Distribution characteristics of lipids in hadal sediment in the Yap Trench*
YAN Yixin , SUN Chengjun , HUANG Yuhuan CAO Wei JIANG Fenghua YANG Guipeng , , , DING Haibing , , ,
1 Key Laboratory of Marine Chemistry Theory and Technology, Ocean University of China, Qingdao 266100, China 2 College of Chemistry and Chemical Engineering, Ocean University of China, Qingdao 266100, China
3 Key Laboratory of Marine Eco-environmental Science and Technology, Marine Bioresource and Environment Research Center, First Institute of Oceanography, Ministry of Natural Resources, Qingdao 266061, China
4 Qingdao National Laboratory for Marine Science and Technology, Qingdao 266237, China
5 Qingdao Collaborative Innovation Center of Marine Science and Technology, Qingdao 266100, China
Received May 2, 2018; accepted in principle Jun. 28, 2018; accepted for publication May 21, 2019 © Chinese Society for Oceanology and Limnology, Science Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Abstract In this study, cored sediment samples collected by the Jiaolong Submersible at 6 779 m depth from the hadal zone of the Yap Trench in May 2016 were sliced in 1-cm interval from top to bottom, and lipids in each sediment layer were quantitatively and qualitatively analyzed. The vertical distribution profi les of the lipids in the sediment sample, their main existing forms, and their possible sources were investigated. The results show that the concentration of lipid in the surface sediment was the highest with the carbon number from 12 to 27, dominated by medium and short-chain lipids. The total concentration of fatty acids in surface sediment was much higher than those in the ofference shore and deep-sea areas, being up to 325.77 μg/g due to the funnel efference ect caused by the “V” terrain of the trench. Fatty acid 18׃0 was the most abundant lipids in the sediment sample. Abnormal high concentrations of fatty acid 18׃1ω7 and alkanes indicated the existence of hydrothermal fl uids in the study area. In addition, saturated fatty acids and monounsaturated fatty acids existed mainly in free form, and polyunsaturated fatty acids existed mainly in bound form. Most of the alkanes were in bound form, and their major source was autochthounous input. The carbon number of alcohols in the sediment sample ranged from 12 to 20, mainly existed in bound form. The source of fatty acids was mainly autochthonous input, and the neutral lipids had both marine and terrestrial origin. This is the fi rst study of lipids in hadal sediment of the Yap Trench. The results will promote deeper understanding of organic carbon cycle in marine environment.
Keyword: hadal zone; the Yap Trench; lipids; fatty acids; sediment
1 INTRODUCTION
In marine environment, the hadal zone is 6 000 m below sea level. It is the deepest area of the ocean and has unique biological community. Hadal sediments constitute one of the most important benthic environments for hadal biological communities, providing abundant, continuous, and long-time scale environmental information (Chen, 2002). To study the biogeochemistry of sediments, biomarkers are considered as a powerful tool for the uniqueness of the source and the structural stability. Studying biomarkers allow us to track the sources and migration and transformation pattern of substance, to investigate the sedimentary characteristics, and to provide information for understanding biogeochemical processes in sediment. Biomarker can also provide useful information on paleoceanography.
Lipids are important biomarkers in the ocean, especially in sediments, organisms, and suspend particles. They are widely used in marine biogeochemical research. For example, studies about biomarkers in surface sediment in the Yellow Sea and the Bohai Sea indicated that the main components of their sediments were a mixture of terrestrial and marine sources. In the middle Yellow Sea, lipids in its muddy sediment were mostly terrestrial origin (Gao et al., 2017). Connelly et al. (2016) studied the seasonal distributions of fatty acids in the sediment and analyzed the fatty acids in suspended particulate organic matter of the Beaufort Sea in the Canadian Arctic. Their results show that the composition of fatty acids in winter was mainly insoluble saturated fatty acids. Budge and Parrish’s (1998) study of fatty acids in sediment of a fjord-like bay in the eastern Newfoundland, Canada, show that fatty acids were from diatom and dinofl agellates and were efference ectively recycled through the diagenetic process at the sediment-water interface. The main source of fatty acids in the sediments is bacterial and terrigenous inputs (Budge and Parrish, 1998; Budge et al., 2001). In studies of deep-sea sediments (3 000-3 300 m) in the Indian Ocean and the Atlantic Ocean, Peng et al. (2011) inferred that the sources of organic matter were mostly from marine photoautotrophic organism production and terrestrial input based on the isotopic composition of n-alkanes. However, due to the diき culties of arrival and sampling, current study of lipids in sediments is mostly concentrated in the ofference shore areas. Until now, few studies focused on lipids in hadal sediments (Guan et al., 2019), and characteristics of lipids in abyss and hadal zone sediment of the Yap Trench are still undocumented.
The Yap Trench is located in the southeastern part of the Philippine Sea Plate in the Western Pacifi c Ocean. It is a complex structural area among the Philippine Sea Plate, the Caroline Plate, and the Pacifi c Plate. Its deepest point is at 8 527 m. Recently, China has conducted a comprehensive study of marine benthos in the trench, including their biodiversity and population structure, distribution of benthic animals, environmental adaptability of seabed microbes, and cellular components of various organisms. Zhu et al. (2015) identifi ed a kind of blind shrimp Rimicaris exoculata collected from the Yap Trench, and they found that the concentrations of fatty acids 16׃1 and 18׃1 in it was signifi cantly higher than those in the coastal shrimps. This blind shrimp was found in the sea bottom under extreme hydrothermal conditions. The lipids in the cells play a crucial role in its survival in extreme environments and provide a new perspective for studying lipid biomarkers (Zhu et al., 2015).
The “V” terrain and hydrothermal activity at the Yap Trench have remarkable infl uence on the concentrations and distributions of various substances in the sediment from its abyss and hadal zone. Exploring lipid biomarkers in the hadal sediment will be helpful to understand the biogeochemical processes, and the degradation and burial of organic matter in the hadal environments. This study is the fi rst report about lipid characteristics in the sediment from the Yap Trench. Various lipids, including saturated fatty acids (SFA), monounsaturated fatty acids (MUFA), and polyunsaturated fatty acids (PUFA) as well as neutral lipids such as alkanes, alcohols, sterols, and phytol, were analyzed qualitatively and quantitatively. Their vertical distribution characteristics, sources, and morphological changes in the sediment were tracked. This research will be signifi cant on studying biogeochemical processes in deepsea area, especially in hadal zone.
2 MATERIAL AND METHOD
2.1 Study area
Sediment samples were cored and collected by the Jiaolong manned submersible carried by R/V Xiangyanghong 09 in May, 2016. A metal multi-tube sampler carried by the submersible was used to collect sediment sample in the Dive111 station (138°30.94′E, 9°52.06′N) at 6 779 m depth on the west side of the Yap Trench. After the submersible returned to R/V Xiangyanghong 09, sediment samples were sliced in a 1-cm interval into 7 layers and frozen stored at -20°C for future analysis. The sediment contained khaki clay and black iron-manganese nodules. Ironmanganese nodules were mainly distributed in 3-5 cm layers. Figure 1 shows the sampling location and the station map.
2.2 Lipids extraction
In this study, concentrations of various lipids in free and bound forms in the sediment layers were determined. The method was based on a published procedure (Ding and Sun, 2005). The method can be described briefl y as follows.
