Moringa oleifera leaf ethanol extract ameliorates lead-induced hepato-nephrotoxicity in rabbits
Nancy B.Mohamed, Amira H.Mohamed, Nashwa A.Abu-Aita, Soad M.Nasr, Somia A.Nassar, Kawkab A.Ahmed
1Directorate of Veterinary Medicine, 3 Teraet El Zomor St., Nasr El Din, El haram, Giza, Egypt
2Department of Clinical Pathology, Faculty of Veterinary Medicine, Cairo University, Giza, 12211, Egypt
3Department of Parasitology & Animal Diseases, National Research Centre, 33 Bohouth St., Dokki, Giza, 12622, Egypt
4Department of Laboratory Medical Sciences, College of Applied Medical Sciences; Prince Sattam bin Abdulaziz University, Saudi Arabia
5Department of Pathology, Faculty of Veterinary Medicine, Cairo University, Giza, 12211, Egypt
ABSTRACT
KEYWORDS: Lead toxicity; Moringa oleifera; Rabbits; Liver functions; Kidney functions; Histopathology
1.Introduction
Lead (Pb) has been extensively used in different industries; it is one of the most influential air pollutants, and it is toxic even in low concentrations causing physiological, mental and histological disorders[1].Lead is the well-elaborated heavy toxic metals and its biological effect is based on the concentration and time of the exposure.Exposure to environmentally harmful doses of lead is one of the main health risk factors concerning human and animal populations, particularly children and infants who are more at risk than adults due to their lowered tolerance and immature immune systems[2].Lead-intoxication induces hematopoietic and hematological alterations, cardiac impairment, and dysfunction of kidney and liver[3,4].Lead toxicity induces reactive oxygen species and can cause genotoxicity resulting from induction of cellular oxidative stress, inhibition of DNA metabolism[5], and metabolic and reproductive disturbances[6].
Medicinal plants are promising sources of drugs used for the treatment of many diseases.Form these plants, Moringa oleifera(M.oleifera), a native plant to India, is multi-nutrient with multiple medicinal uses.It is widely cultivated and mainly distributed in most of Southeast Asian countries, Australia, Egypt, Tropical Africa, and Mexico[7].M.oleifera contains important minerals(potassium, calcium, and iron) and is a good source of protein,vitamins (vitamin A, vitamin C), and amino acids[8].M.oleifera leaves also contain large amounts of various phenolics (flavonoids,anthocyanins, proanthocyanidin, and cinnamates), glucosinolates,flavonol glycosides (glucosides, rutinosides, and malonyl glucosides), quercetin, kaempferol, and many other phytochemical substances[8,9].Different parts of the plant such as leaves, roots, seed,bark, fruits,flowers and immature pods act as cardiac and circulatory stimulants and possess anticancer effects[10].Other main medicinal characteristics of this plant include hypoglycemic[11], cholesterollowering, antioxidant, and hepatoprotective effects[9,12].
Although the extracts of different parts of M.oleifera have been tested against liver and kidney toxicity in different models, its effectiveness in the treatment of lead-induced hepato-renal toxicity has not yet been investigated.Therefore, the aim of this study was to evaluate the effect of M.oleifera ethanol extract as an adjunct treatment on lead acetate induced hepato-nephrotoxicity in male rabbits.
2.Materials and methods
2.1.Preparation of ethanol extract of M.oleifera leaves
M.oleifera leaves (Family: Moringaceae) were obtained from the Egyptian Scientific Society for Moringa at National Research Centre, Dokki, Giza where the plant was identified by Professor Dr.Aboelfetoh M.Abdalla, and a voucher sample number (MO19) was kept in the Horticulture and Crops Technology Department, National Research Centre, Egypt.
M.oleifera leaves were air-dried and ground to coarse powder.Then 50 g of the powdered plant was macerated several times with 500 mL ethanol 70% at room temperature with stirring for 72 h and filtered using filter paper Whatman No.1.The extract was concentrated using a rotary evaporator (Heidolph 2000, Germany) under reduced pressure and at 40 ℃.The extract was placed into evaporating dishes to dry at room temperature under normal atmospheric pressure and stored at -4 ℃ for further use.The extract yield was (w/w) 8.75g(17.50%).
