Unraveling enhanced membrane lipid biosynthesis in Chlamydomonas reinhardtii starchless mutant sta6 by using an electrospray ionization mass spectrometry-based lipidomics method*
ZANG Zhengrong , , LI Yanhua , HU Qiang , HAN Danxiang
1 Center for Microalgal Biotechnology and Biofuels, Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan 430072, China 2 Key Laboratory for Algal Biology, Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan 430072, China
3 University of Chinese Academy of Sciences, Beijing 100049, China
4 State Key Laboratory of Freshwater Ecology and Biotechnology, Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan 430072, China
5 Laboratory for Marine Biology and Biotechnology, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266071, China
6 Beijing Key Laboratory of Algae Biomass, Microalgae Biotechnology Center, SDIC Biotech Investment Co. Ltd., State Development & Investment Corp., Beijing 100142, China
Received May 17, 2019; accepted in principle Jul. 2, 2019; accepted for publication Aug. 22, 2019 © Chinese Society for Oceanology and Limnology, Science Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Abstract The unicellular green alga Chlamydomonas reinhardtii, a well-established model organism, has been widely used in dissecting glycerolipid metabolism in oxygenating photosynthetic organisms. In previous studies, it has been found that shunting carbon precursors from the starch synthesis pathway can lead to a 10-fold increase in TAG content as compared to the wild type, but it is unknown whether inactivation of AGPase may afference ect membrane lipids biosynthesis. The study aims to investigate global changes in lipid metabolism and homeostasis in the starchless mutant C. reinhardtii st a 6. By utilizing an electrospray ionization/mass spectrometry (ESI/MS)-based lipidomics approach, a total of 105 membrane lipid molecules of C. reinhardtii were resolved, including 16 monogalactosyldiacylglycerol (MGDG), 16 digalactosyldiacylglycerol (DGDG), 11 phosphatidylglycerol (PG), 6 sulfoquinovosyldiacylglycerol (SQDG), 49 diacylglyceryl- N, N, N-trimethylhomoserine (DGTS), 2 phosphatidylethanolamine (PE), and 5 phosphatidylinositol (PI) molecules. The quantitative results indicated that the membrane lipid profi les were similar between the two C. reinhardtii strains grown under both low- and high-light conditions, but the cellular contents of a great number of lipids were altered in sta6 due to the defect in starch biosynthesis. Under low-light conditions, sta6 accumulated more PI, MGDG, DGDG but less amounts of DGTS as compared to WT. Under high light, s ta6 cells contained higher content membrane lipids than cc-124, except for PG, which is more or less similar in both strains. Our results demonstrate that the cellular membrane lipid homeostasis underwent profound changes in the starchless mutant, and thereby its physiological impact remains to be explored.
Keyword: Chlamydomonas reinhardtii; chloroplast lipidomes; extraplastidic lipidomes; electrospray ionization mass spectrometry
1 INTRODUCTION
The unicellular green microalga Chlamydomonas reinhardtii has been widely used as a model organism for studying lipid metabolism in photosynthetic organisms (Harris, 2001; Siaut et al., 2011; Merchant et al., 2012; Li-Beisson et al., 2015). Lipids possess crucial functions in many biological processes in plants and microalgae, such as maintaining cellular membrane structures, storing energy and carbons, and mediating signaling pathways (Guschina et al., 2006; Hu et al., 2008; Barbaglia and Hofference mann-Benning, 2016). Lipid profi les of algal cells may be afference ected by various environment stresses, such as high light and nutrient deprivation conditions (Solovchenko et al., 2008).
The traditional methods for lipids analysis include thin layer chromatography (TLC), gas chromatography (GC) and GC-mass spectrometry (GC/MS) (Diehl et al., 1995; Xu et al., 2002; Rainville et al., 2007). However, there are many disadvantages for these methods. TLC, GC, and GC/MS involve timeconsuming and laborious sample pre-treatment procedures. Moreover, these methods cannot be used to quantify the cellular content of a given lipid molecule, with limited applications in functional genomics studies (Rainville et al., 2007; Carrasco-Pancorbo et al., 2009).
In recent years, mass spectrometry (MS) based analytical methods have been used in analysis of lipidomes of biological samples with difference erent genetic backgrounds or physiological interferences (Han and Gross, 2003, 2005; Welti et al., 2003; Rainville et al., 2007). Since microalgal glycerolipids have attracted great interest due to their potentials in energy and nutraceutical applications, lipidomes of a large number of microalgae including both membrane glycerolipids and triacylglycerols have been resolved (reviewed by De Costa et al., 2016). Lipidomics is a very efference ective biochemical analytical tool for precise identifi cation of glycerollipids, which is not only able to resolve lipid classes and fatty acyl composition, but also can facilitate absolute quantifi cation at molecular level, especially when coupled with ultra-high performance liquid chromatography that can essentially improve the sensitivity and reproducibility (Cutignano et al., 2016; Han et al., 2017; Řezanka et al., 2018).
For C. reinhardtii, the model species for dissecting lipid metabolism in microalgae, its lipidomes have been characterized in a few previous studies which showed monogalactosyldiacylglyceride (MGDG), digalactosydiacylglyceride (DGDG), sulfoquinovosyldiacylglycerol (SQDG), phosphatidylglycerol (PG), diacylglyceryl- N, N, N-trimethylhomoserine (DGTS), phosphatidylinositol (PI) and phosphatidyl ethanolamine (PE) are the major membrane lipids of
C. reinhardtii (Yoon et al., 2012; Yang et al., 2015). MGDG, DGDG, SQDG, and PG constitute the chloroplast membranes in algal cells, playing important functional roles in photosynthesis (Harwood and Guschina, 2009; Kobayashi et al., 2016). In microalgae like C. reinhardtii, the extraplastidic membrane lipids are mainly composed of DGTS, PI and PE (Moellering et al., 2009), among which the betain lipid DGTS was the major class (Klug and Benning, 2001).
In this study, the analytical tool of ultra performance liquid chromatography (UPLC)-ESI-MS/MS was used to quantitatively analyze the lipidomes of C. reinhardtii wild type and starchless mutant sta 6 cells. The starchless mutant sta6 was defi cient of the gene encoding an ADP-glucose pyrophosphorylase subunit and hence incapable of synthesizing starch (Zabawinski et al., 2001). In previous study, shunting carbon precursors from the starch synthesis pathway in C. reinhardtii led to a 10-fold increase in triacylglycerol (TAG) content as compared to the wild type (Li et al., 2010a, b), but it is unknown whether inactivation of AGPase may have any efference ect on the cellular contents of membrane lipids. The study aims to investigate global changes in lipid metabolism and homeostasis in the starchless mutant. In addition, responses of such two C. reinardtii strains to the changing illumination conditions at the level of lipid molecules were investigated by using the lipidomics approach as well, which will underpin our understanding about the impact of blocking starch biosynthesis on cellular capabilities in adapting changing environmental conditions.