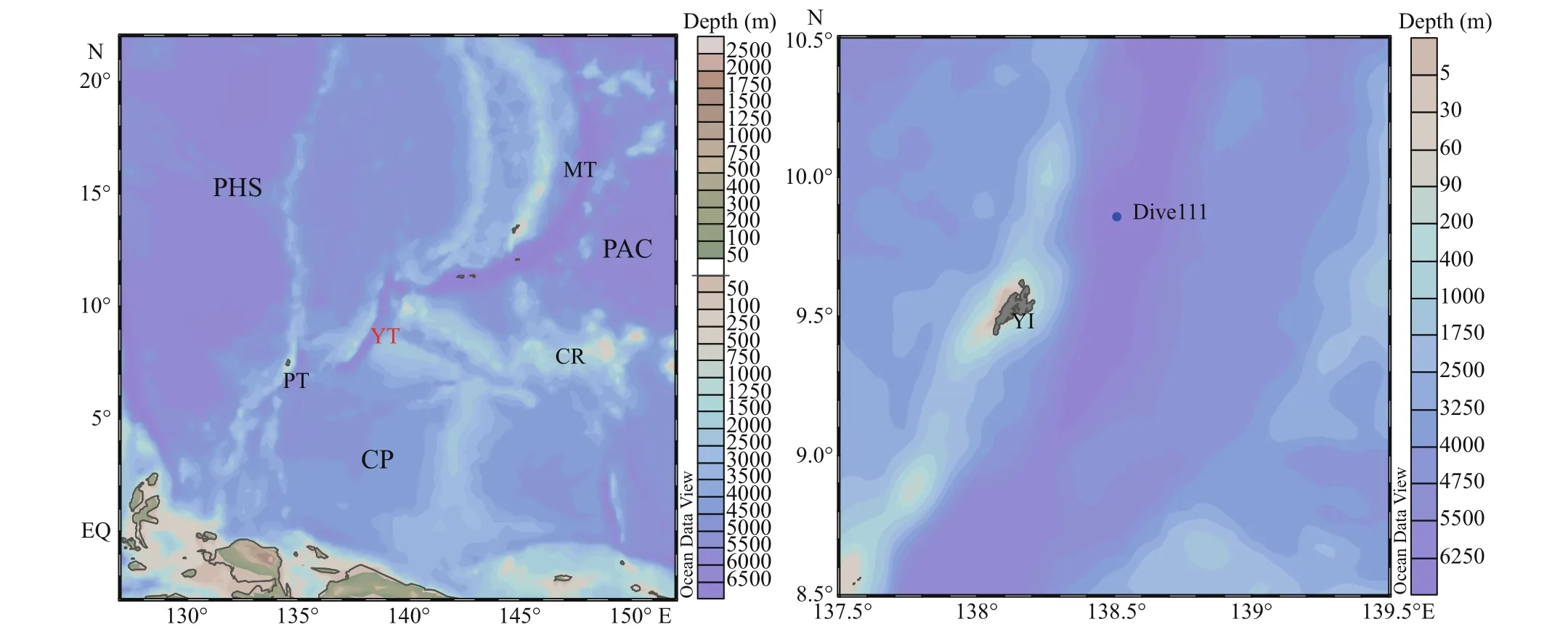
Fig.1 Geographic information of the study area and the sampling station
After the sliced sediment sample thawing, about 0.5 g sediment was used to measure its water content. The sediment sample was transferred in a glass centrifuge tube and placed in a vacuum freeze drier (Christ ALAPH 4-LD Plus). After freeze-drying 48 h, the sediment was taken out for weighing. After 30 min, repeated the above steps until the constant weight was reached. The water content was calculated by the subtraction method (Yue et al., 2018). About 1.5-2 g of the sediment sample was used for lipid analysis. For free lipids analysis, the sediment was extracted once with about 10 mL of methanol, and three times with 10 mL CH2Cl2-CH3OH (v׃v=2׃1) solution. Each extraction was subjected to vortex mixing (1-2 min), sonication (1-2 min), and centrifugation (2 500 r/min for 10 min at 4°C). The supernatant of each extraction was transferred to a separate funnel. Then a small amount of 5% NaCl solution was added to the combined supernatant to form a CH2Cl2phases. After standing for 6 h, the CH2Cl2phase was released into a pyriform fl ask and about 10 mL of CH2Cl2was added to the separate funnel to extract the supernatant again. After three extractions, the organic solvent in the pyriform fl ask was evaporated in a rotary evaporator. Then 0.5 mol/L KOH-CH3OH solution (3×3 mL) was used to wash the lipids into a 20-mL test tube and saponifi ed in a 100°C metal bath for 2 h (SCILOGEX HB120-S). The neutral lipids were extracted by 5 mL n-hexane three times from the solution. After rotary evaporation, the neural lipids were transferred into a 4-mL vial by 3×1 mL hexane. The hexane was blow-dried by nitrogen gas and then the neutral lipids were reacted with 100 μL BSTFA (Bis (trimethylsilyl) trifl uoroacetamide) in 100-μL acetonitrile at 100°C for 2 h. After blowing-ofference BSTFA and acetonitrile, 50 μL 5α (H)-cholestane internal standard (2 mg/mL cholestane/hexane solution) was added in the neutral lipids for future analysis. For fatty acid extraction, HCl solution (6 mol/L) was added to the remaining solution in the test tube until pH<2. The fatty acids were extracted by 3×5 mL n-hexane and transferred into a pyriform fl ask. After rotary evaporation, the fatty acids were washed by 3×1 mL BF3-methonl and 3×1 mL methanol into a 20-mL test tube and then esterifi ed at a 100°C for 2 h to form fatty acid methyl esters (FAMEs). The FAMEs were extracted by 3×5 mL hexane. The FAMEs were transferred into a 4-mL vial by 3×1 mL hexane after rotary evaporation. The hexane was blow-dried by pure N2and internal standard nonadecanoic acid methyl ester (1 mg/mL in hexane) was added in the vial for future analysis of FAMEs. After extraction of free lipids, about 20 mL 0.5 mol/L KOH-CH3OH solution was added to the sediment residue and then heated at 100°C for 2 h. The following steps were the same as the procedures used for separation and extraction of free lipids as described above. Recovery test showed that relative standard deviation of the whole extraction methods was ±2.6% (Sun et al., 1997).
All the extracted lipids were qualitative analyzed by gas chromatography-mass spectrometry (GC-MS: Agilent 7890A-5975C) and quantitative analyzed by gas chromatography (GC: ECHROM A90) with automatic injector and fl ame ionization detector (FID). The lipids were separated by a 30.0 m× 250 μm×0.25 μm column (HP-5MS, Agilent) coated with (5%-phenyl)-methylpolysiloxane copolymer. The oven temperature gradients of the GC and GCMS were as follows: for neutral lipids, the temperature was raised from 50°C to 200°C at 30°C/min, stayed for 10 min, then increased to 215°C at 5°C/min, followed by 220°C at 1°C/min and stayed for 2 min, and fi nally increased to 280°C and stayed for 30 min; for fatty acids, the temperature was raised from 40°C at 30°C/min to 200°C, then at 5°C/min to 215°C, continued heated at 1°C/min to 220°C, stayed at 220°C for 2 min, then increase at 10°C/min to 280°C for 4 min, fi nally increase to 310°C at 20°C/min and stayed for 5 min. The errors of peaks area and retention time by this GC analysis was less than 3%.
2.3 Measurement of δ 13 C of FMAEs
The FAME samples were further analyzed using GC-IRMS (Thermal Finnigan, Mat 253 Plus) to gain δ13C information to track the source of the fatty acids in the sediment samples (MacAvoy et al., 2002). The temperature was raised from 100°C to 190°C at 20°C/min, then increased to 235°Cat 1.5°C/min, followed by 295°C at 20°C/min and stayed for 15 min. The FAMEs were separated by a 50.0 m × 200 μm×0.3 μm column (HP-5). The injection volume was 2 μL. The combustion temperature of the oven was set at 850°C, which can turn all organic substances into CO2. The carbon isotope standard was VPDB (Vienna Peedee Belemnite) based on defi nition of Coplen (1995). All FAMEs δ13C values were corrected for the addition of the methyl group to the original fatty acids based on the equation:
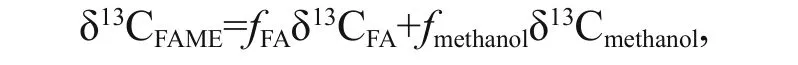
where δ13CFAME, δ13CFAand δ13Cmethanolare the δ13C values of the FAME, the underivatized fatty acid and the methanol, respectively. fFAand fmethanolare the fractions of the underivatized fatty acid and methanol in the FAME , respectively (Ballentine et al., 1996; Uhle et al., 1997). In this study, the δ13Cmethanolwas 43%.
3 RESULT
3.1 Distributions of fatty acids in the sediment sample
There were 25 free and bound fatty acids identifi ed in the sediment samples at the Dive111 station, including 7 SFAs (12׃0, 14׃0, 15׃0, 16׃0, 17׃0, 18׃0, 20׃ 0), 12 MUFAs (12׃1, 13׃1, 14׃1, 15׃1, 16׃1, 17׃1, 18׃1ω7, 18׃1ω9, 20׃1, 21׃1, 22׃1, 23׃1), and 6 PUFAs (22׃3, 22׃4, 22׃6, 23׃1, 23׃2, 24׃4) (Fig.2). Except for the 4-cm layer, the concentration of 18׃0 in all the other layers was the highest, followed by 16׃0. The highest concentration of 18׃0 was detected in the surface sediment, up to 120.97 μg/g. In addition, the concentrations of 18׃1ω9 and 24׃4 were also high (above 50 μg/g). Concentrations of other fatty acids were below 20 μg/g, and the lowest value was 1.51 μg/g (13׃1). In all the sediment layers, shortchain and medium-chain fatty acids were dominated, and long-chain fatty acid (carbon number>20) only occupied a small fraction. For example, in 2-cm sediment depth, short and medium-chain fatty acids accounted for 95% of the total fatty acids. In addition, long-chain fatty acids were all unsaturated.
The vertical distribution profi les of fatty acids showed that the surface sediment layer had the highest concentration of total fatty acids, up to 325.77 μg/g. The concentrations of difference erent fatty acids in the surface sediments varied signifi cantly. SFA was the main component, accounting for 70% of the total fatty acids; followed by MUFA, accounting for 19% of the total fatty acids. The concentrations of SFA, MUFA and PUFA had difference erent vertical variation trends (Fig.3). Concentrations of SFA decreased rapidly from surface to subsurface layer, and reached minimum at 4-cm depth. Then its concentration showed an upward trend in deeper sediment. As a comparison, the concentrations of MUFA and PUFA were the highest in the surface sediment layer; reached a sub-maximal at 4-cm depth, and varied insignifi cantly in deeper sediments. Except in the 4-cm sediment layer, SFA was the main fatty acids in all the other layers. At the 4-cm layer, MUFA was the main component, accounting for 52% of the total fatty acids, and PUFA, accounting for 36% of the total fatty acids. Concentration of PUFA 22׃6 was the highest in the 4-cm layer, accounting for 17% of the total fatty acids, followed by MUFA 16׃1 and 17׃1, accounting for about 8% of total fatty acids, respectively. SFA 12׃0, 16׃0, 18׃0, and 20׃0 were not detected in this layer. In other sediment layers, concentration of SFA 18׃0 was the highest in both free and bound forms. SFA 12׃0 was only detected in the surface sediment layer as free form.
Generally, the main form of the fatty acids in the sediments sample was free, but for various fatty acid species, their main forms were difference erent. SFA and MUFA mainly existed in free form, and PUFA mainly existed in bound form. In the sediment sample, the concentration of free SFA reached the maximum at 2-cm depth. Its lowest concentration occurred at 4-cm depth. The bound SFA had the highest concentration in the surface sediment, and its concentration decreased with increasing sediment depth. The concentrations of free MUFA and PUFA were much lower than that of SFA, and their vertical distribution trends varied in signifi cantly. The concentrations of bound MUFA and PUFA reached the highest in the surface layer, and the sub-maximal value appeared in 4 cm depth.