2.2.Lead acetate
Lead (Ⅱ) acetate trihydrate [Pb(CH3CO2)2·3H2O, molecular weight: 379.33 g/mol] was purchased from Sigma-Aldrich (Merck),Germany.It was dissolved in distilled water (DH2O) at a dose of 40 mg/mL for oral administration.The dose of lead acetate [40 mg/kg body weight (b.w.)/day, 5 days/week for 8 weeks] was selected according to the pilot experiment which was assessed before the experiment to confirm the time when intoxication signs appeared on rabbits after administration of lead acetate.Five rabbits were administered 40 mg/kg b.w.lead acetate/day, 5 days/week for 8 weeks orally.Liver and kidney function tests and histopathological examinations were performed every 15 days.A typical sign of lead acetate intoxication was recorded after 8 weeks which was confirmed by significant increases of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and creatinine in the serum of animals.Histopathological alterations were also observed in the liver and kidneys.
2.3.Animal
Thirty-six male white New Zealand rabbits, weighing 1-1.5 kg and aged 4-6 weeks, were purchased from a farm belonging to Animal Production Institute, Ministry of Agriculture, Dokki, Giza.
2.4.Ethics statement
This experiment was carried out according to the guidelines of the Institutional Animal Care and Use Committee, Cairo University, and the protocol approval No.: CU-II-F-94-18 on 11/2018.
2.5.Experimental design
The experiment was carried out at the Experimental Rabbit Unit of Lab Animal House, National Research Center, Dokki, Giza, Egypt.Rabbits were housed under hygienic conditions in separate stainless steel cages at a controlled temperature (23±2) ℃, with 12 h light/dark cycle, and relative humidity of 50%-60%.All rabbits were fed a balanced growing rabbit commercial pelleted diet, with ad libitum fresh tap water.After two weeks of acclimatization period,a total of 36 rabbits were divided randomly into two main groups:normal control (14 rabbits) and lead intoxicated group (22 rabbits).Blood samples were taken from five animals and three animals of each group were sacrificed at the 4th, 8th, 10th and 12th week of the experiment for tissue sampling.The first group as a normal control group received phosphate-buffered saline at a dose of 1 mL/kg b.w.The second group was administered orally with lead acetate at a dose of 40 mg/kg b.w./day (5 days/week) for 8 weeks.The criteria of lead acetate intoxication signs (from clinical sign and biochemical analysis) included loss of appetite, depression (rabbits often appeared as limited movement and a low response to normal stimuli or fear), lethargy and hair loss.At the 4th and 8th week of treatment,6 animals (3 animals each period) were randomly selected and sacrificed while the remaining animals (16 rabbits) were assigned randomly into 2 subgroups (8 rabbits each): treated and non-treated.The 1st subgroup (non-treated) was orally given 1 mL phosphatebuffered saline for further 4 weeks while the 2nd subgroup (treated)was administered orally with M.oleifera leaf ethanol extract freshly dissolved in phosphate buffer saline at a dose of 400 mg/kg b.w./day for 4 weeks.The dose of M.oleifera ethanol extract (400 mg/kg b.w./day for 4 weeks) was selected as previously described[11]based on acceptable safety and efficacy in a similar experiment.All experimental animals were kept under close observation for abnormal clinical signs and mortalities.Body weight was recorded before the start of the experiment (zero time) and every 2 weeks thereafter.The body weight gain was calculated by deducting the weight of the animal at the start of the experiment (zero time) from the actual body weight gained at the 2nd, 4th, 6th and 8th week of the experiment.Body weights of rabbits at the 10th and 12th week were compared to that at the 8th week of the experiment.
2.6.Sampling
2.6.1.Blood samples
Blood samples (5 mL) were collected by the ear-vein puncture early in the morning before feeding from 5 rabbits randomly selected from all groups (normal control, lead-intoxicated, and M.oleifera ethanol extract-treated groups) at the 0, 4th, 8th, 10th and 12th week of the experiment.One mL blood sample was placed into EDTA anticoagulant tubes for the hematological investigations, and the remaining 4 mL was left to clot in plain centrifuge tubes for serum separation.The serum samples were stored at -20 ℃ for further biochemical analysis.
2.6.2.Liver and kidney tissue samples
From the normal control (at the 4th, 8th, 10th, and 12th week), lead intoxicated (at the 4th and 8th week), as well as 2 lead intoxicated subgroups (non-treated and treated) (at the 10th and 12th week), 3 rabbits were randomly selected and sacrificed.Tissue specimens from the liver and kidneys were obtained for histopathological examinations and determination of antioxidant markers in the liver tissue.