2 MATERIAL AND METHOD
2.1 Strains and growth conditions
Chlamydomonas reinhardtii cc-124 (WT) was purchased from Chlamydomonas resource center at the University of Minnesota (https://www.chlamycollection.org), and sta6 was kindly provided by Arizona State University. Both strains were precultured in the TAP growth medium (Gorman and Levine, 1965) at 25°C on an orbital shaker incubator (NBS Innova 44R, Eppendorf, Germany) at a constant rate of 100 r/min. The light intensity was kept at 50 μmol/(m2·s), which was measured by using a photosynthetically active radiation (PAR) meter (Mastercycler pro S, Eppendorf, Germany). Algal cells from the logarithmic phase were inoculated into the 800 mL column photobioreactor (i.d., 5 cm) containing 750 mL HSM medium (Sueoka, 1960), with an initial cell concentration of (2.0-2.5)×106cells/mL. Algal cells were cultivated under 5 μmol/(m2·s) (low light, LL) for 3 days and then transferred to 150 μmol/(m2·s) (high light, HL) for 12 h. The algal samples from both illumination conditions were harvested by centrifugation at 3 000× g for 5 min at 4°C, and then freeze-dried.
2.2 Lipid extraction
Total lipids were extracted according to the method described previously (Wang and Benning, 2011) with minor modifi cations. The freeze-dried samples (about 10 mg, dry weight) were ground in liquid nitrogen and extracted with 6 mL solvents composed of methanol, chloroform and formic acid (20׃10׃1, v/v/v). The extracts were vigorously vortexed for 5 min followed by adding 3 mL of 0.2 mol/L H3PO4and 1 mol/L KCl, and then vortexed briefl y. After centrifuging at 1 000× g for 5 min, lipids dissolved in the lower chloroform phase were collected by using a glass pipette. Solvents were then evaporated under nitrogen stream. Lipid samples were stored at -80°C prior to use.
2.3 Lipidomes analysis and quantifi cation with ESI/MS
Lipidomes analyses were performed on a triple quadrupole MS/MS (Xevo TQ-S, Waters, USA) with electrospray ionization (ESI) source coupled with an Acquity Ultra-Performance Liquid Chromatography (UPLC) system (Waters, USA). Lipid samples were separated on a BEH C18column (length 50 mm, internal diameter 2.1 mm, particle size 1.7 μm; Waters) prior to the ESI/MS analysis. The column temperature was kept at 30°C and 35°C for the positive and negative ion mode, respectively. The ESI/MS analysis was carried out according to the method developed by Yoon et al previously (Yoon et al., 2012) with modifi cations. Samples were recovered in 1 mL chloroform/methanol (1:1, v/v). For absolute quantifi cation, lipids samples were mixed with internal standards (ISTD), including MGDG 18:0/18:0, DGDG 18:0/18:0, DGTS 16:0/16:0 d9, PE 17:0/14:1, PG 17:0/20:4 and PI 17:0/20:4. Among these ISTD, PG 17:0/20:4 was used for both PG and SQDG quantifi cation. The external standards (ESTD) for calibration included MGDG mixture standard containing MGDG 16:3/18:3, MGDG 16:3/18:2 and MGDG 16:1/18:3, DGDG mixture standard containing DGDG 16:3/18:3, DGDG 16:3/18:2, DGDG 16:1/18:3 and DGDG 16:0/18:3, DGTS 16:0/16:0, PG 16:0/18:1, PG 18:0/18:1, PE 18:0/18:1, PI 18:1/18:1 and SQDG 16:0/18:3 were used as ETSDs for the corresponding classes of membrane lipids. Lipid standards were all purchased from Avanti Polar Lipids Ltd. (USA) except that MGDG 18:0/18:0 and DGDG 18:0/18:0 were obtained from Matreya LLC (USA). For quantifi cation, ESTD were titrated relative to a constant amount of ISTD to establish the correlation between the ratio of the analyte signal to the ISTD signal and the ratio of their concentrations (Wang et al., 2014). MGDG, DGDG, and DGTS were detected in the positive mode, while PG, SQDG, PE, and PI were analyzed in the negative mode. Multiple reaction monitoring (MRM) was employed for quantitative analysis.
2.4 Data processing
Raw fi les of the ESI/MS data were retrieved and analyzed with Masslynx v4.1 software (Waters). Student’s t-test was used to compare the cellular contents of given lipids between WT and sta 6 ( n=4, including two biological replicates and two technical replicates). If the test gives P value ≤0.05, the difference erences between two samples were interpreted as being signifi cant.
3 RESULT
3.1 Growth of cc-124 and sta6 under low light and high light
The biomass of cc-124 increased slightly in the fi rst 2 h and then was fl uctuated over 12 h under the LL conditions (Fig.1a). On the contrast, WT grew fast under the HL conditions, the biomass of which achieved 0.4 g/L after 12 h (Fig.1a). Distinct from cc-124, sta6 showed slight growth under two conditions and no signifi cant difference erence was observed (Fig.1b).
3.2 Lipidomes of cc-124 and sta6 cells grown under low light
The lipidomics platform established in this study enabled absolute quantifi cation of 7 classes of major membrane lipids of C. reinhardtii (Table 1). Under LL, the content of total membrane lipids was 180.57±14.75 and 283.63±11.90 nmol/mg ( P <0.001) for WT and sta6, respectively. Under HL, the content of total membrane lipids was reduced by 11.7% to 159.13±7.14 nmol/mg in WT, while it was increased by 36.4% and reached 386.82±34.52 nmol/mg in sta6.
