Efference ects of organic carbon consumption on denitrifi er community composition and diversity along dissolved oxygen vertical profi les in lake sediment surface*
HONG Pei , GONG Shihao , WANG Chunbo , SHU Yilin , WU Xingqiang , TIAN Cuicui , Oscar Omondi DONDE , CAI Pei , WU Huaming , XIAO Bangding ,
1 Key Laboratory of Algal Biology of the Chinese Academy of Sciences, Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan 430072, China
2 University of Chinese Academy of Sciences, Beijing 100049, China
3 College of Life Sciences, Anhui Normal University, Wuhu 241000, China
Received Apr. 18, 2019; accepted in principle Jul. 24, 2019; accepted for publication Aug. 19, 2019
© Chinese Society for Oceanology and Limnology, Science Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Abstract At present, the understanding of the dynamics of denitrifi ers at difference erent dissolved oxygen (DO) layers under organic carbon consumption within the surface sediments remains inadequate. In this study, high-throughput sequencing and quantitative PCR targeting nirS gene were used to analyze the denitrifi er abundance dynamics, community composition, and structure for aerobic (DO 0.5-6.9 mg/L), hypoxic-anoxic (DO 0-0.5 mg/L), and anoxic (DO 0 mg/L) layers in surface sediments under organic carbon consumption. Based on the analysis of nirS gene abundance, the values of denitrifying bacteria decreased with organic carbon consumption at difference erent DO layers. When the bacterial species abundance at the genus level were compared between the high-carbon and low-carbon sediments, there was signifi cant increase in 6 out of 36, 7 out of 36 and 6 out of 36 genera respectively for the aerobic, hypoxic-anoxic and anoxic layers. On the other hand, 14 out of 36, 9 out of 36 and 15 out of 36 genera showed signifi cant decrease in bacterial species abundance respectively for the aerobic, hypoxic-anoxic and anoxic layers. Additionally, 14 out of 36, 20 out of 36, and 15 out of 36 genera had no change in bacterial species abundance respectively for the aerobic, hypoxic-anoxic, and anoxic layers. This indicates that the carbon utilization ability of difference erent denitrifi ers on each DO layers was generally difference erent from each other. Diversity of denitrifying bacteria also presented signifi cant difference erences in difference erent DO layers between the high- and low-carbon content sediment layers. Moreover, under the high-carbon and low-carbon content, the abundance of nirS gene showed a high peak within the hypoxic-anoxic regions, suggesting that this region might be the main distribution area for the denitrifying bacteria within the surface sediments. Furthermore, community of unique denitrifi ers occurred in difference erent DO layers and the adaptive changes of the denitrifi er community followed the organic carbon consumption.
Keyword: eutrophic freshwater lake; surface sediments; dissolved oxygen profi les; denitrifi er; organic carbon consumption
1 INTRODUCTION
Nitrate pollution in both surface and groundwater has for a long time been one of the most important water quality issues worldwide (Nolan, 2001; Puckett et al., 2011). Sediment denitrifi cation is the main process of nitrogen removal in shallow lakes (Sirivedhin and Gray, 2006). Normally, reduced oxygen concentrations and the availability of electron donors (i.e. organic carbon) and N sources were the three conditions necessary for the denitrifi cation. In nature, the organic carbon compounds are the most common carbon source for the denitrifi cation process (Van Rijn et al., 2006) and the nature of organic carbon has infl uence on the denitrifi cation performance, microbial community structure as well as denitrifi cation genes (Srinandan et al., 2012; Xu et al., 2018). Previous studies have reported on the efference ects of organic matter on denitrifi cation in soil and sediments of the lakes, oceans and rivers (Grofference man et al., 2009; Huang et al., 2011; Jenerette and Chatterjee, 2012; Wu et al., 2013; Jia et al., 2016; Liu et al., 2018a). Dissolved oxygen (DO) is usually considered the most critical proximal regulator of microbial denitrifi cation (Cao et al., 2017). Difference erent denitrifying enzymes have varying sensitivity to difference erent oxygen concentrations (McKenney et al., 2001). Nitrogen removal in wastewater treatment systems with difference erent DO concentrations has been signifi cantly studied (Chen et al., 2016; Waki et al., 2018; Zou et al., 2018), but the response of denitrifying bacteria is still not adequately reported. Previous studies on the physical and chemical properties of the lake sediment-water interface found that the surface sediments had severe redox gradient changes within the range of several millimeters (Santschi et al., 1990). However, under condition of continuous degradation of organic matter, the characteristics of denitrifying bacteria in surface sediments on the vertical scale based on changes of DO content are still not adequately known.
Various reductases catalyzed nitrate to nitrite, nitric oxide, nitrous oxide and dinitrogen gas through the process of denitrifi cation (Zumft, 1997). Through this process, nirS gene that encodes for cytochrome-cd1nitrite reductase, catalyzes the fi rst step in the evolution of the gaseous product (NO2ˉ-NO). The nirS gene is more widely distributed amongst the bacteria from the natural environments (Braker et al., 1998) and has been most frequently used as functional biomarkers of the denitrifying community (Bulow et al., 2008; Francis et al., 2013; Yang et al., 2013; Gao et al., 2016).
In the current study, three lake sediment layers: aerobic zone (AEZ), hypoxic-anoxic zone (HAZ) and anoxic zone (ANZ), distinguished using an oxygen microsensor on a vertical scale over organic carbon consumption rate, were stratifi ed and sampled. The aim of this study was to explore the dynamics in denitrifying bacteria abundance, community composition, structure, and diversity along the vertical DO boundary within the surface sediment profi les using nirS gene. This was to provide a deeper understanding on the traits of denitrifying bacteria based on the consumption of organic carbon within a eutrophic lake. The information generated from this study is helpful in providing accurate understanding on the contribution of lake surface sediments to denitrifi cation process, providing insights necessary in developing eutrophication control strategies.
2 MATERIAL AND METHOD
2.1 Experimental design
Surface sediment was collected in May 2018 from Dianchi Lake, located in Kunming, China (102°38′0.84′′E, 24°58′41.61′′N) using specifi c sampling methods described previously by Tian et al. (2015). Surface sediment cores were sampled to a depth of 20 mm and carefully transferred to PVC cylinders in situ (30 mm diameter × 110 mm high) before being subject to laboratory microcosm incubations. A simulation of the overlying lake water contained in mg/L; 48.6 NaNO3, 5.1 MgSO4·7H2O, 3.8 NH4Cl, 5.6 K2HPO4, 4.4 KH2PO4, 0.1 mL/L of trace elements (Nancharaiah et al., 2008) and pH of 7.2. A 20-mL aliquot of synthetic lake water was then gently overlaid onto the sediment using a siphon to avoid disturbance. The depth of the added synthetic lake water was approximately 30 mm above the sediment, resulting in a water׃sediment ratio as described in Rong et al. (2016). The nitrate concentration in the overlaid water was measured 1to 2 times per day with an ion chromatography (ICS5000þ, Thermo Fisher Scientifi c, MA, USA), and when it was lower than 1 mg/L, the water was replaced. All cylinders were stored in dark incubators at 25°C for 30 days. Sampling for denitrifi er bacterial richness was set up at day 10 when the organic carbon content was high and at day 30 when the organic carbon content was low.
2.2 Sediment analyses
At days 10 and 30, the sediment was acquired and parameters measured in triplicate. Micro-sensors with a 90-110 μm tip diameter were used to detect DO levels (Unisense, Denmark). The sediment cores were cut into 3 parts of the following intervals; 0-2 mm, 2-4 mm, 4-6 mm. Frozen dried sediments were sieved to measure the total organic carbon (TOC) by an elementar vario TOC system (Elementar, Germany). Dissolved organic carbon (DOC) was extracted by water at a ratio of 1:10 (sediment/water) and measured using a DRB200 digital reactor (HACA, USA).