2.7.Analytical methods
2.7.1.Hematological examination
Complete blood count was evaluated in blood samples of rabbits using a hematological analyzer (Exigo Vet, Sweden).
Erythrogram included red blood cell count (RBC), hematocrit (HCT),hemoglobin (Hb) concentration and red cell indices; mean corpuscular volume (MCV), and mean corpuscular hemoglobin concentration(MCHC).Leukogram included leukocyte, lymphocytes, monocytes,granulocytes and platelet count (PLT).
2.7.2.Serum biochemical analysis
Activities of ALT, AST, and alkaline phosphatase (ALP) and the level of blood glucose, creatinine, urea, total cholesterol (TC), triglycerides(TG), high-density lipoprotein cholesterol (HDL-C), total proteins,and albumin were determined spectrophotometrically (T80 UV/VIS PG instrument Ltd, UK) using commercial Test kits (Biodiagnostic Co, Egypt) according to the manufacturer’ instructions.Low-density lipoprotein cholesterol (LDL-C) was calculated according to the Friedewald equation[13].Total globulins were calculated by subtracting the obtained value of albumin from the total proteins.
2.7.3.Histopathological studies
Specimens from the liver and kidney collected from all experimental groups were fixed in neutral buffered formalin 10%, washed, dehydrated, cleared and embedded in paraffin.Paraffin blocks were sectioned at 4-5 μm thickness and stained with hematoxylin and eosin (H&E)[14].The slides were examined by a light microscope (Olympus BX50, Japan) under high power magnification (×400) for histopathological examination.
2.7.4.Oxidant/antioxidant parameters
A portion of liver tissue was weighed, washed and homogenized with ice-cold normal saline (10% w/v) using a homogenizer (Witeg,Germany).The homogenate was then centrifuged at 2 773 ×g for 5 min at 4 ℃ using a cooling centrifuge (Sigma 3-18KS, Germany).The supernatant was collected and stored at -80 ℃ to determine the levels of reduced glutathione (GSH)[15] and malondialdehyde(MDA) level[16].
2.8.Statistical analysis
All data were expressed as mean±SD.The data were normally distributed and the differences between normal control and lead intoxicated groups at the 0, 4th and 8th week of the experiment were analyzed using the Student’s t-test.At the 10th and the 12th week,differences between the normal control and the treated groups were tested for significance using one-way analysis of variance (ANOVA)followed by Duncan’s multiple range test using Statistical Package for Social Science (SPSS) software version 17 computer program(SPSS Inc, Chicago, IL, USA).The difference was considered significant at P<0.05.
3.Results
3.1.Symptoms of lead intoxication
Rabbits exposed to lead acetate showed loss of appetite, depression(rabbits often appeared as limited movement and a low response to normal stimuli or fear), lethargy and the hair was easily detached.At the end of the experiment, two animals died after blood collection from the lead-intoxicated group and no mortalities occurred in the other groups during the experimental periods.
3.2.Body weight gain
The mean body weight gain of the lead acetate-intoxicated animals decreased significantly (P<0.01) at the 2nd, 4th, 6th and 8th week after lead acetate exposure compared to the control group.After treatment with M.oleifera ethanol extract, body weight gain was still lower than that of the control group (Figure 1).
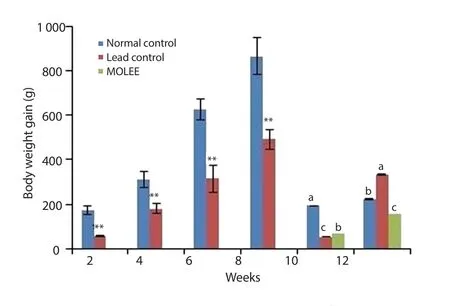
Figure 1.Body weight gain of male rabbits (Mean±SD).**P<0.01 compared with the normal control group.Means with different letters (a, b, c) analyzed by ANOVA test at the same period are significant at P<0.05.MOLEE:Moringa oleifera leaf ethanol extract.