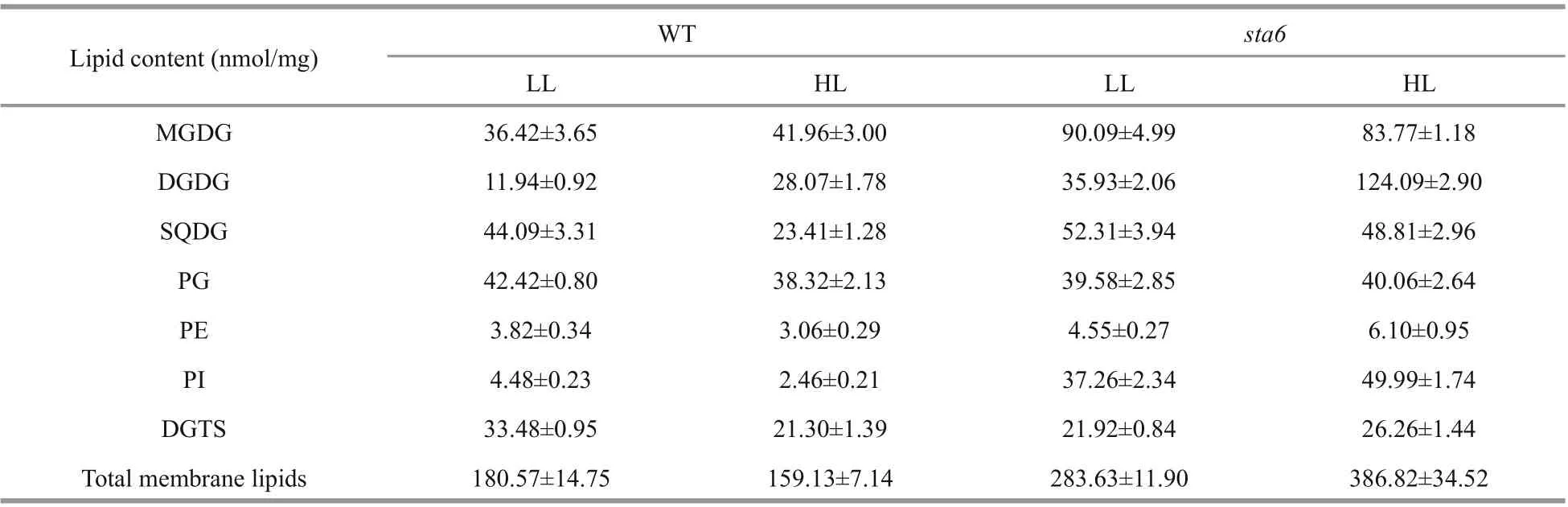
Table 1 The contents of total membrane lipid classes and total membrane lipids in WT and sta6 cultivated under low light (LL) and high light (HL) conditions
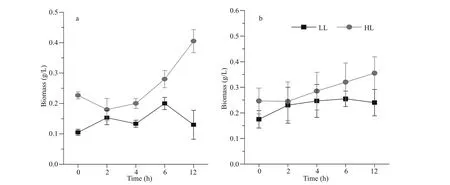
Fig.1 Biomass production of WT (a) and sta6 (b) cultivated under low-light (LL) and high-light (HL) conditions
The galactolipids including MGDG and DGDG were the most abundant membrane lipids in WT and starchless mutant sta6 grown under LL, accounting for 27.38% and 44.74% of the total membrane lipids in them, respectively (Fig.2, Table 1). In WT, the content of MGDG and DGDG was 36.42 nmol/mg DW and 11.94 nmol/mg DW, respectively. The content of MGDG and DGDG in sta6 was greater than that of WT by 147.35% ( P <0.001) and 200.78% ( P <0.001), respectively.
Under LL, the most abundant MGDG molecular species was MGDG 18׃3/16:4 in WT, of which the content was 20.59 nmol/mg DW (Fig.3a), accounting for 14.50% of the total MGDG. Difference erent from WT, MGDG 18:1/16:1, 18:1/16:4, and 18:3/16:4 were the most abundant MGDG species in sta6 cells grown under LL, accounting for 25.77% (corresponding to 23.11 nmol/mg DW), 23.27% (20.86 nmol/mg DW), and 23.90% (21.43 nmol/mg DW) of its total MGDG pool, respectively. The major DGDG species in WT and sta 6 under LL was both DGDG 18:3/16:1, which was 7.6 and 30.87 nmol/mg DW (Fig.3b), corresponding to 65.07% and 86.78% of the total DGDG, respectively.
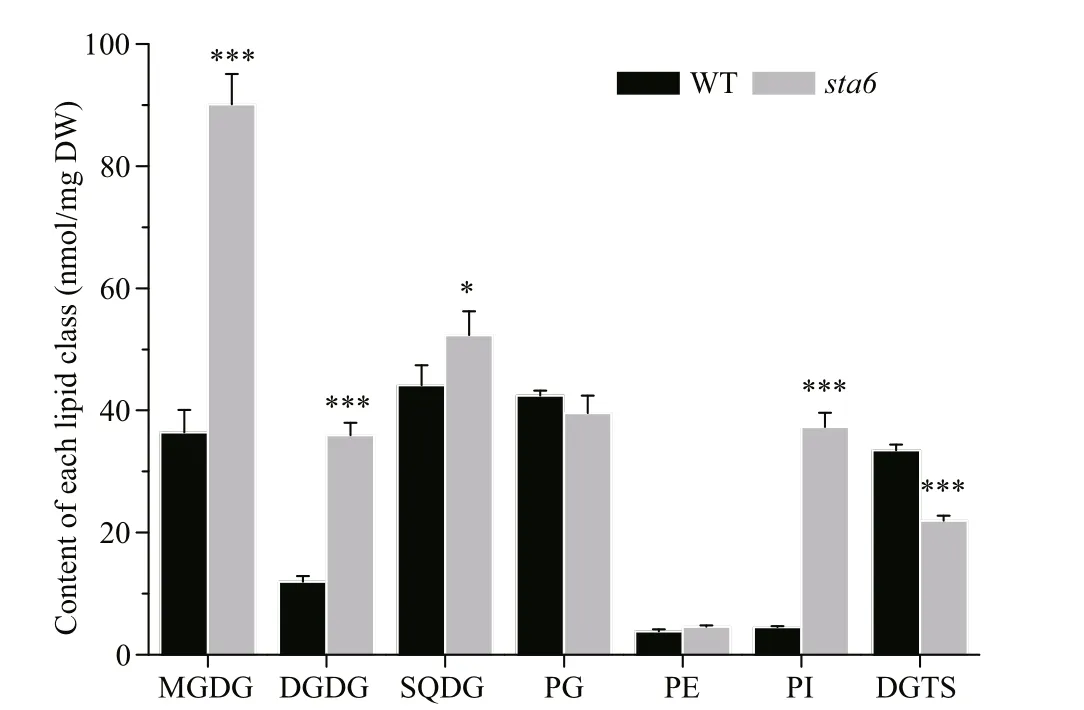
Fig.2 Contents of membrane lipids in C. reinhardtii WT and sta6 cells under low light (LL)
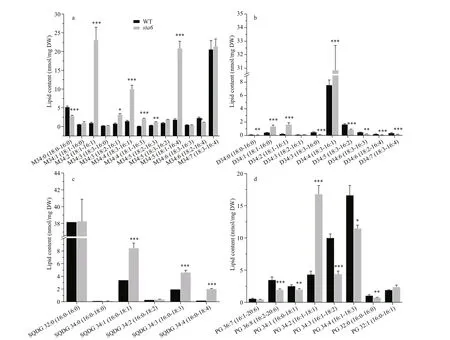
Fig.3 Contents of chloroplast membrane lipids molecular species in C. reinhardtii WT and sta6 under low light (LL)
SQDG and PG were two types of polar lipids present in the chloroplast membranes. The total SQDG content in sta6 under LL was 53.92 nmol/mg DW, which was 18.64% higher than in WT (44.09 nmol/mg DW, P <0.05) (Fig.2). Among SQDG molecular species, the content of SQDG 16:0/16:0 was 38.20 and 38.29 nmol/mg DW in WT and sta6, respectively (Fig.3c), which was the major species in both WT and sta6. Among SQDG molecules, one of the highly unsaturated species SQDG 16:0/18:4 was the one enhanced to the most extent, which was increased by 975.84% ( P <0.001) in sta6 as compared with WT. As for PG, the only bulk phospholipid found in chloroplast (Wada and Murata, 2007), WT and sta6 contained similar amounts of them, of which the cellular content was 42.42 and 39.58 nmol/mg DW, respectively (Fig.2). The most abundant PG species were PG 16:1/18:3 in WT, whereas it was replaced by PG 16:1/18:1 in sta6 (Fig.3d), which accounted for 41.09% and 41.81% of their total PG, respectively. In addition, PG 16:1/18:1 showed the greatest change with an increase by 287.80% ( P <0.001) in sta6 compared to WT.