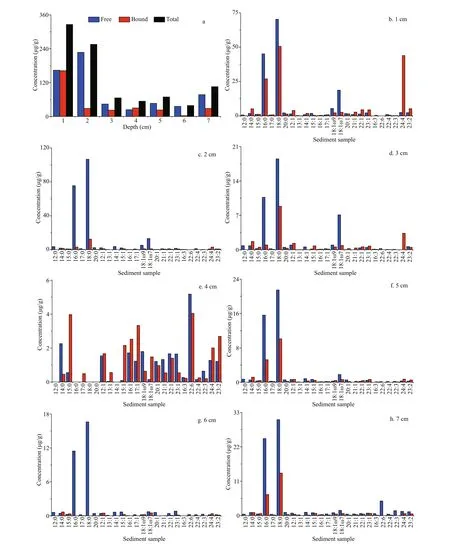
Fig.2 The distributions of difference erent kinds fatty acids in difference erent sediments layers (a. total fatty acids)
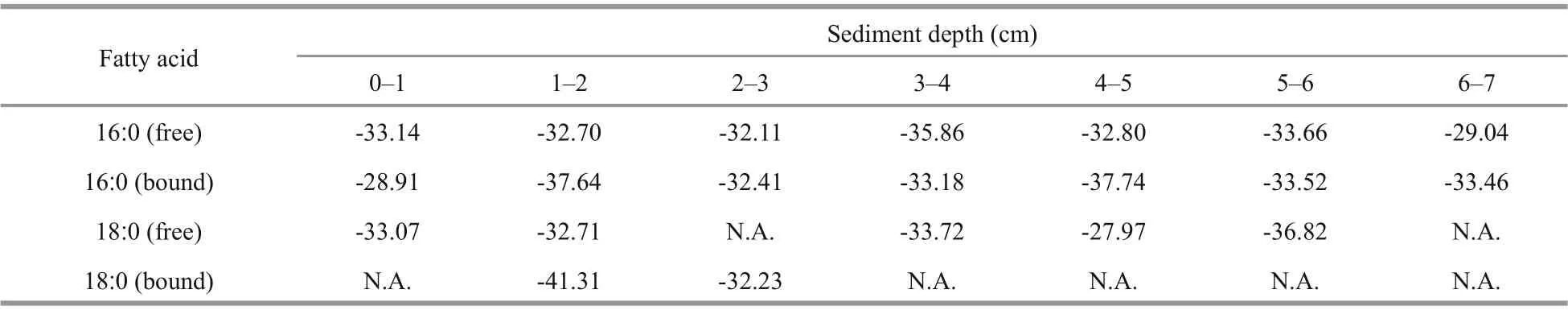
Table 1 δ 13 C values (‰) of fatty acids 16׃0 and 18׃0 in the sediment samples
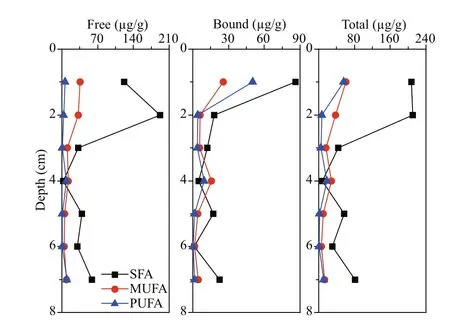
Fig.3 Vertical distributions of various fatty acids in difference erent forms
3.2 δ 13 C values of fatty acids 16׃0 and 18׃0 in the sediment samples
Table 1 showed the δ13C values of two typical fatty acids 16׃0 and 18׃0 in the sediment samples. Overall, the δ13C values of fatty acids 16׃0 and 18׃0 ranged from -28.91‰ to -37.74‰, and from -27.97‰ to -41.31‰, respectively, with mean values -33.36‰ and -33.98‰, respectively. In 0-1 cm and 3-4 cm layer, the δ13C values of free fatty acid 20׃0 were -26.52‰ and -30.74‰, respectively.
3.3 Distributions of neutral lipids in the sediment samples
In the Dive111 station, 31 neutral lipids were identifi ed in free or bound form in the sediment sample, including 14 alkanes (14׃0, 15׃0, 16׃0, 17׃0, 18׃0, 19׃0, 20׃0, 21׃0, 22׃0, 23׃0, 24׃0, 25׃0, 26׃0, 27׃0), 3 kinds of alkenes (17׃1, 24׃1, 26׃1), 8 alcohols (12׃0, 14׃0, 15׃0, 16׃0, 17׃0, 18׃0, 19׃0, 20׃0), 3 kinds of sterols (cholesterol, stigmasterol, sitosterol) and one isoprenoid alcohol (phytol) (Fig.4). The concentration of total neutral lipids in the surface sediment layer was the highest (150.21 μg/g); and the lowest concentration was observed in the 4-cm sediment depth, only 23.78 μg/g.
Throughout the core, except for the 4-cm sediment layer, n-alkanes were the most abundant neutral lipids, accounting for 15%-53% of total neutral lipids in difference erent layers. Their carbon number was between 14 and 27, dominant by <C20, and only 3 long-chain alkanes with carbon number 25, 26, and 27 were identifi ed. The vertical distribution profi les of alkanes showed that their total concentration was the highest in the surface sediments (67.67 μg/g), and the concentration of C15 alkane was the highest (25.56 μg/g), followed by C16 (20.31 μg/g). The concentrations of C19-C21 alkanes were all less than 1 μg/g. In the sediment sample, alkanes were mainly in bound form. Especially in 6-cm depth, only bound alkanes were found in this layer. In addition, alkanes C17, C21, C22, C23, and C24 existed all in bound form. In fact, alkane is a typical hydrophobic compound. Strong hydrophobic action should be an important to promote absorption of alkane on the sediment. However, unlike other layers, bound alkane only accounted for 44% of total amount of alkanes in the 4-cm sediment layer. In this depth, the concentrations of free alkanes C15 and C18 were the highest, up to 4.63 and 4.52 μg/g, respectively. Free alkanes C20 was only found in the 4-cm layer, its concentration was as low as 0.01 μg/g, and alkanes C19 and C20 all existed in free form. Low concentrations of alkane C19 and C20 might indicate that they were early degraded in the sediment. It is possible that unlike other alkanes, alkanes C19 and C20 might originate from difference erent sources and became easy degrade. In the sediment sample, concentrations of both free and bound alkanes reached a maximum at the surface layer and tended to decrease with increased sediment depth.
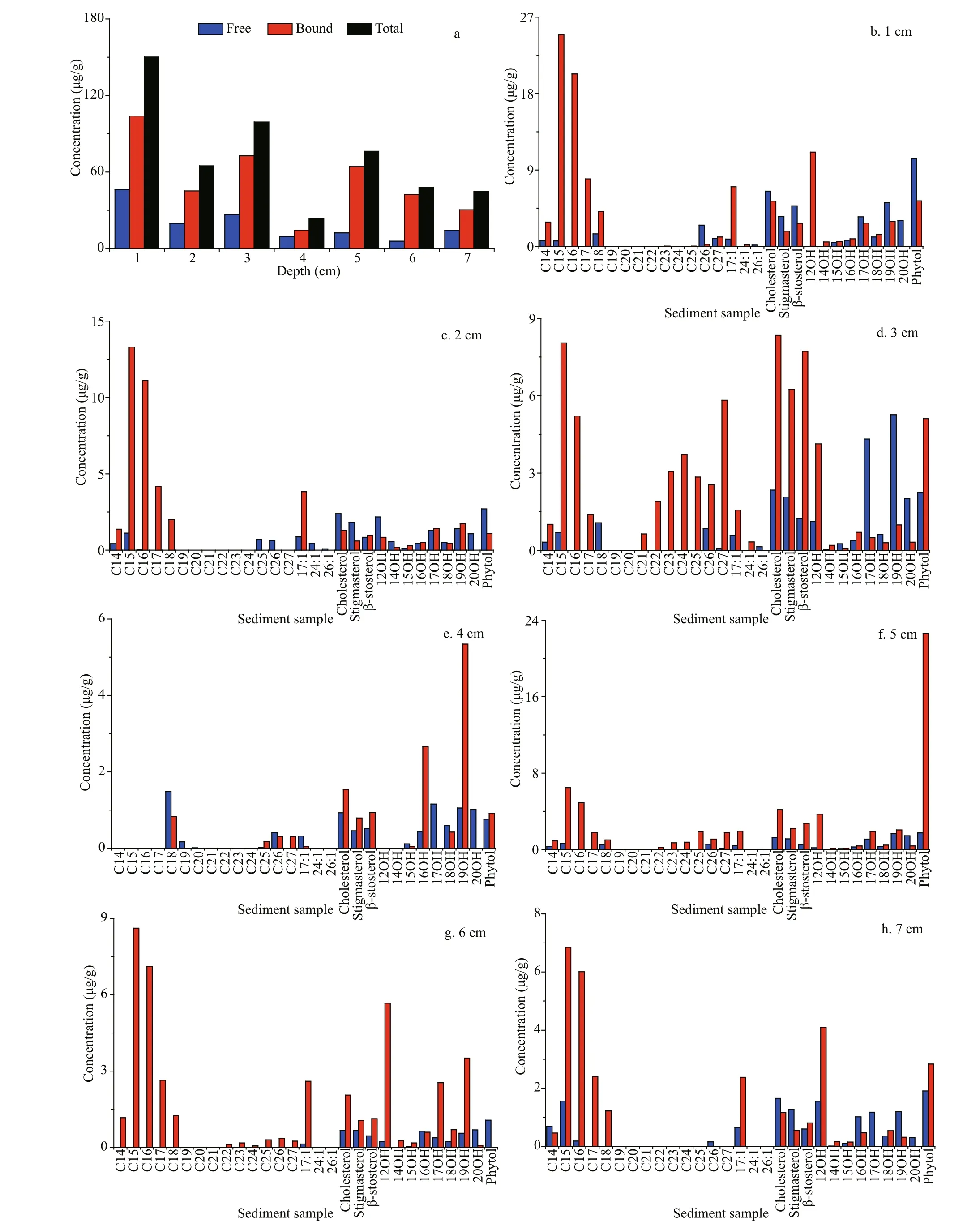
Fig.4 The distributions of difference erent kinds neutral lipids in difference erent sediments layers (a. total neutral lipids)
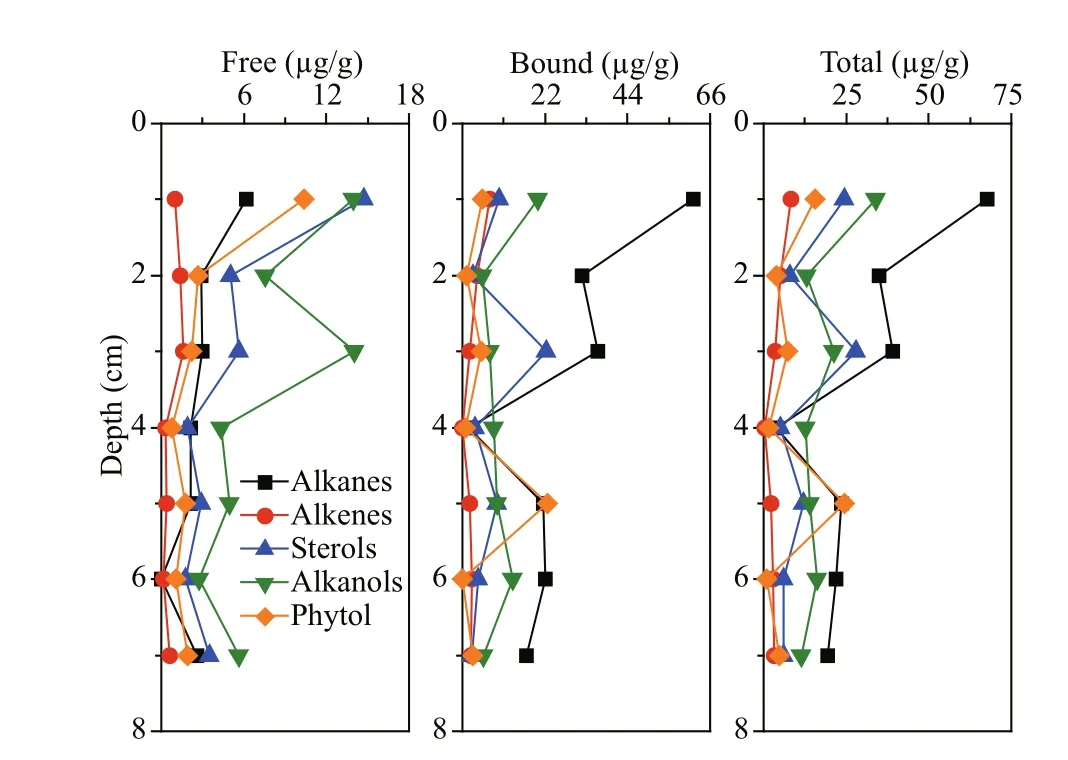
Fig.5 Vertical distributions of various neutral lipids in difference erent forms
In the sediment sample, the carbon number of alcohols distributed between 12 and 20. The proportion of alcohols in the 4-cm sediment layer was the highest, accounting for 54% of the total amount of neutral lipids in this depth. The value in 5-cm sediment depth was the lowest, accounting for only 18% of neutral lipids. In the surface sediment, two most abundant alcohols were 12׃0 and 19׃0, with concentrations of 34.78 and 33.93 μg/g, respectively. The concentration of total alcohols was highest in the surface sediment, and it showed a down decreasing trend. The main form of alcohols in the sediments was bound, especially in the 4-cm and 6-cm layers, accounting for 68% and 83% of total alcohols, respectively. Concentrations of both free and bound alcohols were found to be the highest in the surface sediment. With increasing sediment depth, their concentrations kept decreasing in general.
Vertical distribution patterns of concentrations of three sterols: sitosterol, stigmasterol, and cholesterol were similar in the core (Fig.5). Their high concentrations appeared in the surface and 3-cm sediment layers. The concentrations of free sterols were the highest in the surface sediment with downcore decreasing trends. The concentrations of bound sterols was the highest in the 3-cm sediment layer. In general, the sterols existed mainly in bound form in the sediment sample. However, in the difference erent sediment layers, free and bound sterols had various distribution patterns. They mainly existed in free form in the surface and subsurface sediment, accounting for 60% and 64% of the total amount of sterols, respectively. In the medium-deep sediment layers (3-7 cm), their dominant form was bound, accounting for 42% to 80% of total amount of sterols.