2.3 DNA extraction
DNA was extracted from approximately 0.8 g of each sediment sample using an EZNA Soil DNA Kit (Omega, USA) in line with instructions of the manufacturer. DNA concentration and quality were assessed by spectrophotometry and 1% agarose gel electrophoresis, respectively. All DNA extracts were stored at -20°C until further processing.
2.4 qPCR and high-throughput sequencing
Quantitative PCR analysis was undertaken for the denitrifi cation bacterial abundance based on the quantifi cation of nirS gene and the primer pair nirScd3Af and nirSR3cd (Kandeler et al., 2006), and 16S rRNA gene with the primer pair 338F and 806R (Xu et al., 2016). Their abundance was determined by the qPCR standard curves (Bio-Rad, USA) using SYBR Green as the signal dye. Standard curve was obtained by tenfold serial dilution of standard plasmids containing target functional gene. Each 20-μL reaction mixture contained 1 μL of template DNA, 10 μL of iTaq Universal SYBR Green Supermix (Bio-Rad), 1 μL of each primer, and 7 μL of water. The data were analyzed using the Bio-Rad software. Plasmid containing target gene and nuclease-free water as positive and negative controls respectively were run together with each sample.
The primer nirScd3aF/nirSR3cd of nirS was also used for sequencing amplifi cation on an Illumina MiSeq platform (Illumina, USA) by LC-Bio Technology Co. (Hangzhou, China). Raw fastq fi les were demultiplexed and quality-fi ltered with Trimmomatic and merged with FLASH. Sequences with 97% similarity were clustered as Operational Taxonomic units (OTUs) with UCHIME software (version 7.1 http://drive5.com/uparse/). Taxonomy of nirS gene was analyzed using the RDP Classifi er (http://rdp.cme.msu.edu/).
2.5 Statistical analysis
Alpha diversity was applied in analyzing the complexity of species diversity of the samples using the Observation and Chao indices with Mothur (version v.1.30.1 http://www.mothur.org/wiki/Schloss_SOP#Alpha_diversity). Beta diversity analysis was used to evaluate difference erences in species complexity among the samples. Beta diversity was calculated through Analysis of Similarities (ANOSIM) with weighted Non-metric Multidimensional Scaling analysis (NMDS) in the R “vegan” package (v3.2.3). One-way and two-way ANOVA, and Pearson’s correlation analysis were conducted using SPSS version 19.0 software. Other fi gures were drawn using the Origin 2017 program.
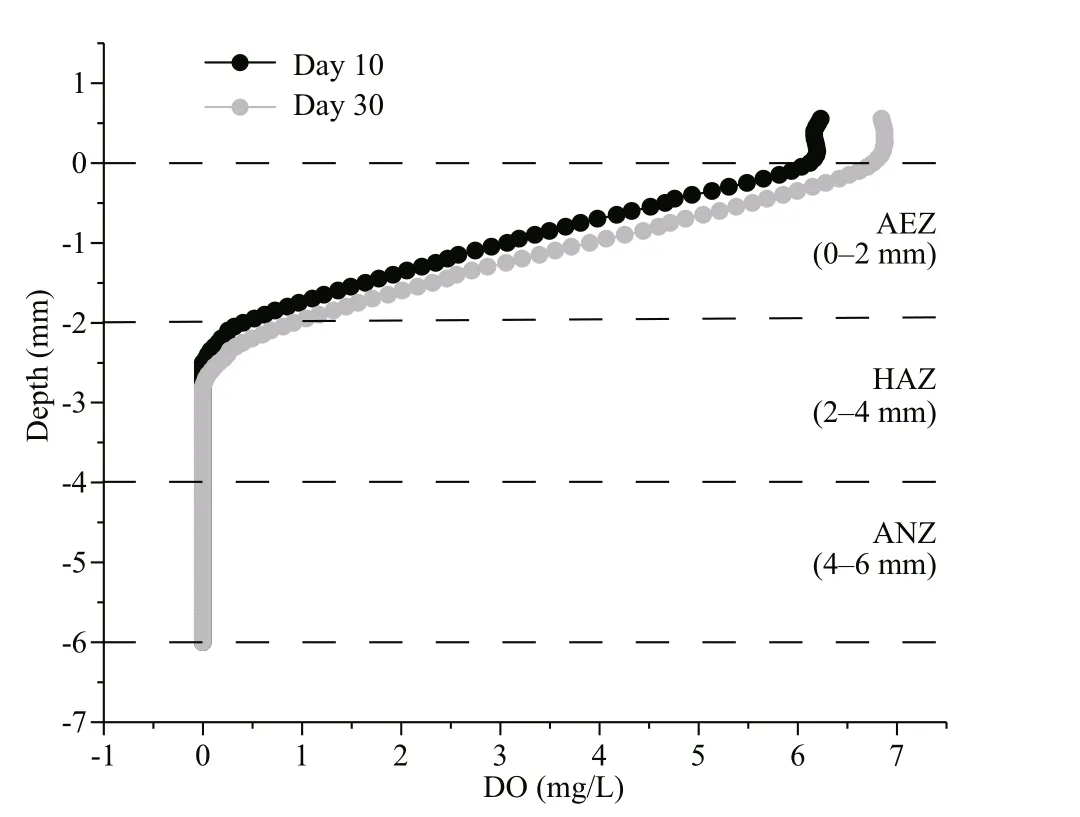
Fig.1 Dissolved oxygen profi les at the sediment-water interface on days 10 and 30
3 RESULT
3.1 Chemical properties of surface sediments among vertical DO profi les
Oxygen penetration depth is a measure of infi ltration depth of oxygen from the water into the sediment and determines the thickness of the aerobic sediment. The experimental results revealed that oxygen penetrated into the sediment, and that the depth of penetration for oxygen concentration of 0.5 mg/L varied within the range of 2.0-2.5 mm during the 10thand 30thsampling days (Fig.1). Based on the DO penetration depth data, it was determined that 0-2 mm, 2-4 mm, and 4-6 mm corresponded to the aerobic, hypoxia-anoxic and anoxic DO layers, respectively. Two-way ANOVA showed that TOC content ( P <0.01) and DOC content ( P <0.01) were signifi cantly consumed over time at the three layers. Similarly, TOC content ( P <0.01) was found to be signifi cantly difference erent between the depths in the surface sediments (two-way ANOVA, Table 1). Furthermore, interactions between sampling days and depth were found in DOC content ( P <0.01).