PI and PE were two types of phospholipids constituting the extraplastidic membranes in C. reinhardtii. Under LL, the total PI content was 4.36 nmol/mg DW in WT (Fig.2), among which PI 16:0/18:1 was the major species accounting for 94.24% of the total PI (Fig.4a). In sta6, the content of PI was increased dramatically by 731.58% ( P <0.001) compared to that of WT. There was no signifi cant change in total PE content between WT and sta6, which were 3.82 and 4.55 nmol/mg DW, respectively (Fig.2). Two PE species including 18:0/18:3 and 18:2/18:3 were identifi ed in both strains, among which PE 18:0/18:3 was the major species (Fig.4a).
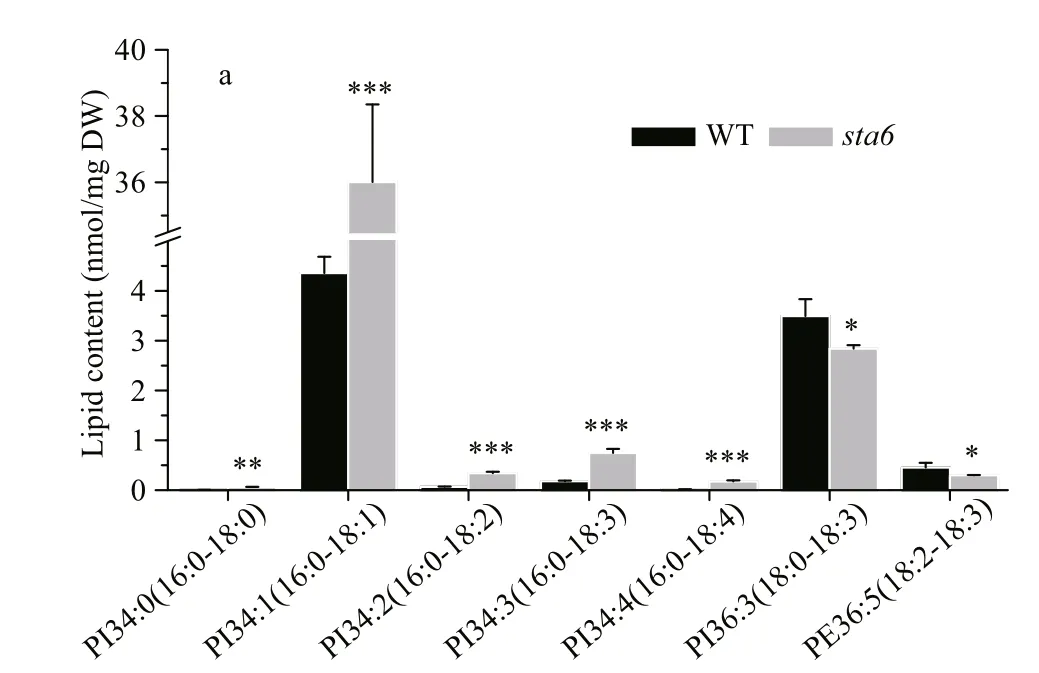
A large number of DGTS molecular species were identifi ed in both strains. DGTS 16:0/18:3 was the most abundant species in WT (7.61 nmol/mg DW), whereas DGTS 16:0/16:1, 16:0/18:3 and 16:0/18:4 were three species with the highest contents in sta6, which was 3.08, 3.9, and 4.27 nmol/mg DW, respectively (Fig.4b). Among the membrane lipids, DGTS was the only group of lipid class that was reduced in sta6 compared with WT (Fig.2, 33.48 vs 21.92 nmol/mg DW, P <0.001).
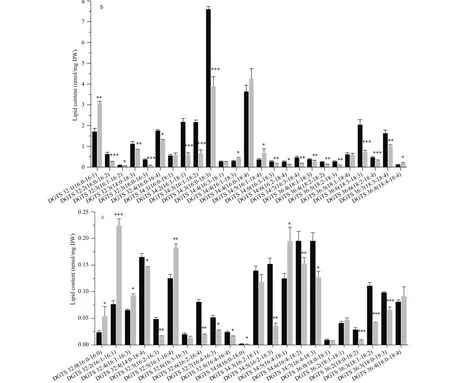
Fig.4 Contents of extraplastidic membrane lipids molecular species in C. reinhardtii WT and sta6 under low light (LL)
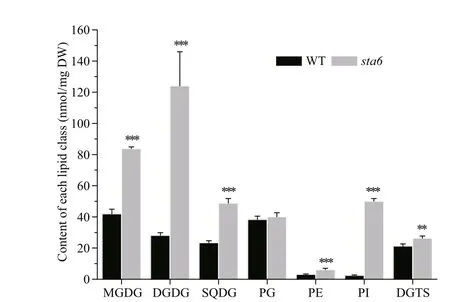
Fig.5 Contents of membrane lipids in C. reinhardtii WT and sta6 cells under high light (HL)
3.3 Lipidomes of cc-124 and sta6 cells grown under high light
After high light acclimation for 12 h, the contents of all the membrane lipids were signifi cantly increased in sta6 as compared to WT, except for PG. The chloroplast membrane lipid MGDG, DGDG, and SQDG was increased by 99.62% ( P <0.001), 342.14% ( P <0.001) and 108.50% ( P <0.001), respectively, while the extraplastidic membrane lipids PI, PE, and DGTS was increased by 19-fold ( P <0.001), 99.35% ( P <0.001) and 23.32% ( P <0.01), respectively (Fig.5).