Generally, phytol and sterols shared similar vertical distribution patterns. In the surface sediment layers, free form phytol was dominant. While in the mediumdeep sediment layers, it mainly existed in bound form. Concentration of free phytol was the highest in the surface sediment, up to 10.37 μg/g. Below the surface layer, the concentration decreased with increasing sediment depth. The concentration of bound phytol was the highest in the 5-cm sediment layer (22.61 μg/g). In the sediment sample, phytol mainly existed in bound form. The vertical distribution of total phytol concentration was similar to that of bound phytol. Its highest concentration was 24.34 μg/g in the 5 cm sediment layer. While in the surface sediment, the value decreased to 15.71 μg/g.
Only three alkenes were detected as rarest neutral lipids in all depths of the sediment sample. Alkene 17׃1 was the most abundant alkene in all the sediment layers. Its concentration was the highest in the surface sediment (7.86 μg/g), and decreased with sediment depth. Alkenes 24׃1 was only found in three top sediment layers and its concentration was less than 0.5 μg/g. Alkene 17׃1 and 24׃1 existed in both bound and free forms, and 26׃1 existed only in free form.
4 DISCUSSION
4.1 Distributions of lipids in the Yap Trench
In difference erent layers of the sediment sample, the ratios between concentrations of total fatty acids and neutral lipid varied remarkably, from 0.82 in the 6-cm sediment depth to 3.94 in the subsurface sediment. In most sediment layers, the ratios were higher than 1, indicating fatty acids were dominant lipids in the sample. Concentration of total fatty acids in surface sediment of the sample from the Yap Trench was 325.77 μg/g. As a comparison, the concentration of total fatty acid in the surface sediment of the Yellow Sea were between 20.65 and 44.72 μg/g (Yang and Wang, 2012). The concentrations of total fatty acids in the surface sediments of the muddy areas in the East China Sea were between 11.58 and 31.58 μg/g (Wang et al., 2011), and the concentrations ranged from 7.85 to 47.37 μg/g in the surface sediments of the South China Sea (Cao, 2017). In surface sediment, the concentration of total fatty acids in the samples from these marginal seas was signifi cantly lower than that in the sediment sample from the Yap Trench hadal zone. High concentrations of fatty acids in the hadal sediment sample might refl ect the funnel efference ect of the hadal zone. In fact, the “V” shaped trench has a steeper lateral gradient. This topographical feature is conducive to the accumulation of organic matter from the upper ocean layer to the bottom of the trench, resulting in higher levels of lipids in the surface sediments. Similar to the marginal seawater along China coast, the fatty acids identifi ed in the Yap Trench were even carbon number dominant. However, unlike ofference shore sediment, concentration of fatty acid 18׃0 was the highest in most sediment layers from the hadal zone, while in sediments from the ofference shore areas, fatty acids 16׃0 or 16׃1 had the highest concentrations. The presence of high concentration of 18׃0 in the sediments from Dive111 station is an abnormal feature of the Yap Trench hadal sediment, indicating some special source of lipids in the hadal zone of the trench. However, more future studies are necessary to explain this phenomenon.
The source of organic carbon in deep-sea sediments is mainly due to the deposition of plankton in surface seawater. Previous studies show that average percentage of organic carbon in ordinary bathypelagic sediments is 0.20% (Degens and Mopper, 1976). In sediments from the South China Sea, organic carbon content was between 0.53% and 0.70% (Duan, 2000). In the Pacifi c Ocean, the content ranged from 0.36% to 1.34% (Chen et al., 1986). Specially, in the sediment from the Western Pacifi c, organic carbon content was between 0.37% and 1.18% (Bao, 1987). The total organic carbon content in the sediment samples at Dive111 station was low and varied in difference erent sediment layers. The highest content was in the 1 cm layer, up to 0.52%. Between 3-cm and 7-cm sediment depths, the organic carbon content varied insignifi cantly, around 0.07% (Yue, 2017). Overall, the organic carbon content decreased with increased depth in the sediment sample. In general, the concentration total amount of fatty acids varied consistently with variation of organic carbon content (Bradley and Summons, 2010) in sediment. However, concentrations of the fatty acids in the sediment from the Dive111 station were much higher than those in the sediment from other sea areas and had poor correlation with the organic carbon content. One possibility may attribute to the presence of hydrothermal fl uids. Previous studies have shown that the infl uence of hydrothermal fl uids on fatty acids is more important than organic carbon (Simoneit et al., 1990; Rushdi and Simoneit, 2002a, b). The increase in organic carbon content in sediment at many hydrothermal zones was not signifi cant, but concentrations of fatty acids in it was high (Hedrick et al., 1992), up to 247 μg/g. Our result indicated the existence of hydrothermal fl uids as a source of fatty acids in the hadal zone of the trench.