3.2 Abundance of denitrifi ers along DO profi les
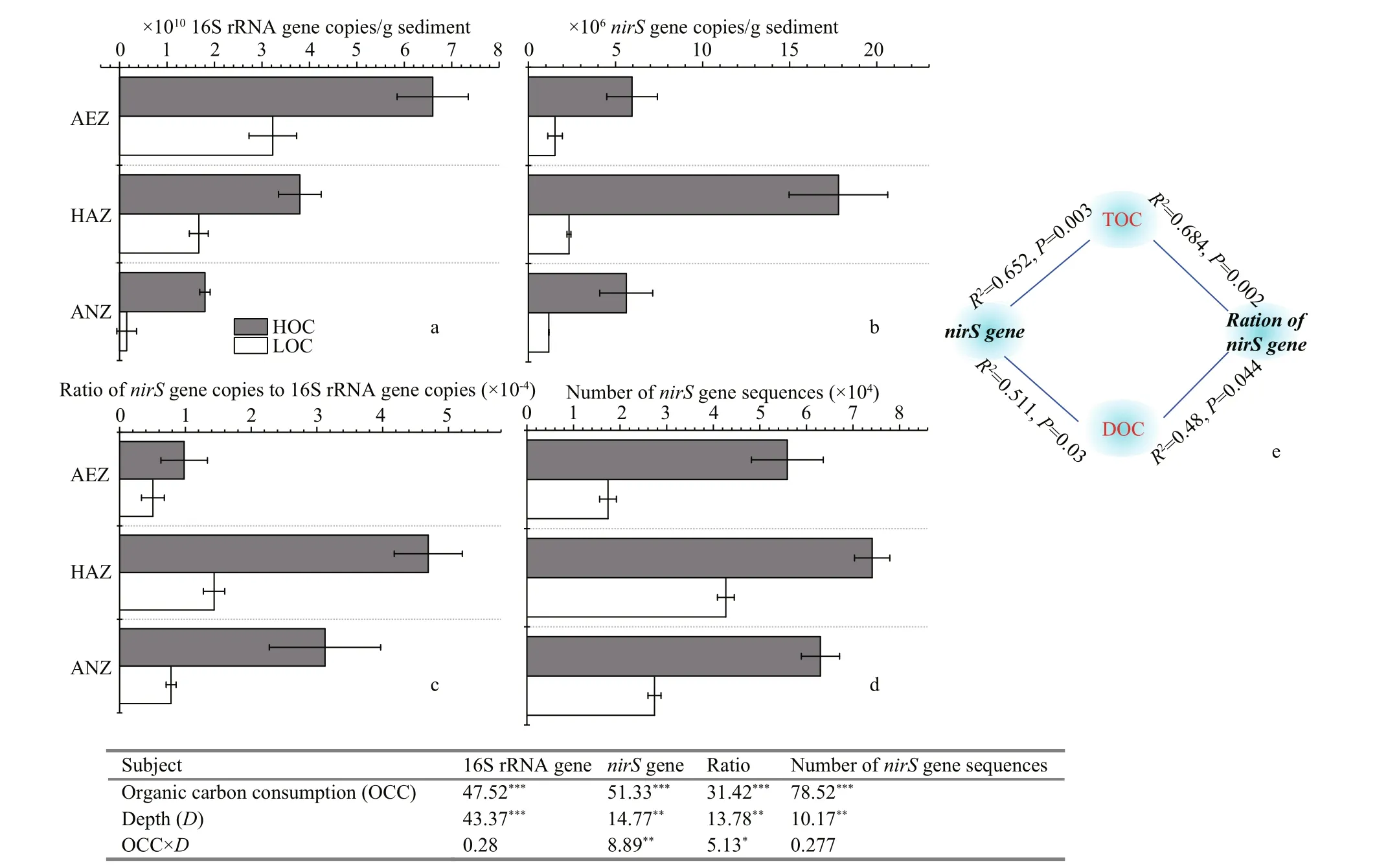
Fig.2 The values of 16S rRNA bacterial gene (a), the denitrifi er nirS gene abundance (b), the ratio of nirS gene to 16S rRNA gene according to qPCR (c), the number of nirS gene sequences based on high-throughput sequencing (d), along the aerobic (AEZ), hypoxic-anoxic (HAZ) and anoxic (ANZ) layers on high and low organic carbon sediment surface
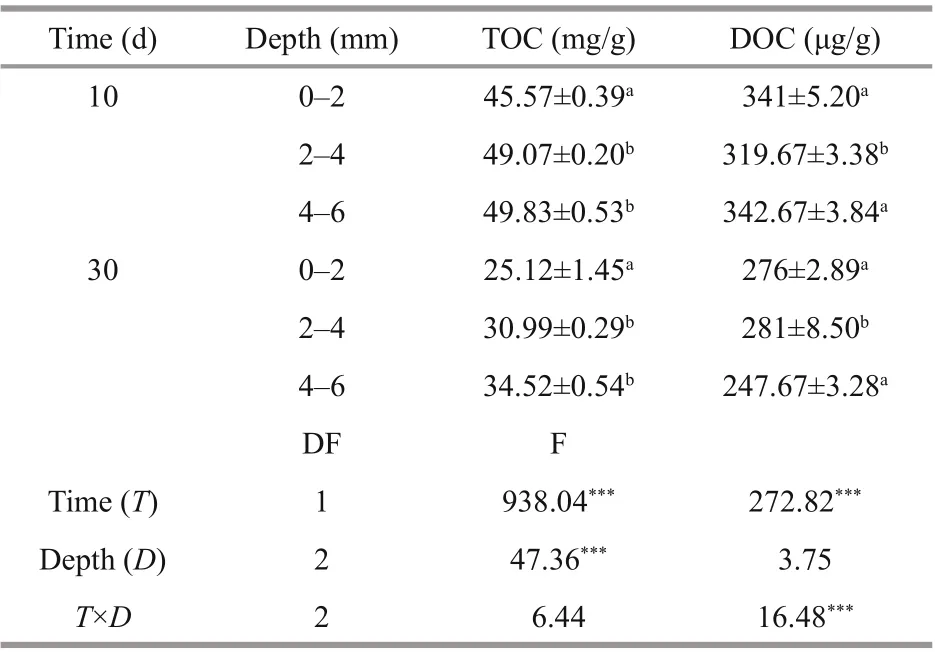
Table 1 Vertical distribution and two-way ANOVA of carbon properties in sediments
The qPCR analysis of 16S rRNA and nirS gene copies revealed that the abundance of total bacteria decreased gradually with increasing depth, while the abundance and relative abundance of nirS gene increased to a peak at hypoxia-anoxic DO layers (2-4 mm) both at high and low organic carbon sediment content (Fig.2). Moreover, two-way ANOVA showed that the abundance of total bacteria as well as the abundance and relative abundance (ratio of nirS/16S rRNA gene copies) of nirS gene were signifi cantly difference erent based on the consumption of organic carbon and increase in the depth ( P <0.01; Fig.2a, b, c). Additionally, the interaction of depth and organic carbon consumption through the abundance and relative abundance of nirS gene was signifi cantly difference erent ( P <0.05). The number of nirS gene sequences based on Illumina Miseq-based sequencing also showed the same trend (Fig.2d). Moreover, Pearson’s correlation showed that TOC and DOC contents were the signifi cant factors correlating with the abundance and relative abundance of nirS gene (Fig.2e).