The cellular contents of the chloroplast membrane lipid molecules were shown in Fig.6. For galactolipids, most MGDG and DGDG species in sta6 were higher than that in WT (Fig.6a & b). The cellular content of MGDG 18:1/16:3 in WT was 0.14 nmol/mg DW, whereas it was 4.96 nmol/mg DW in sta6, increased by 33-fold (Fig.6a, P <0.001). The predominant DGDG 18:3/16:1 was 66.55 nmol/mg DW in sta6, upregulated by 392.51% compared to WT (Fig.6b, 13.51 nmol/mg DW, P <0.001). Similar to galactolipids, most SQDG species were up-regulated in sta6 compared to in WT under HL. Among SQDG species, the most abundant SQDG 16:0/18:0 changed dramatically from 0.14 nmol/mg DW in WT to 0.63 nmol/mg DW in sta6, increased by 364.67% (Fig.6c, P <0.001). The total PG in two strains showed no signifi cant change (Fig.5), because the cellular contents of two predominant species PG 16:1/18:2 and PG 16:1/18:3 were unchanged (Fig.6d). However, several minor PG species were increased in sta6 compared to WT, including PG 16:1/18:1 increased by 138.63% ( P <0.01) and PG 16:0/16:1 decreased by 41.41% ( P <0.01) in sta6 as compared with WT (Fig.6d).
Similar to the drastic changes in the chloroplast membrane lipids, the cellular content of extraplastidic membrane lipids including PI, PE, and DGTS showed drastic increase in sta6 by 19-fold ( P <0.001), 99.35% ( P <0.001), and 23.32% ( P <0.01), respectively, as compared to that in WT (Fig.7). Each PI molecular species in sta6 was increased compared to that of WT under HL, among which the highly saturated PI 16:0/18:0 showed the largest increase by 22-fold ( P <0.001) (Fig.7a). For the two PE species that identifi ed in both WT and sta6, PE 18:0/18:3 rapidly increased by 46.84% in sta6 as compared with that in WT (Fig.7a, 2.35 nmol/mg DW, P <0.05), whereas PE 18:2/18:3 remained unchanged. In numerous DGTS species, DGTS 16:0/16:1 increased to the most in sta6 (Fig.7b, P <0.001), which was 182.70% higher than that of WT. However, a large number of DGTS species remained relatively stable in both strains, such as the dominant DGTS species, DGTS 16:0/18:3 (Fig.7b). By contrast, two DGTS species with highly unsaturated fatty acids, DGTS 18:3/18:4 and DGTS 18:4/18:4, were reduced by 31.33% (Fig.7b, P <0.01), and 47.93% (Fig.7c, P <0.001) in sta6, respectively, when compared to that of WT.
4 DISCUSSION
The starchless mutant sta6 has been intensively investigated due to its capabilities in overaccumulating triacylglycerols under stress conditions (Krishnan et al., 2015; Fan et al., 2017; Tran et al., 2019). However, mechanisms underlying enhanced TAG biosynthesis in sta6 remained elusive. There are emerging studies showing sta6 exhibited comprehensive phenotypes, including retarded cellular growth under certain conditions, increased central carbon metabolism, and slowed NADPH reoxidation (Blaby et al., 2013; Krishnan et al., 2015). This study aimed at dissecting the alterations in the cellular structures with respect to the membrane lipids compositions, which will provide insights into the physiological consequences of blocking starch biosynthesis in microalgae.
By using the ESI/MS method, a total of 16 MGDG molecules, 16 DGDG molecules, 6 SQDG molecules, 11 PG molecules, 5 PI molecules, 2 PE molecules and 49 DGTS molecules were identifi ed in both C. reinhardtii WT and sta6, indicative of that the membrane profi les are very similar between these two strains. On the other hand, quantitative analysis revealed that the cellular contents of a large number of lipid molecules were changed in sta6, due to the defi ciency in starch synthesis.
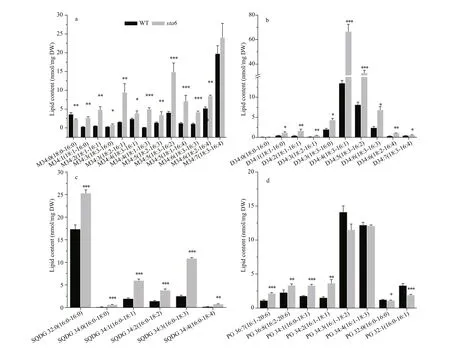
Fig.6 Contents of chloroplast membrane lipids molecular species in C. reinhardtii WT and sta6 under high light (HL)
In general, the chloroplast membranes of C. reinhardtii are composed of the same lipid classes as the chloroplasts of plants and algae (Goss and Wilhelm, 2009). The chloroplast membranes of C. reinhardtii are enriched in uncharged galactolipids, MGDG and DGDG (Riekhof et al., 2003), corresponding to 60%-80% of total chloroplast lipids in oxygenic photosynthetic organisms and MGDG is the predominant one (Block et al., 1983; Riekhof et al., 2003; Sakurai et al., 2006). However, in this study, although galactolipids MGDG and DGDG are still the prevalent chloroplast lipids in both C. reinhardtii strains under LL and HL, DGDG became dominant in sta6 under HL (Fig.5). This result suggests that the MGDG/DGDG ratio was somehow changed in starchless mutant, probably to maintain the structure and stability of the thylakoid membranes under HL (Murphy, 1982; Demé et al., 2014), because DGDG is a typical bilayer forming lipids while MGDG tended non-lamellar and hexagonal structures due to its conelike molecular shape (Sen et al., 1981; Webb and Green, 1991).
Starch and lipids are two major energy storage forms in most unicellular green algae, especially under many stress conditions, such as high light. When the starch synthesis pathway was switched ofference , the metabolic shift of carbon fl ux away from starch synthesis into lipids synthesis pathways (Li et al., 2010b). It was found in this study that most chloroplast membrane lipids, except PG, showed higher concentrations in sta6 than in WT under difference erent light conditions, especially under HL. These results indicated that the formation of more thylakoid membranes and photosynthetic complexes might have occurred in the starchless mutant. Similar to two diatoms wild type strains Cyclotella meneghiniana and Phaeodactylum tricornutum (Lepetit et al., 2012), illumination of sta6 with HL leads to a further increase of the concentration of anionic lipid SQDG, as compared with WT.