Among the identifi ed fatty acids, in addition to the high concentrations of 16׃0 and 18׃0, another high concentration fatty acid in the sample was free bacterial fatty acid 18׃1ω7. In surface sediment, its concentration was up to 21.34 μg/g. On the other hand, no typical photosynthetic bacteria fatty acids such as i-15׃0, a-15׃0 etc. (Rütters et al., 2002) was found in the sediment sample. Fatty acid 18׃1ω7 is a typical sulfur-oxidizing bacterial marker (Conway and McDowell Capuzzo, 1990; Mori et al., 2015, Giovannelli et al., 2016), and is abundant in some other hydrothermal microorganisms (Abraham et al., 2013). Sulfur-oxidizing bacteria are considered as the basis of hydrothermal vent food web, and then 18׃1(7) was detected in many difference erent organisms in hydrothermal vent (Fullarton et al., 1995; Phleger et al., 2005; Giovannelli et al., 2016). Therefore, abundant fatty acid 18׃1(7) in sediment were often associated with hydrothermal vent and hydrothermal fl uids, which can lead to high abundance of bacterial communities (Yamanaka and Sakata, 2004). The higher concentration of 18׃1ω7 in the sediment sample further shows that some of the fatty acids in the Yap Trench might come from hydrothermal zones, refl ecting a typical characteristic of the microbial community in the zone. Previous studies have shown that the total concentration of alkanes in the surface sediments of the North Yellow Sea and the Bohai Sea ranged from 0.21 to 3.11 μg/g (Cao et al., 2018). Normally, the concentrations of alkanes in bathypelagic sediments are at ng/g level (Rushdi and Simoneit, 2002a, b) with short-chain alkane dominance. For example, in the sediments from the southwestern African continental margin (1 488 m) (Vogts et al., 2012), the concentrations of alkanes were only 910 ng/g. In the sediment from the Dive111 station, abnormal high concentrations of alkanes were detected. Especially in its surface layer, the concentration of total alkanes was as high as 67.67 μg/g. High concentrations of alkanes also indicated the existence of hydrothermal fl uids (Konn et al., 2012), which could promote the conversion of fatty acids and alcohols to alkanes (Venkatesan et al., 2003). In hydrothemal vent, abiotic process could also promote formation of hydrocabon. For example, Delacour et al. (2008) found higher abundance of n-C16 to n-C20 alkane in the rock obtained from the Lost City Hydrothermal System. They suggested that the vent fl uids with high concentrations of methane and hydrogen might cause abiotic origin of hydrocarbons. In addition, hydrothermal fl uids also facilitated formation of short-chain alkanes (Huang and Pu, 2017) and our results supported this conclusion. Abundant alkane could support growth of alkane-oxidizing bacteria in hydrothermal vent. Fatty acid 18׃1(7) is also a dominant lipid in some kinds of alkane-oxidizing bacteria (Rosario-Passapera et al., 2012). This correlation between fatty acid 18׃1(7) and alkane further supported possible existence of hydrothermal vent in the study area.
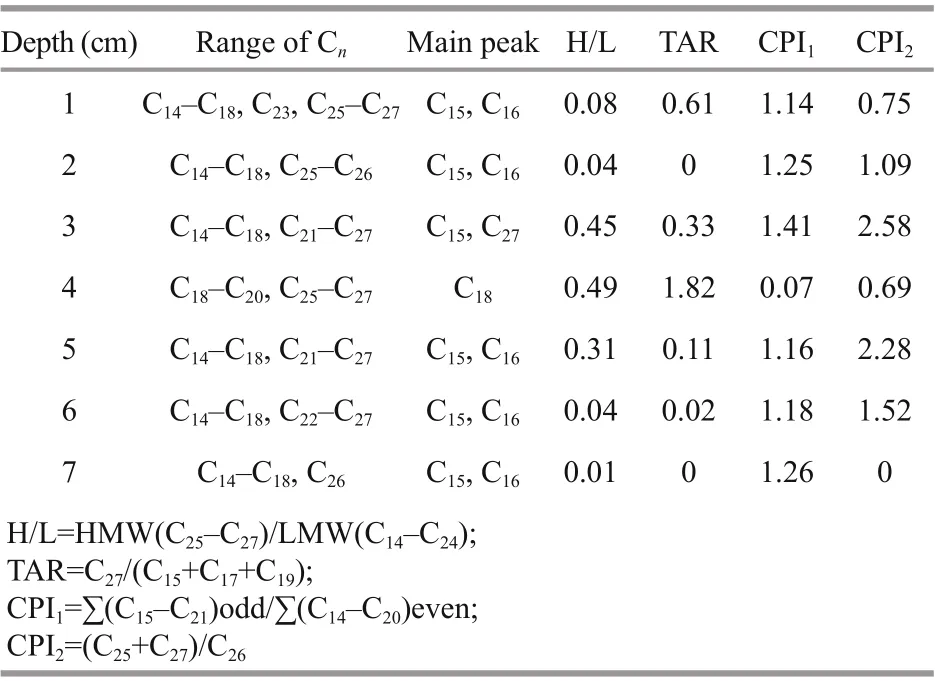
Table 2 Characteristics index of alkane molecule composition
In this study, the δ13C values of fatty acids 16׃0 and 18׃0 varied between -41.31‰ and -27.97‰. Previous study in Shenhu, a typical oil and gas enrichment fi eld, shows that the short-chain fatty acids (ScFAs; n-C12 to n-C18) in the sediment mainly from marine microorganisms had average δ13C values of 26.7‰ to 28.2‰ (Zhu et al., 2014). Overall, our results were signifi cantly lighter than their values, indicating that other organic carbon source might be utilized by various organisms to synthesis fatty acids. MacAvoy et al. (2002) reported that in the methanotrophic mussel Bathymodiolus childressi from hydrocarbon seep in Gulf of Mecico, its fatty acidδ13C values ranged from -45.4‰ to -39.6‰ or from -78.8‰ to -68.4‰, depending on whether thermogenic or biogenic methane was utilized by the methanotrophic symbionts. It is possible that some organisms in the research area will utilize methane released from hydrothermal vent of the study area to synthesis organic carbon with low δ13C values, and then depleted the δ13C values of fatty acids in the sediment samples.
4.2 Abnormal characteristics of lipids in the 4-cm sediment layer
In the sediment sample from the Dive111 station, the 4-cm sediment layer was the main distribution area of iron-manganese nodules. In this layer, the total concentrations of various neutral lipids and saturated fatty acids reached minimal values. The composition of lipids in this layer was signifi cantly difference erent from that of other layers. For example, only in this layer, unsaturated fatty acids were dominant. Concentrations of MUFA and PUFA were high and accounted for 52% and 33% of the total fatty acids in the layer, respectively. In other layers, SFA accounted for the largest proportion, followed by MUFA. In this layer, fatty acids 16׃0 and 18׃0 were not detected, while in other layers they were the two most abundant fatty acids. The difference erence among concentrations of various fatty acids in this layer was insignifi cant. For example, concentration of acid 22׃6 was the highest but only accounted for 17% of the total fatty acids. Concentration of total neutral lipids was low in this layer, only 23.78 μg/g. In this layer, alcohols were main neutral lipids and accounted for 54% of the total neutral lipids, followed by sterols, which accounted for 22% of the total neutral lipids. Unlike other sediment layers, alcohol 12׃0 and 14׃0 were not detected in this layer, and the concentration of alcohol 19׃0 was the highest (6.40 μg/g), accounting for 27% of the total neutral lipids. The alcohol was mainly in bound form in this layer. Compared with other sediment layers, fewer species of alkanes were identifi ed in this 4 cm layer, including C18, C19, C20,C25, C26, C27and even carbon number alkanes were dominant. Alkanes C19and C20were only detected this layer, existing mainly in free form. In this layer, we also found signifi cant difference erence of CPI1between this layer and the other layers. CPI (carbon preference index) is an indicator that refl ects the maturity of organic matter contained in sediments. The parameter is mostly used to refl ect the relative abundance between odd-numbered carbon atoms and evennumbered carbon atoms in n-alkanes. In this layer, the CPI1, which refl ects the relative abundance between odd-numbered carbon and even-numbered carbon of short chain alkanes (C15-C20), was only 0.07, much lower than that in the other layers (Table 2). In addition, we also checked the ratio of H/L (light/heavy hydrocarbons) and TAR (terrestrial/aquatic lipids ratios), which are commonly used parameters to evaluate the relative contributions of terrestrial n-alkanes and sea-derived n-alkanes (Silliman and Schelske, 2003). In general, if H/L is less than 1, the alkanes are primarily composed of bacteria, algae, and marine origin (Yao and Shen, 2003). For TAR value, the range of 1.6-8.5, 1.2-1.6 and <1.1 indicates a predominance of terrestrial origin, mixed terrestrial and marine origin and marine phytoplankton origin, respectively (Lu et al., 2011). The values of H/L and TAR indicated that the alkanes in this layer were mainly terrestrial and marine inputs. Our δ13C value of fatty acid 20׃0 was close to that of Zhu et al. (2014) in the Shenhu area. Similar to their conclusion, our results also show a mixed signal of carbon source in the trench. On the other side, it is possible that due to the presence of iron-manganese nodules as large particles, the specifi c surface area of the 4-cm layer sediment was small, which is not conducive to the adsorption and preservation of organic matter, causing a signifi cant reduction in organic carbon content (Yue et al., 2018) and concentrations of lipids.
4.3 The source of lipids
Fatty acids are typical biomarkers in marine sediments. It is generally considered that saturated fatty acids with a carbon chain length larger than 20 are mainly from terrestrial origin. Even-carbon saturated fatty acids with carbon number less than 20 (12׃0, 14׃0, 16׃0, 18׃0, and 20׃0) and 18׃1ω9 have mixed origins (terrestrial and marine). PUFA (22׃3, 22׃4, 22׃6, 23׃1, 23׃2, and 24׃4) and 16׃1 are derived from marine phytoplankton, of which 22׃6 is derived from intracellular component (Ding and Sun, 2005). Odd-carbon fatty acids (15׃0, 17׃0, etc.) and 18׃1ω7 are mainly derived from bacteria (Budge et al., 2001). Previous studies show that the sediments in the Yap Trench has marine biological sources, terrestrial sources and volcanic sources (Yue et al., 2018). However, we did not fi nd saturated fatty acids with carbon chain length over 20 in the sediment sample from the Dive111 station. The Yap Trench is far from mainland, and it is diき cult for terrestrial origin material to reach the hadal zone of the trench through rivers input or atmospheric deposition. In the sediment samples, the proportion of fatty acids with carbon number more than 20 was only 18%, and most of them were polyunsaturated. Overall, our results indicated that the source of fatty acids in the sediment around the study area should be in marine origin.
