of the brown crab Cancer pagurus. Front. Physiol., 9: 1164.
Wang Y J, Li L S, Hu M H, Lu W Q. 2015. Physiological energetics of the thick shell mussel Mytilus coruscus exposed to seawater acidifi cation and thermal stress. Sci. Total Environ., 514: 261-272, https://doi.org/10.1016/j.scitotenv.2015.01.092.
Watson S A, Fields J B, Munday P L. 2017. Ocean acidifi cation alters predator behaviour and reduces predation rate. Biol. Lett., 13(2): 20160797, https://doi.org/10.1098/rsbl.2016. 0797.
Watson S A, Lefevre S, McCormick M I, Domenici P, Nilsson G E, Munday P L. 2014. Marine mollusc predator-escape behaviour altered by near-future carbon dioxide levels. Proc. Biol. Sci., 281(1774): 20132377, https://doi.org/10. 1098/rspb.2013.2377.
Webster N S, Uthicke S, Botté E S, Flores F, Negri A P. 2013. Ocean acidifi cation reduces induction of coral settlement by crustose coralline algae. Global Change Biol., 19(1): 303-315, https://doi.org/10.1111/gcb.12008.
Widdicombe S, Needham H R. 2007. Impact of CO2-induced seawater acidifi cation on the burrowing activity of Nereis virens and sediment nutrient fl ux. Mar. Ecol. Prog. Ser., 341: 111-122, https://doi.org/10.3354/meps341111.
Widdicombe S, Spicer J I. 2008. Predicting the impact of ocean acidifi cation on benthic biodiversity: what can animal physiology tell us? J. Exp. Mar. Biol. Ecol., 366(1-2): 187-197, https://doi.org/10.1016/j.jembe.2008.07.024.
Wright J M, O’Connor W A, Parker L M, Ross P M. 2018a. Predation by the endemic whelk Tenguella marginalba (Blainville, 1832) on the invasive Pacifi c oyster Crassostrea gigas (Thunberg, 1793). Molluscan Res., 38(2): 130-136, https://doi.org/10.1080/13235818.2017.1 420397.
Wright J M, Parker L M, O’Connor W A, Scanes E, Ross P M. 2018b. Ocean acidifi cation afference ects both the predator and prey to alter interactions between the oyster Crassostrea gigas (Thunberg, 1793) and the whelk Tenguella marginalba (Blainville, 1832). Mar. Biol., 165(3): 46, https://doi.org/10.1007/s00227-018-3302-6.
Wu F L, Wang T, Cui S K, Xie Z, Dupont S, Zeng J N, Gu H X, Kong H, Hu M H, Lu W Q, Wang Y J. 2017. Efference ects of seawater pH and temperature on foraging behavior of the Japanese stone crab Charybdis japonica. Mar. Pollut. Bull., 120(1-2): 99-108, https://doi.org/10.1016/j.marpolbul.2017.04.053.
Xu X Y, Yip K R, Shin P K S, Cheung S G. 2017. Predator-prey interaction between muricid gastropods and mussels under ocean acidifi cation. Mar. Pollut. Bull., 124(2): 911-916, https://doi.org/10.1016/j.marpolbul.2017.01.003.
Xu X, Yang F, Zhao L Q, Yan X W. 2016. Seawater acidifi cation afference ects the physiological energetics and spawning capacity of the Manila clam Ruditapes philippinarum during gonadal maturation. Comp. Biochem. Physiol. A: Mol. Integr. Physiol., 196: 20-29, https://doi.org/10.1016/j.cbpa.2016.02.014.
Zhao X G, Guo C, Han Y, Che Z M, Wang Y C, Wang X Y, Chai X L, Wu H X, Liu G X. 2017b. Ocean acidifi cation decreases mussel byssal attachment strength and induces molecular byssal responses. Mar. Ecol. Prog. Ser., 565: 67-77, https://doi.org/10.3354/meps11992.
Zhao X G, Shi W, Han Y, Liu S X, Guo C, Fu W D, Chai X L, Liu G X. 2017a. Ocean acidifi cation adversely infl uences metabolism, extracellular pH and calcifi cation of an economically important marine bivalve, Tegillarca granosa. Mar. Environ. Res., 125: 82-89, https://doi.org/10.1016/j.marenvres.2017.01.007.
Zittier Z M C, Hirse T, Pörtner H O. 2013. The synergistic efference ects of increasing temperature and CO2levels on activity capacity and acid-base balance in the spider crab, Hyas araneus. Mar. Biol., 160(8): 2 049-2 062, https://doi.org/10.1007/s00227-012-2073-8.
 Journal of Oceanology and Limnology2020年3期
Journal of Oceanology and Limnology2020年3期
- Journal of Oceanology and Limnology的其它文章
- List of the Most Outstanding Papers Published by CJOL/JOL in 2017-2018
- Who is the “murderer” of the bloom in coastal waters of Fujian, China, in 2019?*
- The investigation of internal solitary waves over a continental shelf-slope*
- Efference ect of diets on the feeding behavior and physiological properties of suspension-feeding sea cucumber Cucumaria frondosa*
- Efference ects of light quality on growth rates and pigments of Chaetoceros gracilis (Bacillariophyceae)*
- Marine bacterial surfactin CS30-2 induced necrosis-like cell death in Huh7.5 liver cancer cells*