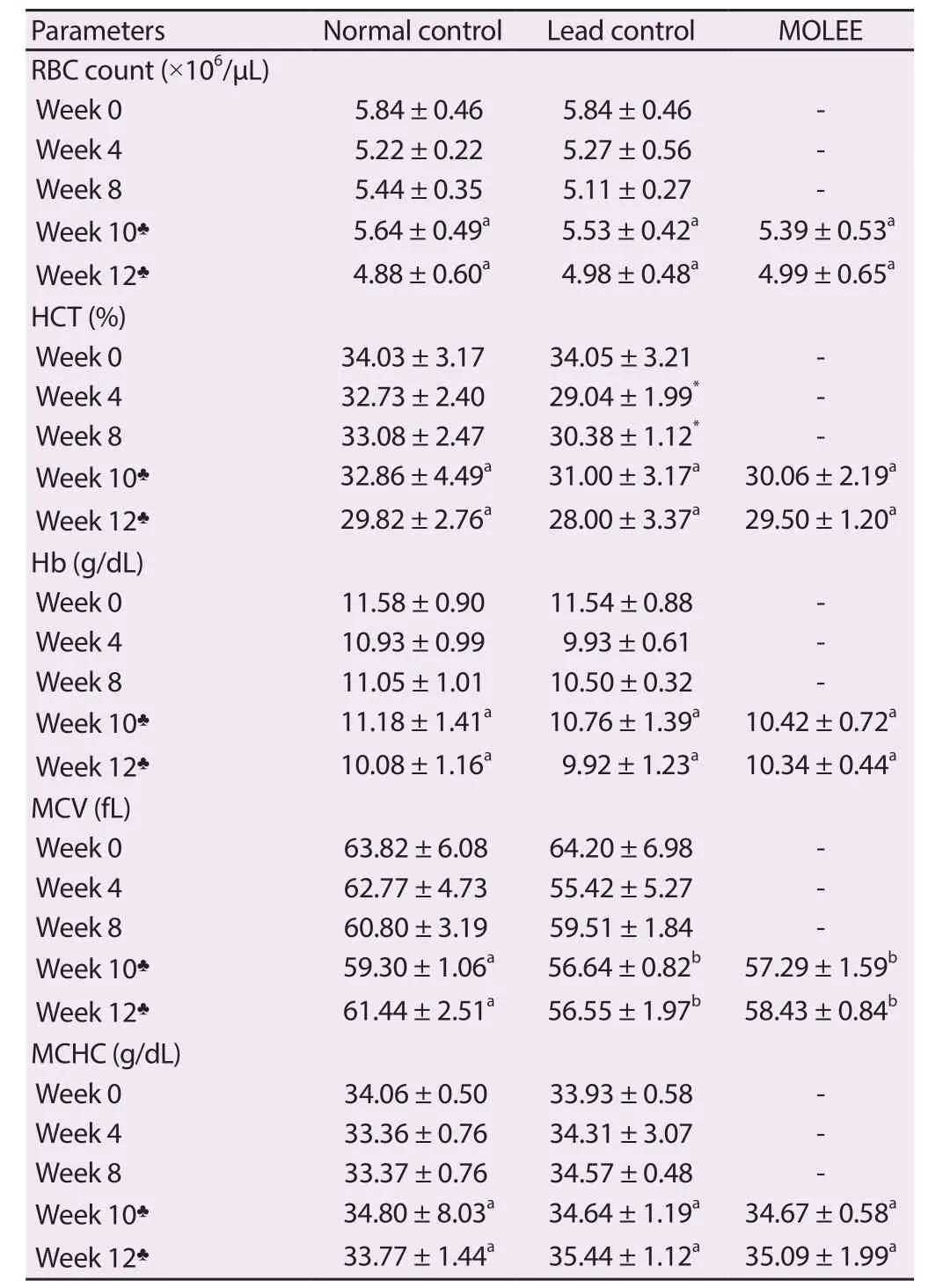
Table 1.Erythrogram of male rabbits (Mean ± SD).