Among the sea-derived fatty acids, PUFA such as 22׃6, 18׃3 and 18׃3 are often closely related to eukaryotic algae. In general, the concentrations of these fatty acids were very low in the sediment sample from Dive111 station, indicating that the fatty acids produced by eukaryotic algae were diき cult to reach or exist in hadal sediment of the trench. The ratios of 16׃1 and 16׃0 in all the sediment layers were less than 1. Previous studies have shown that if the ratio is higher than 1.6, the existence of diatoms should be considered. We also did not detect diatom specifi c fatty acids 16׃4ω1 in the sediment samples. In the identifi ed neutral lipids, the concentration of diatom indicator brassicasterol was zero. No evidence supports the existence of diatoms in the sediment from the Dive111. However, by detecting biological fossils in the Yap Trench, diatoms in the sediment from Dive111 station were relatively abundant (Huang et al., 2019). These confl ict results show that although lipids were more stable than proteins and sugars, they could still be degraded completely when the algae sank from sea surface to sediment, while diatom shells composed mainly of inorganic components could be preserved in the sediment. Compared with SFA, in particular, MUFA and PUFA had lower stability (Ding and Sun, 2005). As a result, various diatom lipid biomarkers could not be tracked in the sediment. A typical feature of the sediment sample from the Dive111 stations was that most lipids were more abundant in surface sediments than in subsurface layers. In sediment, the concentrations of lipids were controlled by fresh organic matter input and organic matter degradation. In fact, the seawater in the sampling station of the trench was oxic. In sediment-seawater interface, continuously fresh organic matter input could increase concentrations of lipids in surface sediment. However, existence of oxygen might promote organic matter degradation. Our result indicated that organic matter input over degradation as a dominant factor controlled concentrations of lipids in surface sediment. On the other side, faster degradation of organic matter decreased transport of lipids from surface to subsurface sediment layer. Overall, concentrations of lipids in surface sediment were high than those in subsurface layer.
In alkanes, the C-C bonds make them more stable, so alkane as biomarker can refl ect the source of organic matter in sediment eき ciently (Lu et al., 2004). Alkanes with carbon number less than 21 are generally derived from marine plankton, algae, and bacteria (Blumer et al., 1971) without odd-even predominance. Long-chain alkanes (C>25) are generally derived from higher terrestrial plants with obvious odd predominance (Eglinton and Hamilton, 1967; Goñ et al., 1997). In the sediment sample at Dive111 station, except for the 4-cm sediment layer, the CPI1of the other sediment layers was close to 1 with insignifi cant odd-even predominance. There was a clear odd predominance in the 4-cm layer. CPI2, which refl ects the relative abundance between oddnumbered carbon and even-numbered carbon of long chain alkane (C25-C27), was 0.69-2.58 in difference erent layers. In the 3-cm and 5-cm sediment layers, the CPI2were 2.58 and 2.28, respectively, with obvious odd predominance. In other layers, CPI2had insignifi cant odd-even predominance. Except for the 4-cm sediment layer, in all the other sediment layers, H/L values are 0.01-0.49, indicating marine origin of the alkanes in them. On the other hand, except for the 4-cm sediment layer, TAR values are much less than 1.1 in all other sediment layers, indicating marine phytoplankton origin of alkane in the sediment. Overall, based on the data in Table 1, the main source of alkanes in the sediment sample was marine origin, and the alkane had mixed terrestrial and marine sources in the 4-cm sediment layer.
Although no typical terrestrial fatty acids were detected in sediment samples from the Dive111 station, the existence of stigmasterol and sitosterol, which are mainly found in the roots, stems and leaves of terrestrial higher plants (Xu et al., 2010) and could be used as biomarker of allochthonous (Bataglion et al., 2015), showed that the organic carbon in the sediment sample also had allochthonous input. Unlike stigmasterol and sitosterol, cholesterol, which is considered as biomarker of autochthonous (Bataglion et al., 2015), is not only contained in most marine organisms, but also a metabolitic by-product of mammals (Volkman, 1986). According to previous studies, cholesterol was widely found in surface sediments in difference erent sea area. For example, concentration of cholesterol in the sediment from the Bohai Sea was 0.11-0.25 μg/g (Ma et al., 2009). In the sediment of the sea area around the Nansha Islands (1 584 m), cholesterol accounted for 44.3%-55.2% of total sterols (Duan et al., 1998) as the most abundant sterol. In the bathypelagic sediments (1 670 m) from the Okinawa Trough, the proportion of cholesterol was lower, account for 25.4% of total sterols (Jiang et al., 1994). As a comparison, our results showed that the proportion of stigmasterol and sitosterol in the sediment sample from Dive111 station was signifi cantly higher than that of autochthonous cholesterol, indicating that the sterols in the hadal zone of the Yap Trench were mainly allochthonous input. In fact, for marine and terrestrial organic matter accumulated in sediment, animal inputs are much smaller than plants (Lee et al., 1979). Then, it is reasonable that the proportion of animal produced cholesterol was lower in the hadal sediment of the Yap Trench. Our results about fatty acids did not support the terrestrial origin of the sediment in Dive111 station. However, the existence of stigmasterol and sitosterol indicated that terrestrial input was also a source of sediments in the sediment sample. In the marine environment, fatty acids are usually degraded faster than sterols (Ding and Sun, 2005), which may lead to complete degradation of terrigenous fatty acids before they reach the hadal zone. The sterols are structurally stable and diき cult to be utilized by marine microorganisms. Their slow degradation rates would be helpful for the terrestrial sterols to reach and be preserved in the hadal sediments. In the sediment sample, the carbon number of the alcohols was all short chain (carbon number <20). Normally, long-chain alcohols (carbon number>20) is mainly derived from higher plants, and short-chain alcohols is mainly of microbial origin (Menzel et al., 2003). Our results indicate that the alcohols in the sediment sample are mainly from the ocean.
4.4 Factors afference ecting the morphology of lipids in the sediment sample
Previous studies showed that the morphological distributions of lipids in sediments from difference erent sea areas were difference erent. For example, in the surface sediments of the Yangtze River estuary, bound fatty acids were their main morphological form (Zegouagh et al., 2000). In the sediment from the South China Sea and the Black Sea, the main form of lipids was free (Wakeham, 1999). In the sediment sample from Dive111 station, the morphological distributions of difference erent kinds of lipids varied dramatically (Table 2). Many factors, including primary production of the ocean, chemical characteristics of lipids, the degradation of organic matter in marine environments and adsorption and desorption of sediment might afference ect the morphological distributions of lipids in the sediment of the Yap Trench.
In the sediment sample from the Dive111 station, the proportion of fatty acids, alcohols, phytol, and sterols were relatively high in free form. Their hydrophilic carboxyl group or hydroxyl group in the molecules assisted them to stay in sediment pore water as free lipids. On the other hand, the ratio between the concentrations of free and bound fatty acids in the difference erent sediment layers varied complexly, indicating that various factors controlled the morphological form of the fatty acids. For example, the proportion of free SFA and MUFA in the 2-cm layer was the highest, up to 91.39% and 83.50%, respectively (Table 3). In the surface sediment, their proportion was around 58%. Bound PUFA had the highest percentage in the surface layer, and had the lowest percentage in the 7-cm layer. Generally, due to the lower bioavailability of bound fatty acids, the degree of degradation of bound fatty acids should be lower than that of free fatty acids (Haddad et al., 1992). With increasing sediment depth, the proportion of free fatty acids in total fatty acids should show a decreasing trend. However, this trend was not appeared in the sediment sample from Dive111 station. It is possible that with degradation of free fatty acids, some bound fatty acids might release into the pore water by desorption, then the proportion of free fatty acid did not decline with depth. A study of Zhang et al. (1997) also shows that the degradation difference erences between bound and free sediment was not particularly signifi cant. In deeper sediment layers, more free fatty acids were consumed by microbial degradation in the sediment than bound fatty acids. The faster degradation characteristics of free fatty acids than bound fatty acids might be refl ected gradually (Stefanova and Disnar, 2000). On the other hand, the composition, and component of sediments in the difference erent layers of the sample varied remarkably (unpublished data). Therefore, the adsorption capacity of various sediment layers for fatty acids would be difference erent. Structural efference ect, degradation, absorption capacity and desorption process might result in complex variations of the proportion of concentrations of free and bound fatty acids in difference erent layers of the sample. Similar as fatty acids, the phytol, sterols, and alcohols also showed complex morphological distribution trends in the sediment sample. In surface and subsurface sediment of the sample, most of them were in free form. In the 3-5-cm sediment layers, the proportion of their bound forms increased. Lajat et al. (1990) showed that free sterols were abundant in surface sediments in the Santa Barbara Basin, California, and the proportion of free sterol decreased from surface to medium sediment, which is similar to our results. Yang and Wang (2012) presented that with increasing sediment depth, the neutral lipids degradation activity became weaker and weaker, and the transition from free to bound form enhanced. This kind of morphological transition made the neutral lipids more stable in sediment (Du, 2011). Alkanes and alkenes are typical hydrophobic compounds, so they were mainly in bound form in most layers. An exception occurred in the 4-cm sediment layer, proportion of free alkanes and alkenes were over bound ones. Hydrophilic iron-manganese component might be unfavorable for absorption of alkanes and alkenes in the sediment layer and then more alkanes and alkenes existed as free form. Overall, morphological distributions of lipids in the hadal zone of the Yap Trench were controlled by their structure, their degradation in seawater and sediment, the absorption and desorption of sediment component, etc. However, more studies are necessary to investigate the detail mechanisms of morphology of lipids in sediment of the hadal zone.