3.3 Community composition and structure of denitrifi er bacteria
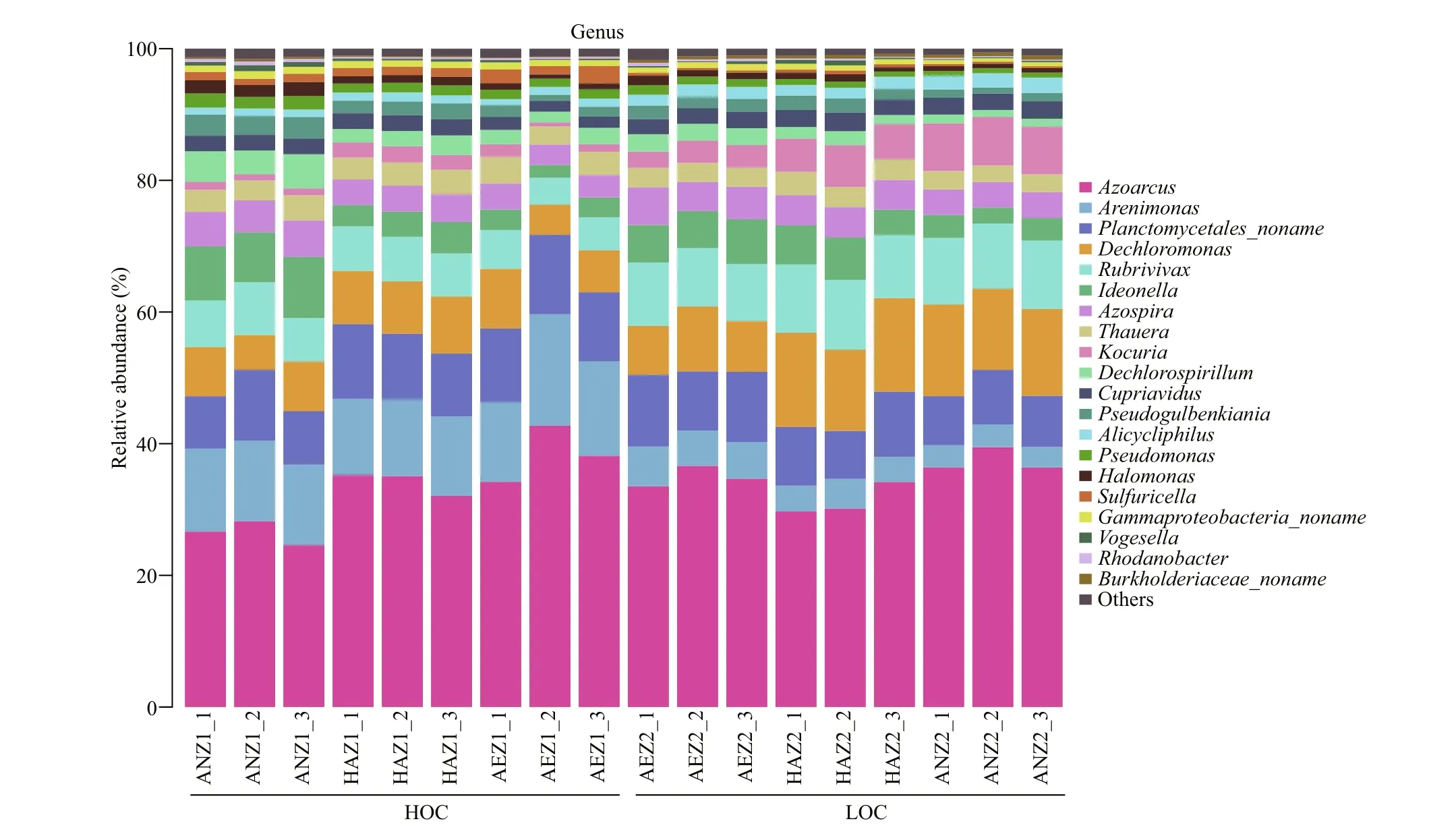
Fig.3 Relative abundance of nirS-based representative denitrifi er communities in the surface sediment
Illumina Miseq-based sequencing identifi ed 36 genera in all the samples, among which only six genera were classifi ed as unknown genera. Azoarcus, Arenimonas, Planctomycetales_noname, Dechloromonas and Rubrivivax were the predominant denitrifi er representatives at the surface sediment (Fig.3). When the bacterial species abundance at the genus level were compared between the high-carbon and low-carbon sediments, there was signifi cant increase in 6 out of 36, 7 out of 36 and 6 out of 36 genera respectively for the aerobic, hypoxic-anoxic and anoxic layers. On the other hand, 14 out of 36, 9 out of 36 and 15 out of 36 genera showed signifi cant decrease in bacterial species abundance respectively for the aerobic, hypoxic-anoxic and anoxic layers. Additionally, 14 out of 36, 20 out of 36, and 15 out of 36 genera had no change in bacterial species abundance respectively for the aerobic, hypoxic-anoxic and anoxic layers. The numbers of bacteria at the genus level were compared. Among them, the dominant genera Azoarcus, Rubrivivax, Kocuria and Alicycliphilus signifi cantly increased, Arenimonas, Ideonella, Dechlorospirillum and Thauera decreased, while Planctomycetales_noname, Dechloromonas, Azospira and Cupriavidus did not show any signifi cant change at the aerobic layer (0-2 mm). At the hypoxic-anoxic layer (2-4 mm), the predominant genera Dechloromonas, Rubrivivax, Kocuria and Azospira signifi cantly increased, Arenimonas, Pseudomonas and Sulfuricella decreased while Azoarcus, Planctomycetales_noname, Ideonella and Thauera had no observable changes with respect to the consumption of organic carbon. With respect to the consumption of organic carbon, the ascendant genera Dechloromonas, Rubrivivax, Kocuria, and Cupriavidus signifi cantly increased, Alphaproteobacteria_noname, Magnetospirillum, Arenimonas, and Planctomycetales_noname signifi cantly decreased, while Dechlorospirillum, Azoarcus, Azospira, and Thauera did not show any signifi cant change at the anoxic layer (4-6 mm) (Fig.4).
ANOSIM was used to analyze the difference erences in the community structure of denitrifi ers across the vertical profi les with the consumption of organic carbon in the surface sediment. These comparisons revealed signifi cant difference erences in denitrifying community structure ( R2=0.93, P=0). NMDS also showed signifi cant difference erence among vertical profi les and between high and low organic carbon sediment surface at the OTU level. On the other hand, the bacterial communities at the same layer and for the same organic carbon content tended to cluster together, with high organic carbon content visibly separated from low organic carbon content, and aerobic (0-2 mm), hypoxic-anoxic (2-4 mm) and anoxic (4-6 mm) clearly parted (Fig.5).
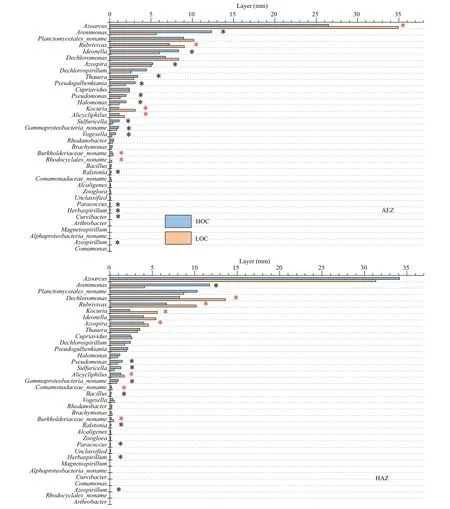
Fig.4 Comparison of the compositions of denitrifi er communities of HOC and LOC content within the sediment at three layers (aerobic, AEZ; hypoxic-anoxic, HAZ; and anoxic, ANZ)
To be continued
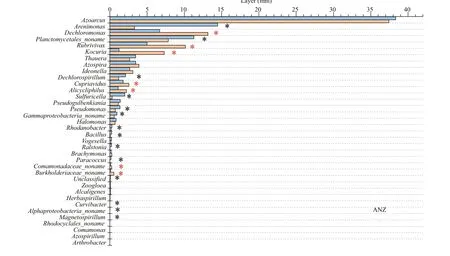
Fig.4 Continued
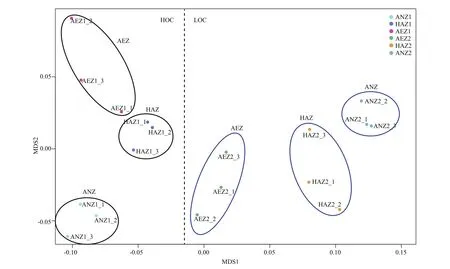
Fig.5 NMDS showed difference erences in nirS-based representative denitrifi er communities among the three layers (AEZ, HAZ and ANZ) between HOC and LOC
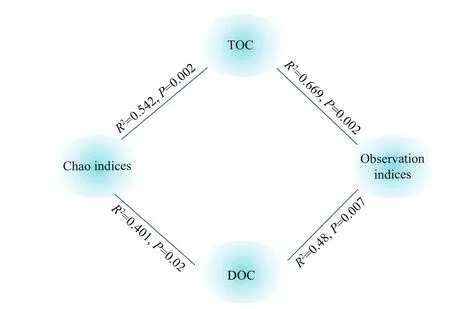
Fig.6 Pearson’s correlation between organic carbon (TOC and DOC) and the indices of Observation and Chao
3.4 Community diversity of denitrifi er bacteria
Based on nirS-type Illumina Miseq-based sequencing results, the alpha diversity indices, Observation and Chao, were used together to evaluate the denitrifi ers community diversity along the surface sediment under the organic carbon consumption. Two-way ANOVA showed that the Observation indices ( P <0.001) and Chao indices ( P <0.01) difference ered signifi cantly between the high and low organic carbon content in the surface sediment. With respect to the consumption of organic carbon, the alpha diversity indices increased at the aerobic (0-2 mm) and anoxic layers (4-6 mm) and decreased at hypoxic-anoxic layers (2-4 mm). Under high organic carbon content, the indices showed peak values at the hypoxic-anoxic layers (2-4 mm), and decreased with the increase in the DO levels at the low organic carbon content. The interaction of depth and organic carbon consumption of the Observation ( P <0.001) and Chao ( P <0.01) indices were signifi cantly difference erent (Table 2). Moreover, Pearson’s correlation analysis revealed that TOC content and DOC content were also potential nutrients that were signifi cantly correlated with the diversity indices (Fig.6).