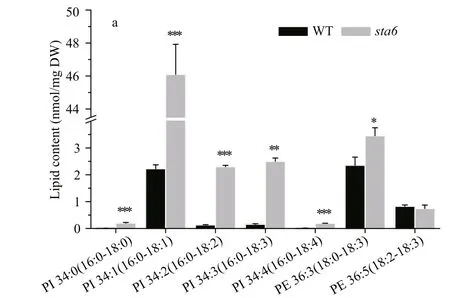
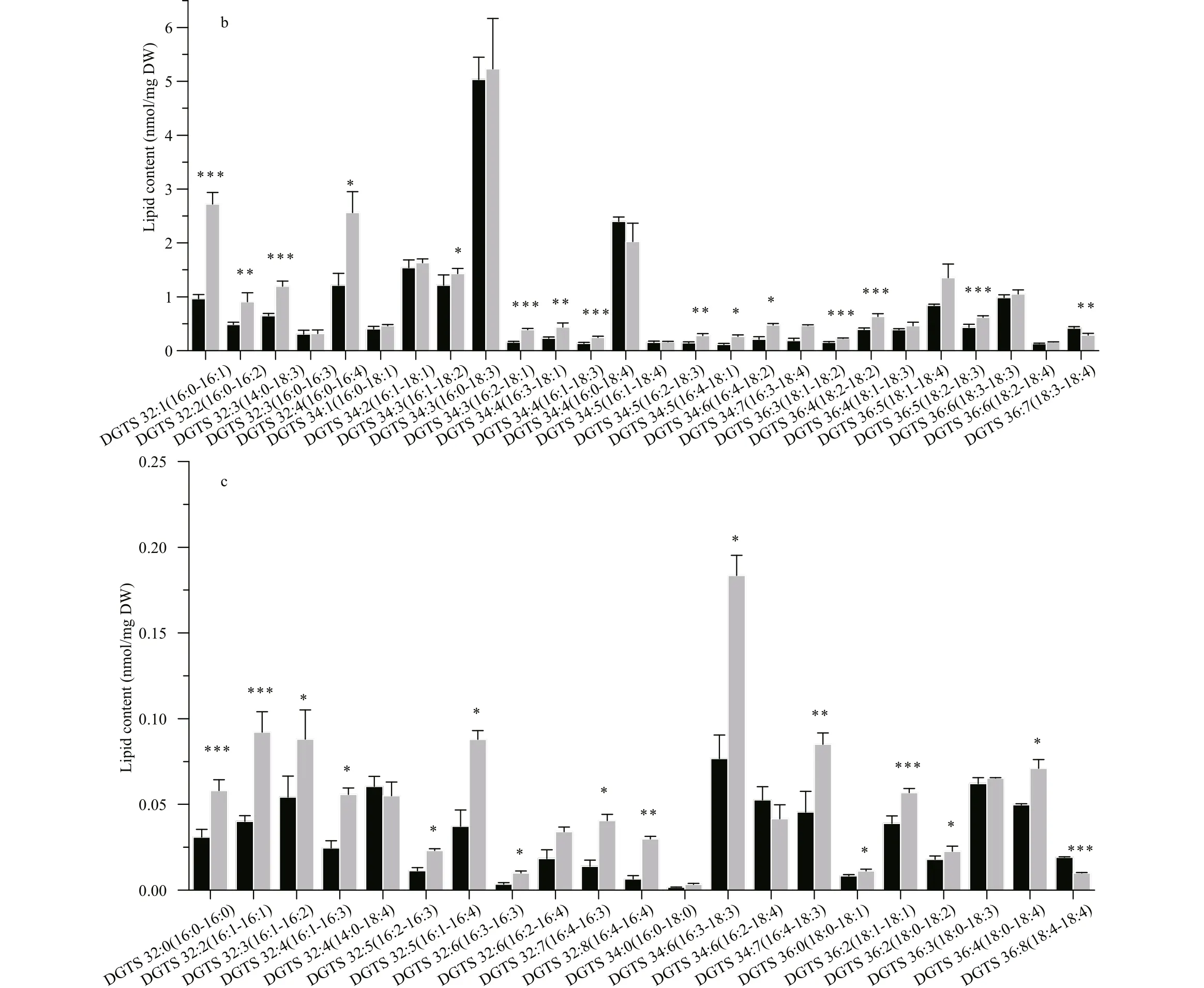
Fig.7 Contents of extraplastidic membrane lipids molecular species in C. reinhardtii WT and sta6 under high light (HL)
The betaine lipid DGTS have been identifi ed as the substitutes for phosphatidylcholine (PC) constituting the extraplastidic membranes in many green algae including C. reinhardtii (Dembitsky, 1996). However, we found that PI replaced DGTS and became the major extraplastidic lipids in sta6. Since PI can be phosphorylated into phosphatidylinositol phosphates, such as phosphatidylinositol 4,5-bisphospate (PI(4,5)P2), participating in signaling pathways (Boss and Im, 2012), we assumed the accumulated PI may play important role in signaling in sta6 to cope with profoundly changed metabolism caused by blocked starch biosynthesis.
5 CONCLUSION
To better understand the impact of blocking starch synthesis on lipid metabolism and homeostasis of C. reinhardtii, we have employed a UPLC-ESI-MS/MS method to qualitatively and quantitatively analyze the membrane lipids of WT and starchless mutant sta6. The results indicated that the lipid profi le of sta6 is similar to that of WT, but cellular content of lipids are dramatically difference erent between these two strains. In sta6, a large number of membrane lipids were upregulated under both LL and HL conditions, which indicated a great amount of photosynthetically-fi xed carbons were shunt into membrane lipid biosynthesis due to blockage of starch biosynthesis.
6 DATA AVAILABILITY STATEMENT
All data generated and/or analyzed during this study are included in this published article.
References
Barbaglia A M, Hofference mann-Benning S. 2016. Long-distance lipid signaling and its role in plant development and stress response. In: Nakamura Y, Li-Beisson Y eds. Lipids in Plant and Algae Development. Springer, Cham. p.339-361.
Blaby I K, Glaesener A G, Mettler T, Fitz-Gibbon S T, Gallaher S D, Liu B S, Boyle N R, Kropat J, Stitt M, Johnson S, Benning C, Pellegrini M, Casero D, Merchant S S. 2013. Systems-level analysis of nitrogen starvation-induced modifi cations of carbon metabolism in a Chlamydomonas reinhardtii starchless mutant. The Plant Cell, 25(11): 4 305-4 323, https://doi.org/10.1105/tpc.113.117580.
Block M A, Dorne A J, Joyard J, Douce R. 1983. Preparation and characterization of membrane fractions enriched in outer and inner envelope membranes from spinach chloroplasts. II. Biochemical characterization. Journal of Biological Chemistry, 258(21): 13 273-13 286.
Boss W F, Im Y J. 2012. Phosphoinositide signaling. Annual Review of Plant Biology, 63(1): 409-429, https://doi.org/10.1146/annurev-arplant-042110-103840.