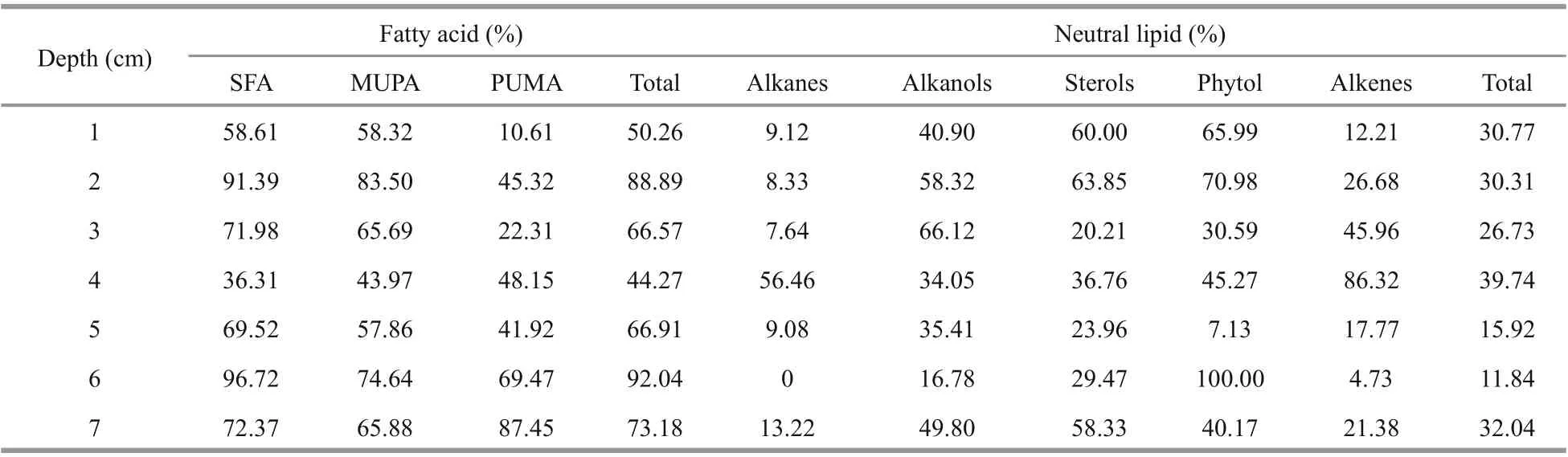
Table 3 Proportions of free-form of various kinds of lipids in the difference erent sediment layers from Dive111 station
5 CONCLUSION
In the sediment of the Dive111 station, fatty acids are the main components of lipids, and 18׃0 is the most abundant fatty acid. Alkanes were the main components of neutral lipids in sediment sample with short-chain predominance. The concentration of total fatty acids in the Yap Trench (325.77 μg/g) was much higher than that in ofference shore area, refl ecting the funnel efference ect of the hadal zone of the trench. High concentration of fatty acid 18׃1ω7 and alkanes indicated existence of hydrothermal fl uids in the study area. In the 4-cm sediment layer, lipids had unique distribution characteristics, attributing to ironmanganese nodules, which constituted the main component in the layer. Fatty acids in the sediment sample were marine origin, and neutral lipids had both marine and terrestrial input. Morphological distributions of various lipids were complex, controlled by their structure, hydrophilicity and hydrophobicity, their degradation processes, the absorption and desorption of the sediment, etc.
6 DATA AVAILABILITY STATEMENT
The data that support the fi ndings of this study are available from the corresponding author upon reasonable request.
7 ACKNOWLEDGMENT
The authors are grateful to all of members help in the Marine Interface Chemistry Lab in the Ocean University of China. The authors also appreciate the help from DING Jinfeng and GAO Fenglei in the Marine Ecology Center, the First Institute of Oceanography, Ministry of Natural Resources for lipid analysis.
References
Abraham W R, Lünsdorf H, Vancanneyt M, Smit J. 2013. Cauliform bacteria lacking phospholipids from an abyssal hydrothermal vent: proposal of Glycocaulis abyssi gen. nov., sp. nov., belonging to the family Hyphomonadaceae. International Journal of Systematic and Evolutionary Microbiology, 63(6): 2 207-2 215.
Ballentine D C, Macko S A, Turekian V C, Gilhooly W P, Martincigh B. 1996. Compound specifi c isotope analysis of fatty acids and polycyclic aromatic hydrocarbons in aerosols: implications for biomass burning. Organic Geochemistry, 25(1-2): 97-104.
Bao G D. 1987. A preliminary study on organic material, nitrogen and phosphorus in the deep-sea sediments of the Pacifi c western region. Acta Sedimentologica Sinica, 5(1): 114-124. (in Chinese with English abstract)
Bataglion G A, Meurer E, de Albergaria-Barbosa A C R, Bícego M C, Weber R R, Eberlin M N. 2015. Determination of geochemically important sterols and triterpenols in sediments using ultrahigh-performance liquid chromatography tandem mass spectrometry (UHPLCMS/MS). Analytical Chemistry, 87(15): 7 771-7 778.
Blumer M, Guillard R R L, Chase T. 1971. Hydrocarbons of marine phytoplankton. Marine Biology, 8(3): 183-189.
Bradley A S, Summons R E. 2010. Multiple origins of methane at the Lost City Hydrothermal Field. Earth and Planetary Science Letters, 297(1-2): 34-41.
Budge S M, Parrish C C, McKenzie C H. 2001. Fatty acid composition of phytoplankton, settling particulate matter and sediments at a sheltered bivalve aquaculture site. Marine Chemistry, 76(4): 285-303.
Budge S M, Parrish C C. 1998. Lipid biogeochemistry of plankton, settling matter and sediments in Trinity Bay, Newfoundland. II. Fatty acids. Organic Geochemistry, 29(5-7): 1 547-1 559.
Cao M L. 2017. The Source and Preservation of Organic Matters in Sediments of Difference erent Areas in the Northern South China Sea. East China Normal University, Shanghai, China. p.1-69. (in Chinese with English abstract)
Cao Y Y, Xing L, Wang X C, Zhao M X. 2018. Study on the indication of n-alkanes in surface sediments from the Bohai Sea and the North Yellow Sea. Periodical of Ocean University of China, 48(3): 104-113. (in Chinese with English abstract)
Chen J F. 2002. New geochemical proxies in paleoceanography studies. Advance in Earth Sciences, 17(3): 402-410. (in Chinese with English abstract)
Chen S, Xu A Y, Luo B K. 1986. Geochemistry of deep-sea sediments in the Pacifi c Ocean. Acta Oceanologica Sinica, 8(6): 694-700. (in Chinese)
Connelly T L, Businski T N, Deibel D, Parrish C C, Trela P. 2016. Annual cycle and spatial trends in fatty acid composition of suspended particulate organic matter across the Beaufort Sea shelf. Estuarine, Coastal and Shelf Science, 181: 170-181.
Conway N, McDowell Capuzzo J. 1990. The use of biochemical indicators in the study of trophic interactions in animal - bacteria symbiosis: Solemya velum, a case study. In: Barnes M, Gibbson R N eds. Trophic Relationships in the Marine Environment. Proceedings of 24th European Marine Biology Symposium. Aberdeen University Press, Aberdeen. p.553-564.
Coplen T B. 1995. Discontinuance of SMOW and PDB. Nature, 375(6529): 285.
Degens E T, Mopper K. 1976. Factors controlling the distribution and early diagenesis of organic material in marine sediments. In: Riley J P, Chester R eds. Chemical Oceanography. Vol. 6. 2ndedn. Academic Press, New York. p.59-113.
Delacour A, Früh-Green G L, Bernasconi S M, Schaefference er P, Kelley D S. 2008. Carbon geochemistry of serpentinites in the Lost City Hydrothermal System (30°N, MAR). Geochimica et Cosmochimica Acta, 72(15): 3 681-3 702.
Ding H B, Sun M Y. 2005. Biochemical degradation of algal fatty acids in oxic and anoxic sediment-seawater interface systems: efference ects of structural association and relative roles of aerobic and anaerobic bacteria. Marine Chemistry, 93(1): 1-19.
Du T J. 2011. Sources and Preservation of Organic Carbon and Lipids in the Intertidal Areas of the Yellow River Delta. Ocean University of China, Qingdao, China. p.1-49. (in Chinese with English abstract)
Duan Y, Cui M Z, Ma L H. 1998. Geochemistry of sterols in marine sediments from the middle continental slope of Nansha Sea. Geochimica, 27(1): 74-80. (in Chinese with English abstract)
Duan Y. 2000. Organic geochemistry of recent marine sediments from the Nansha Sea, China. Organic Geochemistry, 31(2-3): 159-167.
Eglinton G, Hamilton R J. 1967. Leaf epicuticular waxes. Science, 156(3780): 1 322-1 335.
Fullarton J G, Dando P R, Sargent J R, Southwards A J. 1995. Fatty acids of hydrothermal vent Ridgeia piscesae and inshore bivalves containing symbiotic bacteria. Journal of the Marine Biological Association of the United Kingdom, 75(2): 455-468.
Gao H L, Zou L, Wang K, Ye X W. 2017. Compositional distribution and transformation of terrestrial lipid organic matter in the sediments of the Yellow Sea and Bohai Sea. Acta Oceanologica Sinica, 39(2): 53-61. (in Chinese with English abstract)
Giovannelli D, Chung M, Staley J, Starovoytov V, Le Bris N, Vetriani C. 2016. Sulfurovum riftiae sp. nov., a mesophilic, thiosulfate-oxidizing, nitrate-reducing chemolithoautotrophic epsilonproteobacterium isolated from the tube of the deep-sea hydrothermal vent polychaete Riftia pachyptila. International Journal of Systematic and Evolutionary Microbiology, 66(7): 2 697-2 701.
Goñi M A, Ruttenberg K C, Eglinton T I. 1997. Sources and contribution of terrigenous organic carbon to surface sediments in the Gulf of Mexico. Nature, 389(6648): 275-278.
Guan H X, Chen L Y, Luo M, Liu L H, Mao S Y, Ge H M, Zhang M, Fang J S, Chen D F. 2019. Composition and origin of lipid biomarkers in the surface sediments from the southern Challenger Deep, Mariana Trench. Geoscience Frontiers, 10(1): 351-360.
Haddad R I, Martens C S, Farrington J W. 1992. Quantifying early diagenesis of fatty acids in a rapidly accumulating coastal marine sediment. Organic Geochemistry, 19(1-3): 205-216.
Hedrick D B, Pledger R D, White D C, Baross J A. 1992. In situ microbial ecology of hydrothermal vent sediments. FEMS Microbiology Ecology, 10(1): 1-10.
Huang X, Pu X Q. 2017. The infl uence of hydrothermal activities on the organic matter in sediment. Journal of Guangdong Ocean University, 37(1): 117-124. (in Chinese with English abstract)
Huang Y H, Sun C J, Yang G P, Yue X A, Jiang F H, Cao W, Yin X F, Guo C N, Niu J H, Ding H B. 2019. Geochemical characteristics of hadal sediment in the northern Yap Trench. Journal of Oceanology and Limnology, http://link.springer.com/10.1007/s00343-019-9010-3
Jiang S C, O’Leary T, Volkman J K, Zhang H Z, Jia R F, Yu S H, Wang Y, Luan Z F, Sun Z Q, Jiang R H. 1994. Origins and simulated thermal alteration of sterols and ketoalcohols in deep-sea marine sediments of the Okinawa Trough. Organic Geochemistry, 21(3-4): 415-422.