4 DISCUSSION
Nitrate loading and contamination of surface waters (e.g., lakes) has become a common environmental and health problem, especially in developing countries. Microbiological processes can remove excess nitrogen in waterbodies. These processes occur mainly at the surface of the sediment, where microbial activities are higher (Saarenheimo et al., 2017). In this study, the consumption of organic carbon is related to the abundance community composition, community structure and diversity of denitrifying bacteria in surface sediments, and there is a signifi cant difference erence in stratifi ed distribution of denitrifying bacteria under difference erent DO concentrations.
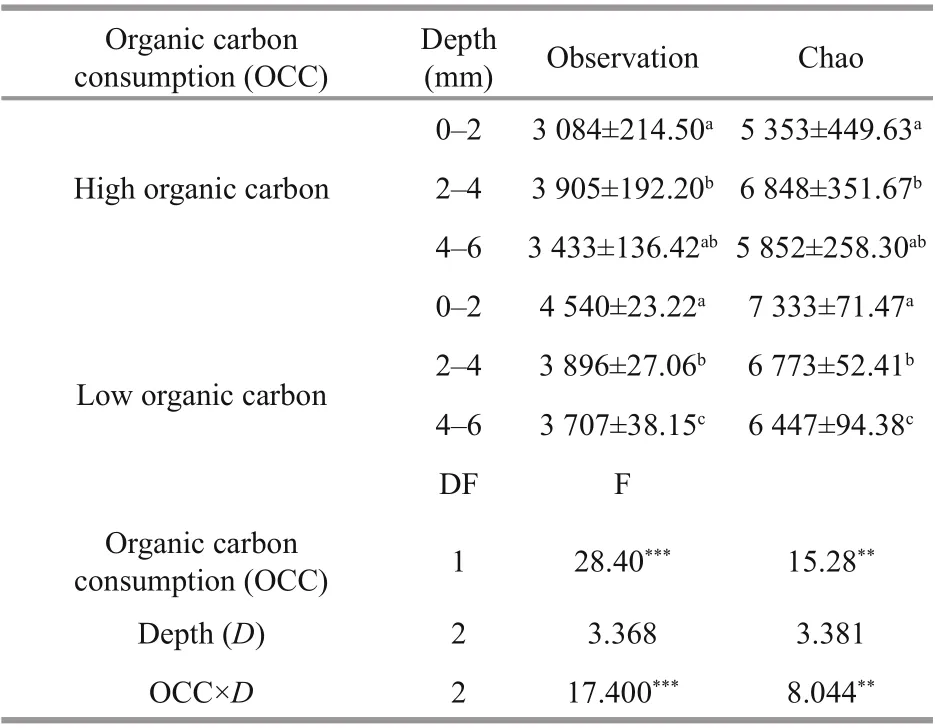
Table 2 Diversity indices of nirS-type denitrifi ers through Illumina Miseq-based sequencing in surface sediment DO profi les between high and low organic carbon content by two-way ANOVA
4.1 Efference ects of organic carbon consumption on denitrifying bacteria in surface sediments
The values of difference erent forms (TOC and DOC) of organic carbon afference ect denitrifi cation in two ways. First, it can provide the substrate needed for the growth of bacteria, which consequently infl uences the denitrifying bacteria richness. Secondly, the decomposition of organic matter depletes oxygen. Generally, lower oxygen content was required for denitrifi cation. Therefore, high organic concentration will create a favorable environment for denitrifi cation (Knowles, 1982; Lee and Rittmanm, 2003; Wu et al., 2019). In the present study, the abundance of nirS gene decreased signifi cantly with the organic carbon consumption, indicating the potential weakening of denitrifi cation ability because of the continuous utilization of organic carbon of lake surface sediment by bacteria.
Microbial community composition, abundance and diversity vary with the environmental gradients (Bier et al., 2015; Fan et al., 2016), and these changes could be explained by the mechanisms of community adaptability to the variation of ecosystem functioning (Liu et al., 2018b). In this study, 36 representatives of denitrifi ers were identifi ed by nirS sequencing. Azoarcus Arenimonas, Planctomycetales_noname,
Dechloromonas, and Rubrivivax were the dominant genera within the lake sediment surface. Such result have also been achieved in agricultural soils, activated sludge, landfi ll leachate and coking wastewater (Thomsen et al., 2007; Remmas et al., 2016; Coyotzi et al., 2017; Li and Lu, 2017). Out of the 36 genera identifi ed through nirS sequencing, six were not identifi ed at the level of genus. This confi rms the existence of many potentially novel bacterial populations that need to be explored and identifi ed. Among the identifi ed genera, the dominant Rubrivivax increased signifi cantly with the consumption of organic carbon for the three DO layers at the sediment surface, indicating that the genus could be properly adapted properly to low carbon content. This could be maintaining its high abundance within the lake sediment surface. In biofi lm reactors, this genus has also showed a relatively high abundance with degradation of exogenous carbon (Qiu et al., 2017). In the present study, the dominant genera of Arenimonas decreased with respect to the consumption of organic carbon in three DO layers within the surface sediment. This indicates that the genus may be driving denitrifi cation process at high organic carbon content, which consequently afference ects their growth under the limitation of organic carbon content. This study also showed no signifi cant change in the genera Azoarcus and Ideonella within the hypoxia-anoxic layer (2-4 mm) and anoxic layer (4-6 mm). This revealed that the content of organic carbon had no efference ect on them within the study conditions. Environmental change is a selective force for the sensitive bacterial individuals, which leads to changes in species and may have cascaded efference ects on ecosystem functions (Carlisle and Clements, 2005). In the present study, the change of organic carbon concentration in eutrophic lake sediment had selective efference ect on the species and abundance of denitrifying bacteria.
The infl uence of organic matter to denitrifi er community structure and diversity has often been mentioned in variable ecosystems (Lu et al., 2014; Chen et al., 2018; Si et al., 2018; Xu et al., 2018). However, there are few studies on denitrifying bacterial community structure and diversity in surface sediments with organic matter consumption under difference erent DO concentrations. In this study, the structure of nirSbased denitrifi er communities in the surface sediments showed signifi cant difference erence between high and low organic carbon content in the surface sediment based on NMDS analysis. Chao and Observation indices also showed variation among the difference erent DO layers with the organic carbon consumption. The interspecifi c competition and coexistence among genera and species of bacteria have been proposed by previous studies (Schmid et al., 2003; Kartal et al., 2007). In the surface sediment, denitrifying bacteria could compete for nutrient with other bacteria and lead to variability in the diversity indices. The signifi cant correlations of DOC and TOC with the diversity indices also hinted as the key factor infl uencing denitrifi er diversity in sediment surface. However, the efference ect of organic carbon on the diversity of denitrifying bacteria and the source of organic carbon such as submerged plant degradation, algal decomposition, or exogenic organic matter input in eutrophic sediment surface needs to be assessed further.