Carrasco-Pancorbo A, Navas-Iglesias N, Cuadros-Rodríguez L. 2009. From lipid analysis towards lipidomics, a new challenge for the analytical chemistry of the 21st century. Part I: Modern lipid analysis. TrAC Trends in Analytical Chemistry, 28(3): 263-278, https://doi.org/10.1016/j.trac.2008.12.004.
Cutignano A, Luongo E, Nuzzo G, Pagano D, Manzo E, Sardo A, Fontana A. 2016. Profi ling of complex lipids in marine microalgae by UHPLC/tandem mass spectrometry. Algal Research, 17: 348-358, https://doi.org/10.1016/j.algal. 2016.05.016.
Da Costa E, Silva J, Mendonça S, Abreu M, Domingues M. 2016. Lipidomic approaches towards deciphering glycolipids from microalgae as a reservoir of bioactive lipids. Marine Drugs, 14(5): 101, https://doi.org/10.3390/md14050101.
Dembitsky V M. 1996. Betaine ether-linked glycerolipids: chemistry and biology. Progress in Lipid Research, 35(1): 1-51, https://doi.org/10.1016/0163-7827(95)00009-7.
Demé B, Cataye C, Block M, Maréchal E, Jouhet J. 2014. Contribution of galactoglycerolipids to the 3-dimensional architecture of thylakoids. The FASEB Journal, 28(8): 3 373-3 383, https://doi.org/10.1096/fj.13-247395.
Diehl B W K, Herling H, Riedl I, Heinz E. 1995.13C-NMR analysis of the positional distribution of fatty acids in plant glycolipids. Chemistry and Physics of Lipids, 77(2): 147-153, https://doi.org/10.1016/0009-3084(95)02462-r.
Fan J H, Zheng L H, Bai Y P, Saroussi S, Grossman A R. 2017. Flocculation of Chlamydomonas reinhardtii with difference erent phenotypic traits by metal cations and high pH. Frontiers in Plant Science, 8: 1 997, https://doi.org/10.3389/fpls. 2017.01997.
Gorman D S, Levine R P. 1965. Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardi. Proceedings of the National Academy of Sciences of the United States of America, 54(6): 1 665-1 669, https://doi.org/10.1073/pnas.54.6.1665.
Goss R, Wilhelm C. 2009. Lipids in algae, lichens and mosses. In: Wada H, Murata N eds. Lipids in Photosynthesis. Springer, Dordrecht. p.117-137.
Guschina I A, Harwood J L. 2006. Lipids and lipid metabolism in eukaryotic algae. Progress in Lipid Research, 45(2): 160-186, https://doi.org/10.1016/j.plipres.2006.01.001.
Han D X, Jia J, Li J, Sommerfeld M, Xu J, Hu Q. 2017. Metabolic remodeling of membrane glycerolipids in the microalga Nannochloropsis oceanica under nitrogen deprivation. Frontiers in Marine Science, 7: 402, https://doi.org/10.3389/fmars.2017.00242.
Han X L, Gross R W. 2003. Global analyses of cellular lipidomes directly from crude extracts of biological samples by ESI mass spectrometry: a bridge to lipidomics. Journal of Lipid Research, 44(6): 1 071-1 079, https://doi.org/10.1194/jlr.R300004-JLR200.
Han X L, Gross R W. 2005. Shotgun lipidomics: electrospray ionization mass spectrometric analysis and quantitation of cellular lipidomes directly from crude extracts of biological samples. Mass Spectrometry Reviews, 24(3): 367-412, https://doi.org/10.1002/mas.20023.
Harris E H. 2001. Chlamydomonas as a model organism. Annu Rev Plant Phys, 52(1): 363-406, https://doi.org/10.1146/annurev.arplant.52.1.363.
Harwood J L, Guschina I A. 2009. The versatility of algae and their lipid metabolism. Biochimie, 91(6): 679-684, https://doi.org/10.1016/j.biochi.2008.11.004.
Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, Darzins A. 2008. Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. The Plant Journal, 54(4): 621-639, https://doi.org/10.1111/j.1365-313X.2008.03492.x.
Klug R M, Benning C. 2001. Two enzymes of diacylglyceryl- O-4′-( N, N, N, -trimethyl) homoserine biosynthesis are encoded by btaA and btaB in the purple bacterium Rhodobacter sphaeroides. Proceedings of the National Academy of Sciences of the United States of America, 98(10): 5 910-5 915, https://doi.org/10.1073/pnas. 101037998.
Kobayashi K, Endo K, Wada H. 2016. Roles of lipids in photosynthesis. In: Nakamura Y, Li-Beisson Y eds. Lipids in Plant and Algae Development. Springer, Cham. p.21-49.
Krishnan A, Kumaraswamy G K, Vinyard D J, Gu H Y, Ananyev G, Posewitz M C, Dismukes G C. 2015. Metabolic and photosynthetic consequences of blocking starch biosynthesis in the green alga Chlamydomonas reinhardtii sta6 mutant. The Plant Journal, 81(6): 947-960, https://doi.org/10.1111/tpj.12783.
Lepetit B, Goss R, Jakob T, Wilhelm C. 2012. Molecular dynamics of the diatom thylakoid membrane under difference erent light conditions. Photosynthesis Research, 111(1-2): 245-257, https://doi.org/10.1007/s11120-011-9633-5.
Li Y T, Han D X, Hu G R, Dauvillee D, Sommerfeld M, Ball S, Hu Q. 2010a. Chlamydomonas starchless mutant defective in ADP-glucose pyrophosphorylase hyperaccumulates triacylglycerol. Metabolic Engineering, 12(4): 387-391, https://doi.org/10.1016/j.ymben.2010. 02.002.
Li Y T, Han D X, Hu G R, Sommerfeld M, Hu Q. 2010b. Inhibition of starch synthesis results in overproduction of lipids in Chlamydomonas reinhardtii. Biotechnology and Bioengineering, 107(2): 258-268, https://doi.org/10.1002/bit.22807.
Li-Beisson Y, Beisson F, Riekhof W. 2015. Metabolism of acyl-lipids in Chlamydomonas reinhardtii. The Plant Journal, 82(3): 504-522, https://doi.org/10.1111/tpj. 12787.
Merchant S S, Kropat J, Liu B S, Shaw J, Warakanont J. 2012. TAG, you’re it! Chlamydomonas as a reference organism for understanding algal triacylglycerol accumulation. Current Opinion in Biotechnology, 23(3): 352-363, https://doi.org/10.1016/j.copbio.2011.12.001.
Moellering E R, Miller R, Benning C. 2009. Molecular genetics of lipid metabolism in the model green alga Chlamydomonas reinhardtii. In: Wada H, Murata N eds. Lipids in Photosynthesis. Springer, Dordrecht. p.139-155.