Konn C, Charlou J L, Donva J P, Holm N G. 2012. Characterisation of dissolved organic compounds in hydrothermal fl uids by stir bar sorptive extraction-gas chomatography-mass spectrometry. Case study: the Rainbow fi eld (36°N, Mid-Atlantic Ridge). Geochemical Transactions, 13: 8.
Lajat M, Saliot A, Schimmelmann A. 1990. Free and bound lipids in recent (1835-1987) sediments from Santa Barbara Basin. Organic Geochemistry, 16(4-6): 793-803.
Lee C, Farrington J W, Gagosian R B. 1979. Sterol geochemistry of sediments from the western North Atlantic Ocean and adjacent coastal areas. Geochimica et Cosmochimica Acta, 43(1): 35-46.
Lu B, Zhou H Y, Chen R H, Zhu C, Ye X R, Xue B. 2004. The composition characteristics of n-alkanes in the modern sediments of the arctic and the comparison with that of sea areas of difference erent latitudes. Chinese Journal of Polar Research, 16(4): 281-294. (in Chinese with English abstract)
Lu X H, Chen Y J, Huang G P, Liu D Y, Tan J H, Li J, Zhang G. 2011. Distribution and sources of lipid biomarkers in surface sediments of the Yellow Sea and Bohai Sea. Ecology and Environmental Sciences, 20(6-7): 1 117-1 122. (in Chinese with English abstract)
Ma H Q, Feng H, Wang X C. 2009. Distribution and sources of sterols in surface sediments from Bohai Bay and Jiaozhou Bay. Marine Sciences, 33(6): 73-79, 85. (in Chinese with English abstract)
MacAvoy S E, Macko S A, Joye S B. 2002. Fatty acid carbon isotope signatures in chemosynthetic mussels and tube worms from gulf of Mexico hydrocarbon seep communities. Chemical Geology, 185(1-2): 1-8.
Menzel D, van Bergen P F, Schouten S, Damsté J S S. 2003. Reconstruction of changes in export productivity during Pliocene sapropel deposition: a biomarker approach. Palaeogeography, Palaeoclimatology, Palaeoecology, 190: 273-287.
Mori K, Suzuki K I, Yamaguchi K, Urabe T, Hanada S. 2015. Thiogranum longum gen. nov., sp. nov., an obligately chemolithoautotrophic, sulfur-oxidizing bacterium of the family Ectothiorhodospiraceae isolated from a deep-sea hydrothermal fi eld, and an emended description of the genus Thiohalomonas. International Journal of Systematic and Evolutionary Microbiology, 65(1): 235-241.
Peng X T, Li J W, Zhou H Y, Wu Z J, Li J T, Chen S, Yao H Q. 2011. Characteristics and source of inorganic and organic compounds in the sediments from two hydrothermal fi elds of the Central Indian and Mid-Atlantic Ridges. Journal of Asian Earth Sciences, 41(3): 355-368.
Phleger C F, Nelson M M, Groce A K, Cary S C, Coyne K J, Nichols P D. 2005. Lipid composition of deep-sea hydrothermal vent tubeworm Riftia pachyptila, crabs Munidopsis subsquamosa and Bythograea thermydron, mussels Bathymodiolus sp. and limpets Lepetodrilus spp. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 141(2): 196-210.
Rosario-Passapera R, Keddis R, Wong R, Lutz R A, Starovoytov V, Vetriani C. 2012. Parvibaculum hydrocarboniclasticum sp. nov., a mesophilic, alkaneoxidizing alphaproteobacterium isolated from a deep-sea hydrothermal vent on the East Pacifi c Rise. International Journal of Systematic and Evolutionary Microbiology, 62(12): 2 921-2 926.
Rushdi A I, Simoneit B R T. 2002a. Hydrothermal alteration of organic matter in sediments of the Northeastern Pacifi c Ocean: Part 1. Middle valley, Juan de Fuca Ridge. Applied Geochemistry, 17(11): 1 401-1 428.
Rushdi A I, Simoneit B R T. 2002b. Hydrothermal alteration of organic matter in sediments of the Northeastern Pacifi c Ocean: Part 2. Escanaba Trough, Gorda Ridge. Applied Geochemistry, 17(11): 1 467-1 494.
Rütters H, Sass H, Cypionka H, Rullkötter J. 2002. Phospholipid analysis as a tool to study complex microbial communities in marine sediments. Journal of Microbiological Methods, 48(2-3): 149-160.
Silliman J E, Schelske C L. 2003. Saturated hydrocarbons in the sediments of Lake Apopka, Florida. Organic Geochemistry, 34(2): 253-260.
Simoneit B R T, Brault M, Saliot A. 1990. Hydrocarbons associated with hydrothermal minerals, vent waters and talus on the East Pacifi c Rise and Mid-Atlantic Ridge. Applied Geochemistry, 5(1-2): 115-124.
Stefanova M, Disnar J R. 2000. Composition and early diagenesis of fatty acids in lacustrine sediments, Lake Aydat (France). Organic Geochemistry, 31(1): 41-55.
Sun M Y, Wakeham S G, Lee C. 1997. Rate and mechanism of fatty acid degradation in oxic and anoxic coastal marine sediment of Long Island Sound, New York, USA. Geochimca et Cosmochimca Acta, 61(2): 341-355, https://doi.org/10.1016/S0016-7037(96)00315-8.
Uhle M E, Macko S A, Spero H J, Engel M H, Lea D W. 1997. Sources of carbon and nitrogen in modern planktonic foraminifera: the role of algal symbionts as determined by bulk and compound specifi c stable isotopic analyses. Organic Geochemistry, 27(3-4): 103-113.
Venkatesan M I, Ruth E, Rao P S, Nath B N, Rao B R. 2003. Hydrothermal petroleum in the sediments of the Andaman Backarc Basin, Indian Ocean. Applied Geochemistry, 18(6): 845-861.
Vogts A, Schefuß E, Badewien T, Rullkötter J. 2012. n -Alkane parameters from a deep sea sediment transect ofference southwest Africa refl ect continental vegetation and climate conditions. Organic Geochemistry, 47: 109-119.
Volkman J K. 1986. A review of sterol markers for marine and terrigenous organic matter. Organic Geochemistry, 9(2): 83-99.
Wakeham S G. 1999. Monocarboxylic, dicarboxylic and hydroxy acids released by sequential treatments of suspended particles and sediments of the Black Sea. Organic Geochemistry, 30(9): 1 059-1 074.
Wang J T, Yang S, Tan L J, Hu J. 2011. Composition and form distribution of lipids biomarkers in a sediment core from southern coastal area of Zhejiang Province. Acta Oceanologica Sinica, 33(5): 83-90. (in Chinese with English abstract)
Xu X, Sun L P, Dong J, Li T J, Mu X F, Shen X F. 2010. Sterol composition analysis of royal jelly by gas chromatography coupled with mass spectrometry. Food Science, 31(18): 317-320. (in Chinese with English abstract)
Yamanaka T, Sakata S. 2004. Abundance and distribution of fatty acids in hydrothermal vent sediments of the western Pacifi c Ocean. Organic Geochemistry, 35(5): 573-582.
Yang S, Wang J T. 2012. Composition and form distribution of lipids biomarkers in surfi cial sediments in southern coastal area of Shandong Peninsula. Marine Environmental Science, 31(1): 11-15. (in Chinese with English abstract)
Yao S C, Shen J. 2003. A preliminary study of n-alkanes in a sedimentary core from Chaohu Lake. Journal of Lake Sciences, 15(3): 200-204. (in Chinese with English abstract)
Yue X A, Yan Y X, Ding H B, Sun C J, Yang G P. 2018. Biological geochemical characteristics of the sediments in the Yap Trench and its oceanographic signifi cance. Periodical of Ocean University of China, 4 8(3): 88-96. (in Chinese with English abstract)
Zegouagh Y, Derenne S, Largeau C, Saliot A. 2000. A geochemical investigation of carboxylic acids released via sequential treatments of two surfi cial sediments from the Changjiang delta and East China Sea. Organic Geochemistry, 31(5): 375-388.
Zhang G, Sheng G Y, Fu J M. 1997. Bound lipids in lacustrine sediments in Guchenghu Lake, East China. Chinese Science Bulletin, 42(21): 1 817-1 820.
Zhu S, Ye M W, Xu J L, Guo C Y, Zheng H K, Hu J B, Chen J J, Wang Y J, Xu S L, Yan X J. 2015. Lipid profi le in difference erent parts of edible jellyfi sh Rhopilema esculentum. Journal of Agricultural and Food Chemistry, 63(37): 8 283-8 291.
Zhu X W, Mao S Y, Wu N Y, Sun Y G, Guan H X. 2014. Molecular and stable carbon isotopic compositions of saturated fatty acids within one sedimentary profi le in the Shenhu, northern South China Sea: source implications. Journal of Asian Earth Sciences, 92: 262-275.
 Journal of Oceanology and Limnology2020年3期
Journal of Oceanology and Limnology2020年3期
- Journal of Oceanology and Limnology的其它文章
- List of the Most Outstanding Papers Published by CJOL/JOL in 2017-2018
- Who is the “murderer” of the bloom in coastal waters of Fujian, China, in 2019?*
- The investigation of internal solitary waves over a continental shelf-slope*
- Efference ect of diets on the feeding behavior and physiological properties of suspension-feeding sea cucumber Cucumaria frondosa*
- Efference ects of light quality on growth rates and pigments of Chaetoceros gracilis (Bacillariophyceae)*
- Marine bacterial surfactin CS30-2 induced necrosis-like cell death in Huh7.5 liver cancer cells*