4.2 Vertical distribution of denitrifying bacteria in difference erent dissolved oxygen layers
The vertical distribution of anaerobic ammoniaoxidizing bacteria under difference erent DO concentrations in lake sediment has been studied and reported (Qin et al., 2018). However, the study on denitrifying bacteria based on DO content within the surface sediments has not been adequately reported. It is generally believed that denitrifi cation can only proceed normally when the DO concentration is kept below 0.5 mg/L in the system (McKenney et al., 2001). In this study, the depth of DO penetration was about 2.0-2.5 mm within the surface sediment, and the oxygen content was below ~0.5 mg/L from a depth of 2.0 mm. The depth of DO penetration recorded here is similar to depths that have been reported in other studies (Laverman et al., 2007; Wang et al., 2014). Based on these fi ndings, we believed that it may be prudent to refer to the zone above a depth of 2 mm as the aerobic layer and below the depth of 2 mm as the hypoxia and anoxic layer based on this study.
The nirS-type denitrifers are more widely distributed in natural environments and is therefore more frequently used as a biomarker of denitrifying bacteria within the sediments (Braker et al., 2001; Bulow et al., 2008). The abundance of nirS denitrifi ers changes with water and sediment depth (Kim et al., 2011; Mao et al., 2017). In the present study, the abundance of nirS gene was higher in hypoxia and anoxic layer than in the other layers within the vertical DO profi les, indicating that denitrifers were most abundant in this hypoxia and anoxic layer. Some studies have also shown that the penetration depth of nitrate is greater than that of oxygen based on microelectrode measurements, inferring that the dominant denitrifi cation layer should be below the oxygen layer (Christensen et al., 1989; Sweerts and de Beer, 1989; Nielsen et al., 1990a).
The change in DO concentration can alter the bacterial community structure. When the DO concentration is from 0-0.7 mg/L, then variations of microbial community structures can be realized in a sulfi de oxidation and nitrate reduction reactor (Wang et al., 2016). The 2.5 mg/L DO concentration also ensures the optimal bacterial community in a moving bed sequencing batch reactor (Cao et al., 2017). In the present study, the structure of nirS-based denitrifi er communities in the surface sediments showed distinctive difference erence among DO profi les both on high and low organic carbon consumption based on ANOSIM and NMDS analyses. This was consistent with vertical structured patterns of denitrifi cation activity within aquifers (Ben Maamar et al., 2015). This indicated the signifi cant variation of denitrifying community structure formed at aerobic (0.5-6.9 mg/L), hypoxic and anoxic (0-0.5 mg/L) and anoxic (0 mg/L) DO layers within lake sediment.
5 CONCLUSION
In this study, specifi c denitrifi er communities existed in difference erent DO layers and the adaptive changes of denitrifi er communities were formed with the consumption of organic carbon. The change of organic carbon concentration in eutrophic lake sediment had selective efference ect on the species and abundance of denitrifying bacteria. In addition, the hypoxic-anoxic layer (2-4 mm) appears to be the main distribution area of denitrifying bacteria in the surface sediments. These fi ndings provide new insights into niche separation based on characteristics of denitrifi er communities within the vertical column of lake surface sediment over a few millimeters.
6 DATA AVAILABILITY STATEMENT
Sequence data that supports the fi ndings of this study have been deposited in NCBI short-read archive under SRA accession PRJNA507510 (https://www.ncbi.nlm.nih.gov/sra/PRJNA507510) and PRJNA5313539 (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA531353).
References
Ben Maamar S, Aquilina L, Quaiser A, Pauwels H, Michon-Coudouel S, Vergnaud-Ayraud V, Labasque T, Roques C, Abbott B W, Dufresne A. 2015. Groundwater isolation governs chemistry and microbial community structure along hydrologic fl owpaths. Front. Microbiol., 6: 1 457, https://doi.org/10.3389/fmicb.2015.01457.
Bier R L, Voss K A, Bernhardt E S. 2015. Bacterial community responses to a gradient of alkaline mountaintop mine drainage in Central Appalachian streams. ISME J., 9(6): 1 378-1 390, https://doi.org/10.1038/ismej.2014.222.
Braker G, Ayala-del-Río H L, Devol A H, Fesefeldt A, Tiedje J M. 2001. Community structure of denitrifi ers, bacteria, and archaea along redox gradients in Pacifi c northwest marine sediments by terminal restriction fragment length polymorphism analysis of amplifi ed nitrite reductase ( nirS) and 16S rRNA genes. Appl. Environ. Microbiol., 67(4): 1 893-1 901, https://doi.org/10.1128/AEM.67.4. 1893-1901.2001.
Braker G, Fesefeldt A, Witzel K P. 1998. Development of PCR primer systems for amplifi cation of nitrite reductase genes ( nirK and nirS) to detect denitrifying bacteria in environmental samples. Appl. Environ. Microbiol., 64(10): 3 769-3 775.
Bulow S E, Francis C A, Jackson G A, Ward B B. 2008. Sediment denitrifi er community composition and nirS gene expression investigated with functional gene microarrays. Environ. Microbiol., 10(11): 3 057-3 069, https://doi.org/10.1111/j.1462-2920.2008.01765.x.
Cao Y F, Zhang C S, Rong H W, Zheng G L, Zhao L M. 2017. The efference ect of dissolved oxygen concentration (DO) on oxygen difference usion and bacterial community structure in moving bed sequencing batch reactor (MBSBR). Water Res., 108: 86-94, https://doi.org/10.1016/j.watres.2016.10.063.
Carlisle D M, Clements W H. 2005. Leaf litter breakdown, microbial respiration and shredder production in metalpolluted streams. Freshw. Biol., 50(2): 380-390, https://doi.org/10.1111/j.1365-2427.2004.01323.x.
Chen X C, Huang Y Y, Chen G Q, Li P P, Shen Y S, Davis T W. 2018. The secretion of organics by living Microcystis under the dark/anoxic condition and its enhancing efference ect on nitrate removal. Chemosphere, 196: 280-287, https://doi.org/10.1016/j.chemosphere.2017.12.197.
Chen Z G, Wang X J, Yang Y Y, Mirino M W, Yuan Y L. 2016. Partial nitrifi cation and denitrifi cation of mature landfi ll leachate using a pilot-scale continuous activated sludge process at low dissolved oxygen. Bioresour. Technol., 218: 580-588, https://doi.org/10.1016/j.biortech.2016.07.008.
Christensen P B, Nielsen L P, Revsbech N P, Sørensen J. 1989. Microzonation of denitrifi cation activity in stream sediments as studied with a combined oxygen and nitrous oxide microsensor. Appl. Environ. Microbiol., 55(5): 1 234-1 241.
Coyotzi S, Doxey A C, Clark I D, Lapen D R, Van Cappellen P, Neufeld J D. 2017. Agricultural soil denitrifi ers possess extensive nitrite reductase gene diversity. Environ. Microbiol., 19(3): 1 189-1 208, https://doi.org/10.1111/1462-2920.13643.
Fan M C, Lin Y B, Huo H B, Liu Y, Zhao L, Wang E T, Chen W M, Wei G H. 2016. Microbial communities in riparian soils of a settling pond for mine drainage treatment. Water Res., 96: 198-207, https://doi.org/10.1016/j.watres.2016.03.061.
Francis C A, O'Mullan G D, Cornwell J C, Ward B B. 2013. Transitions in nirS-type denitrifi er diversity, community composition, and biogeochemical activity along the Chesapeake Bay estuary. Front. Microbiol., 4: 237, https://doi.org/10.3389/fmicb.2013.00237.