Murphy D J. 1982. The importance of non-planar bilayer regions in photosynthetic membranes and their stabilisation by galactolipids. FEBS Letters, 150(1): 19-26, https://doi.org/10.1016/0014-5793(82)81297-0.
Řezanka T, Lukavský J, Vítová M, Nedbalová L, Sigler K. 2018. Lipidomic analysis of Botryococcus (Trebouxiophyceae, Chlorophyta)—Identifi cation of lipid classes containing very long chain fatty acids by oラ ine two-dimensional LC-tandem MS. Phytochemistry, 148: 29-38, https://doi.org/10.1016/j.phytochem.2018.01.011.
Riekhof W R, Ruckle M E, Lydic T A, Sears B B, Benning C. 2003. The sulfolipids 2’- O-acyl- sulfoquinovosyldiacylglycerol and sulfoquinovosyldiacylglycerol are absent from a Chlamydomonas reinhardtii mutant deleted in SQD1. Plant Physiology, 133(2): 864-874, https://doi.org/10.1104/pp.103.029249.
Sakurai I, Shen J R, Leng J, Ohashi S, Kobayashi M, Wada H. 2006. Lipids in oxygen-evolving photosystem II complexes of cyanobacteria and higher plants. The Journal of Biochemistry, 140(2): 201-209, https://doi.org/10.1093/jb/mvj141.
Sen A, Williams W P, Quinn P J. 1981. The structure and thermotropic properties of pure 1, 2-diacylgalactosylglycerols in aqueous systems. Biochimica et Biophysica Acta ( BBA)- Lipids and Lipid Metabolism, 663(2): 380-389, https://doi.org/10.1016/0005-2760(81)90167-3.
Siaut M, Cuiné S, Cagnon C, Fessler B, Nguyen M, Carrier P, Beyly A, Beisson F, Triantaphylidès C, Li-Beisson Y, Peltier G. 2011. Oil accumulation in the model green alga Chlamydomonas reinhardtii: characterization, variability between common laboratory strains and relationship with starch reserves. BMC Biotechnology, 11: 7, https://doi.org/10.1186/1472-6750-11-7.
Solovchenko A E, Khozin-Goldberg I, Didi-Cohen S, Cohen Z, Merzlyak M N. 2008. Efference ects of light intensity and nitrogen starvation on growth, total fatty acids and arachidonic acid in the green microalga Parietochloris incisa. Journal of Applied Phycology, 20(3): 245-251, https://doi.org/10.1007/s10811-007-9233-0.
Sueoka N. 1960. Mitotic replication of deoxyribonucleic acid in Chlamydomonas reinhardi. Proceedings of the National Academy of Sciences of the United States of America, 46(1): 83-91, https://doi.org/10.1073/pnas.46.1.83.
Tran Q G, Cho K, Park S B, Kim U, Lee Y J, Kim H S. 2019. Impairment of starch biosynthesis results in elevated oxidative stress and autophagy activity in Chlamydomonas reinhardtii. Scientifi c Reports, 9: 9 856, https://doi.org/10.1038/s41598-019-46313-6.
UPLC-oaTOF-MS for lipid analysis in complex biological mixtures: A new tool for lipidomics. Journal of Proteome Research, 6(2): 552-558, https://doi.org/10.1021/pr060611b.
Wada H, Murata N. 2007. The essential role of phosphatidylglycerol in photosynthesis. Photosynthesis Research, 92(2): 205-215, https://doi.org/10.1007/s11120-007-9203-z.
Wang B B, Zhang Z, Hu Q, Sommerfeld M, Lu Y H, Han D X. 2014. Cellular capacities for high-light acclimation and changing lipid profi les across life cycle stages of the green alga Haematococcus pluvialis. PLoS One, 9(9): e106679, https://doi.org/10.1371/journal.pone.0106679.
Wang Z, Benning C. 2011. Arabidopsis thaliana polar glycerolipid profi ling by thin layer chromatography (TLC) coupled with gas-liquid chromatography (GLC). Journal of Visualized Experiments, (49): 2 518, https://doi.org/10.3791/2518.
Webb M S, Green B R. 1991. Biochemical and biophysical properties of thylakoid acyl lipids. Biochimica et Biophysica Acta ( BBA)- Bioenergetics, 1060(2): 133-158, https://doi.org/10.1016/S0005-2728(09)91002-7.
Welti R, Wang X M, Williams T D. 2003. Electrospray ionization tandem mass spectrometry scan modes for plant chloroplast lipids. Analytical Biochemistry, 314(1): 149-152, https://doi.org/10.1016/S0003-2697(02)00623-1.
Xu Y N, Wang Z N, Yan X J, Lin W, Li L B, Kuang T Y. 2002. Positional distribution of fatty acids on the glycerol backbone during the biosynthesis of glycerolipids in Ectocarpus fasciculatus. Chinese Science Bulletin, 47(21): 1 802-1 806, https://doi.org/10.1007/BF03183846.
Yang D W, Song D H, Kind T, Ma Y, Hoefkens J, Fiehn O. 2015. Lipidomic analysis of Chlamydomonas reinhardtii under nitrogen and sulfur deprivation. PLoS O ne, 10(9): e0137948, https://doi.org/10.1371/journal.pone.0137948.
Yoon K, Han D X, Li Y T, Sommerfeld M, Hu Q. 2012. Phospholipid: diacylglycerol acyltransferase is a multifunctional enzyme involved in membrane lipid turnover and degradation while synthesizing triacylglycerol in the unicellular green microalga Chlamydomonas reinhardtii. The Plant Cell, 24(9): 3 708-3 724, https://doi.org/10.1105/tpc.112.100701.
Zabawinski C, Van Den Koornhuyse N, D'Hulst C, Schlichting R, Giersch C, Delrue B, Lacroix J M, Preiss J, Ball S. 2001. Starchless mutants of Chlamydomonas reinhardtii lack the small subunit of a heterotetrameric ADP-glucose pyrophosphorylase. Journal of Bacteriology, 183(3): 1 069-1 077, https://doi.org/10.1128/jb.183.3.1069-1077. 2001.
 Journal of Oceanology and Limnology2020年3期
Journal of Oceanology and Limnology2020年3期
- Journal of Oceanology and Limnology的其它文章
- List of the Most Outstanding Papers Published by CJOL/JOL in 2017-2018
- Who is the “murderer” of the bloom in coastal waters of Fujian, China, in 2019?*
- The investigation of internal solitary waves over a continental shelf-slope*
- Efference ect of diets on the feeding behavior and physiological properties of suspension-feeding sea cucumber Cucumaria frondosa*
- Efference ects of light quality on growth rates and pigments of Chaetoceros gracilis (Bacillariophyceae)*
- Marine bacterial surfactin CS30-2 induced necrosis-like cell death in Huh7.5 liver cancer cells*