Gao J, Hou L J, Zheng Y L, Liu M, Yin G Y, Li X F, Lin X B, Yu C D, Wang R, Jiang X F, Sun X R. 2016. nirS-encoding denitrifi er community composition, distribution, and abundance along the coastal wetlands of China. Appl. Microbiol. Biotechnol., 100(19): 8 573-8 582, https://doi.org/10.1007/s00253-016-7659-5.
Grofference man P M, Butterbach-Bahl K, Fulweiler R W, Gold A J, Morse J L, Stander E K, Tague C, Tonitto C, Vidon P. 2009. Challenges to incorporating spatially and temporally explicit phenomena (hotspots and hot moments) in denitrifi cation models. Biogeochemistry, 93(1-2): 49-77, https://doi.org/10.1007/s10533-008-9277-5.
Huang S, Chen C, Yang X, Wu Q, Zhang R. 2011. Distribution of typical denitrifying functional genes and diversity of the nirS-encoding bacterial community related to environmental characteristics of river sediments. Biogeosciences, 8: 3 041-3 051, https://doi.org/10.5194/bg-8-3041-2011.
Jenerette G D, Chatterjee A. 2012. Soil metabolic pulses: water, substrate, and biological regulation. Ecology, 93(5): 959-966, https://doi.org/10.1890/11-1527.1.
Jia Z M, Liu T, Xia X H, Xia N. 2016. Efference ect of particle size and composition of suspended sediment on denitrifi cation in river water. Sci. Total Environ., 541: 934-940, https://doi.org/10.1016/j.scitotenv.2015.10.012.
Kandeler E, Deiglmayr K, Tscherko D, Bru D, Philippot L. 2006. Abundance of narG, nirS, nirK, and nosZ genes of denitrifying bacteria during primary successions of a glacier foreland. Appl. Environ. Microbiol., 72(9): 5 957-5 962, https://doi.org/10.1128/AEM.00439-06.
Kartal B, Rattray J, van Niftrik L A, van de Vossenberg J, Schmid M C, Webb R I, Schouten S, Fuerst J A, Damsté J S, Jetten M S M, Strous M. 2007. Candidatus “ Anammoxoglobus propionicus” a new propionate oxidizing species of anaerobic ammonium oxidizing bacteria. Syst. Appl. Microbiol., 30(1): 39-49, https://doi.org/10.1016/j.syapm.2006.03.004.
Kim O S, Imhofference J F, Witzel K P, Junier P. 2011. Distribution of denitrifying bacterial communities in the stratifi ed water column and sediment-water interface in two freshwater lakes and the Baltic Sea. Aquat. Ecol., 45(1): 99-112, https://doi.org/10.1007/s10452-010-9335-7.
Knowles R. 1982. Denitrifi cation. Microbiol. Rev., 46(1): 43-70.
Laverman A M, Canavan R W, Slomp C P, van Cappellen P. 2007. Potential nitrate removal in a coastal freshwater sediment (Haringvliet Lake, The Netherlands) and response to salinization. Water Res., 41(14): 3 061-3 068, https://doi.org/10.1016/j.watres.2007.04.002.
Lee K C, Rittmann B E. 2003. Efference ects of pH and precipitation on autohydrogenotrophic denitrifi cation using the hollowfi ber membrane-biofi lm reactor. Water Res., 37(7): 1 551-1 556, https://doi.org/10.1016/S0043-1354(02)00519-5.
Li E C, Lu S G. 2017. Denitrifi cation processes and microbial communities in a sequencing batch reactor treating nanofi ltration (NF) concentrate from coking wastewater. Water Sci. Technol., 76(11-12): 3 289-3 298, https://doi.org/10.2166/wst.2017.493.
Liu J X, Li C, Jing J H, Zhao P Y, Luo Z M, Cao M W, Ma Z Z, Jia T, Chai B F. 2018b. Ecological patterns and adaptability of bacterial communities in alkaline copper mine drainage. Water Res., 133: 99-109, https://doi.org/10.1016/j.watres.2018.01.014.
Liu W Z, Yao L, Jiang X L, Guo L D, Cheng X L, Liu G H. 2018a. Sediment denitrifi cation in Yangtze lakes is mainly infl uenced by environmental conditions but not biological communities. Sci. Total. Environ., 616- 617: 978-987, https://doi.org/10.1016/j.scitotenv.2017.10.221.
Lu H J, Chandran K, Stensel D. 2014. Microbial ecology of denitrifi cation in biological wastewater treatment. Water Res., 64: 237-254, https://doi.org/10.1016/j.watres.2014. 06.042.
Mao G Z, Chen L, Yang Y Y, Wu Z, Tong T L, Liu Y, Xie S G. 2017. Vertical profi les of water and sediment denitrifi ers in two plateau freshwater lakes. Appl. Microbiol. Biotechnol., 101(8): 3 361-3 370, https://doi.org/10.1007/s00253-016-8022-6.
McKenney D J, Drury C F, Wang S W. 2001. Efference ects of oxygen on denitrifi cation inhibition, repression, and derepression in soil columns. Soil Sci. Soc. Am. J., 65: 126-132, https://doi.org/10.2136/sssaj2001.651126x.
Nancharaiah Y V, Joshi H M, Hausner M, Venugopalan V P. 2008. Bioaugmentation of aerobic microbial granules with Pseudomonas putida carrying TOL plasmid. Chemosphere, 71(1): 30-35, https://doi.org/10.1016/j.chemosphere.2007.10.062.
Nielsen L P, Christensen P B, Revsbech N P, Sørensen J. 1990a. Denitrifi cation and oxygen respiration in biofi lms studied with a microsensor for nitrous oxide and oxygen. Microb. Ecol., 19(1): 63-72, https://doi.org/10.1007/BF02015054.
Nielsen L P, Christensen P B, Revsbech N P, Sørensen J. 1990b. Denitrifi cation and photosynthesis in stream sediment studied with microsensor and wholecore techniques. Limnol. Oceanogr., 35(5): 1 135-1 144, https:// doi.org/10.4319/lo.1990.35.5.1135.
Nolan B T. 2001. Relating nitrogen sources and aquifer susceptibility to nitrate in shallow ground waters of the United States. Groundwater, 39(2): 290-299, http://dx.doi.org/10.1111/j.1745-6584.2001.tb02311.x.
Puckett L J, Tesoriero A J, Dubrovsky N M. 2011. Nitrogen contamination of surfi cial aquifers-a growing legacy. Environ. Sci. Technol., 45(3): 839-844, https://doi.org/ 10.1021/es1038358.
Qin H Y, Han C, Jin Z W, Wu L, Deng H, Zhu G W, Zhong W H. 2018. Vertical distribution and community composition of anammox bacteria in sediments of a eutrophic shallow lake. J. Appl. Microbiol., 125(1): 121-132, https://doi.org/10.1111/jam.13758.
Qiu T L, Xu Y, Gao M, Han M L, Wang X M. 2017. Bacterial community dynamics in a biodenitrifi cation reactor packed with polylactic acid/poly (3-hydroxybutyrate-co-3-hydroxyvalerate) blend as the carbon source and biofi lm carrier. J. Biosci. Bioeng., 123(5): 606-612, https://doi.org/10.1016/j.jbiosc.2016.12.007.
Remmas N, Melidis P, Katsioupi E, Ntougias S. 2016. Efference ects of high organic load on amoA and nirS gene diversity of an intermittently aerated and fed membrane bioreactor treating landfi ll leachate. Bioresour. Technol., 220: 557-565, https://doi.org/10.1016/j.biortech.2016.09.009.
Rong N, Shan B Q, Wang C. 2016. Determination of sediment oxygen demand in the Ziya River watershed, China: based on laboratory core incubation and microelectrode measurements. Int. J. Environ. Res. Public Health, 13(2): 232, https://doi.org/10.3390/ijerph13020232.
Saarenheimo J, Aalto S L, Rissanen A J, Tiirola M. 2017. Microbial community response on wastewater discharge in boreal lake sediments. Front. Microbiol., 8: 750, https://doi.org/10.3389/fmicb.2017.00750.
Santschi P, Höhener P, Benoit G, Buchholtz-ten Brink M. 1990. Chemical processes at the sediment-water interface. Mar. Chem., 30: 269-315, https://doi.org/10.1016/0304-4203(90)90076-O.
Schmid M, Walsh K, Webb R, Rijpstra W I, van de Pas-Schoonen K, Verbruggen M J, Hill T, Mofference ett B, Fuerst J, Schouten S, Sinninghe Damsté J S, Harris J, Shaw P, Jetten M, Strous M. 2003. Candidatus “ Scalindua brodae”, sp. nov., Candidatus “ Scalindua wagneri”, sp. nov., two new species of anaerobic ammonium oxidizing bacteria. Syst. Appl. Microbiol., 26(4): 529-538, https://doi.org/10.1078/072320203770865837.
Seitzinger S, Harrison J A, Böhlke J K, Bouwman A F, Lowrance R, Peterson B, Tobias C, Van Drecht G. 2006. Denitrifi cation across landscapes and waterscapes: a synthesis. Ecol. Appl., 16(6): 2 064-2 090, https://doi.org/10.1890/1051-0761(2006)016[2064:DALAWA]2.0.CO;2.
Si Z H, Song X S, Wang Y H, Cao X, Zhao Y F, Wang B D, Chen Y, Arefe A. 2018. Intensifi ed heterotrophic denitrifi cation in constructed wetlands using four solid carbon sources: denitrifi cation eき ciency and bacterial community structure. Bioresour. Technol., 267: 416-425, https://doi.org/10.1016/j.biortech.2018.07.029.
Sirivedhin T, Gray K A. 2006. Factors afference ecting denitrifi cation rates in experimental wetlands: fi eld and laboratory studies. Ecol. Eng., 26(2): 167-181, https://doi.org/10. 1016/j.ecoleng.2005.09.001.
Srinandan C S, D’souza G, Srivastava N, Nayak B B, Nerurkar A S. 2012. Carbon sources infl uence the nitrate removal activity, community structure and biofi lm architecture. Bioresour. Technol., 117: 292-299, https://doi.org/10. 1016/j.biortech.2012.04.079.
Sweerts J P R A, de Beer D. 1989. Microelectrode measurements of nitrate gradients in the littoral and profundal sediments of a meso-eutrophic lake (lake Vechten, the Netherlands). Appl. Environ. Microbiol., 55(3): 754-757.
Thomsen T R, Kong Y H, Nielsen P H. 2007. Ecophysiology of abundant denitrifying bacteria in activated sludge. FEMS Microbiol. Ecol., 60(3): 370-382, https://doi.org/10.1111/j.1574-6941.2007.00309.x.
Tian C C, Wang C B, Tian Y Y, Wu X Q, Xiao B D. 2015. Vertical distribution of Fe and Fe(III)-reducing bacteria in the sediments of Lake Donghu, China. Can. J. Microbiol., 61(8): 575-583, https://doi.org/10.1139/cjm-2015-0129.
Van Rijn J, Tal Y, Schreier H J. 2006. Denitrifi cation in recirculating systems: theory and applications. Aquac. Eng., 34(3): 364-376, https://doi.org/10.1016/j.aquaeng. 2005.04.004.
Waki M, Yasuda T, Fukumoto Y, Béline F, Magrí A. 2018. Treatment of swine wastewater in continuous activated sludge systems under difference erent dissolved oxygen conditions: reactor operation and evaluation using modelling. Bioresour. Technol., 250: 574-582, https://doi.org/10.1016/j.biortech.2017.11.078.
Wang C, Shan B Q, Zhang H, Rong N. 2014. Analyzing sediment dissolved oxygen based on microprofi le modeling. Environ. Sci. Pollut. Res. Int., 21(17): 10 320-10 328, https://doi.org/10.1007/s11356-014-2875-y.
Wang X W, Zhang Y, Zhang T T, Zhou J T. 2016. Efference ect of dissolved oxygen on elemental sulfur generation in sulfi de and nitrate removal process: characterization, pathway, and microbial community analysis. Appl. Microbiol. Biotechnol., 100(6): 2 895-2 905, https://doi.org/10.1007/s00253-015-7146-4.
Wen X, Gong B Z, Zhou J, He Q, Qing X X. 2017. Eき cient simultaneous partial nitrifi cation, anammox and denitrifi cation (SNAD) system equipped with a real-time dissolved oxygen (DO) intelligent control system and microbial community shifts of difference erent substrate concentrations. Water Res., 119: 201-211, https://doi.org/10.1016/j.watres.2017.04.052.
Wu S F, Wu Z, Liang Z Y, Liu Y, Wang Y L. 2019. Denitrifi cation and the controlling factors in Yunnan Plateau Lakes (China): exploring the role of enhanced internal nitrogen cycling by algal blooms. J. Environ. Sci., 76: 349-358, https://doi.org/10.1016/j.jes.2018.05.028.
Wu X, Liu G, Butterbach-Bahl K, Fu B, Zheng X, Brüggemann N. 2013. Efference ects of land cover and soil properties on denitrifi cation potential in soils of two semi-arid grasslands in Inner Mongolia, China. J. Arid Environ., 92: 98-101, https://doi.org/10.1016/j.jaridenv.2013.02.003.
Xu N, Tan G C, Wang H Y, Gai X P. 2016. Efference ect of biochar additions to soil on nitrogen leaching, microbial biomass and bacterial community structure. Eur. J. Soil. Biol., 74: 1-8, https://doi.org/10.1016/j.ejsobi.2016.02.004.
Xu Z S, Dai X H, Chai X L. 2018. Efference ect of difference erent carbon sources on denitrifi cation performance, microbial community structure and denitrifi cation genes. Sci. Total Environ., 634: 195-204, https://doi.org/10.1016/j.scitotenv.2018.03.348.
Yang J K, Cheng Z B, Li J, Miao L H. 2013. Community composition of nirS-type denitrifi er in a shallow eutrophic lake. Microb. Ecol., 66(4): 796-805, https://doi.org/10.1007/s00248-013-0265-5.
Zou Y, Xu X C, Wang X J, Yang F L, Zhang S S. 2018. Achieving eき cient nitrogen removal and nutrient recovery from wastewater in a combining simultaneous partial nitrifi cation, anammox and denitrifi cation (SNAD) process with a photobioreactor (PBR) for biomass production and generated dissolved oxygen (DO) recycling. Bioresour. Technol., 268: 539-548, https://doi.org/10.1016/j.biortech.2018.08.015.
Zumft W G. 1997. Cell biology and molecular basis of denitrifi cation. Microbiol. Mol. Biol. Rev., 61(4): 533-616, https://doi.org/10.1016/j.ccr.2004.08.030.
 Journal of Oceanology and Limnology2020年3期
Journal of Oceanology and Limnology2020年3期
- Journal of Oceanology and Limnology的其它文章
- List of the Most Outstanding Papers Published by CJOL/JOL in 2017-2018
- Who is the “murderer” of the bloom in coastal waters of Fujian, China, in 2019?*
- The investigation of internal solitary waves over a continental shelf-slope*
- Efference ect of diets on the feeding behavior and physiological properties of suspension-feeding sea cucumber Cucumaria frondosa*
- Efference ects of light quality on growth rates and pigments of Chaetoceros gracilis (Bacillariophyceae)*
- Marine bacterial surfactin CS30-2 induced necrosis-like cell death in Huh7.5 liver cancer cells*