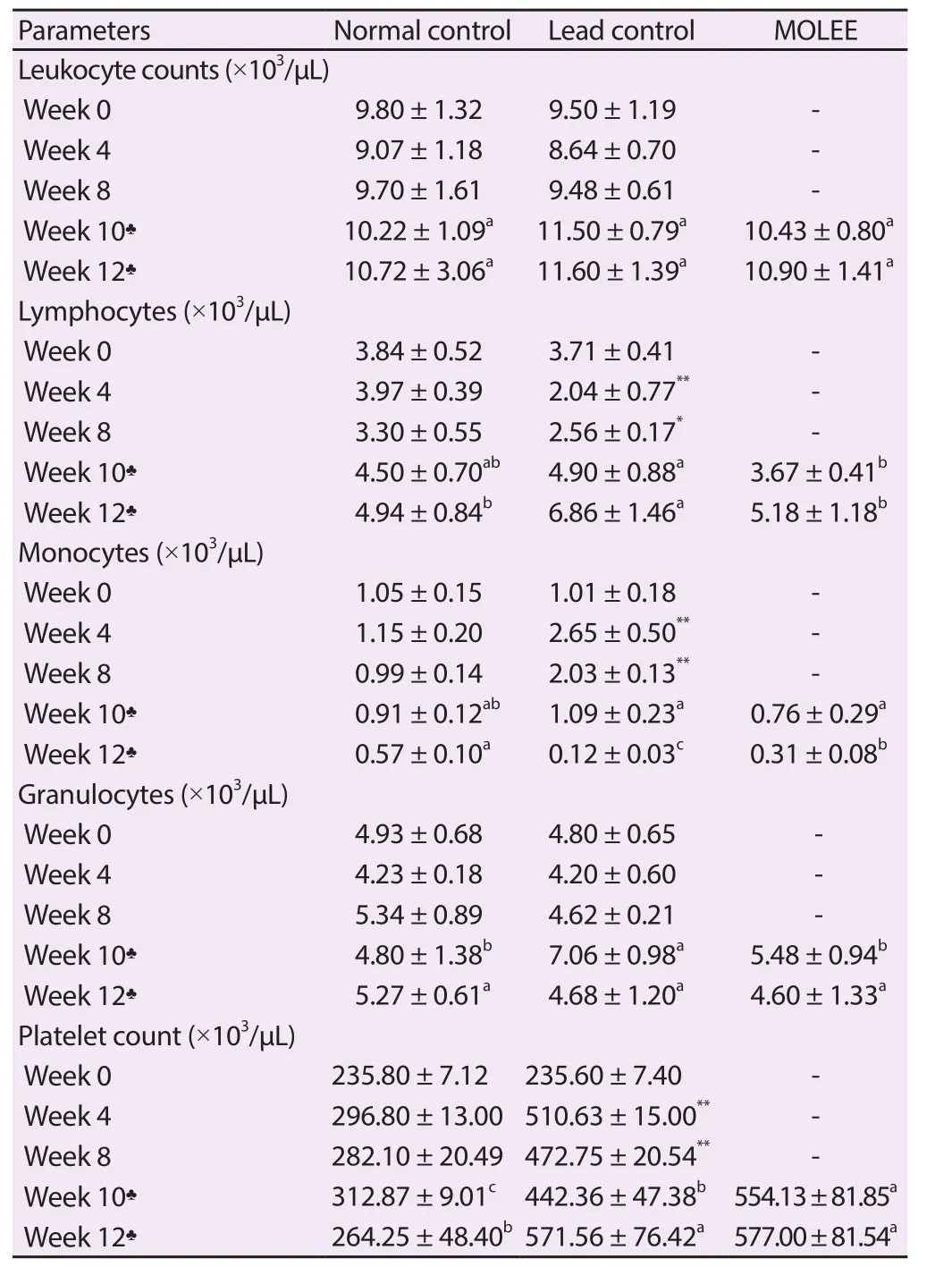
Table 2.Leukogram and platelets of male rabbits (Mean ± SD).
3.3.Hematological findings
No significant changes were observed in RBC, Hb, MCV, or MCHC, while HCT levels were decreased at the 4th and 8th week after lead exposure (Table 1).
The results revealed that there was no change in the leukocyte count.However, there was a significant (P<0.05) decrease in lymphocyte count after 4 and 8 weeks of lead exposure which was associated with a significant (P<0.01) increase in monocyte count.After treatment with M.oleifera ethanol extract, the level of leukocyte was restored (Table 2).In addition, PLT was significantly (P<0.01)increased in the lead acetate-intoxicated animals after 4 and 8 weeks of lead exposure compared with the control group, which was still high after treatment of M.oleifera ethanol extract (Table 2).
3.4.Serum biochemical findings
3.4.1.Effect of lead intoxication on liver functions
At the 10th week of the experiment, there was a significant(P<0.05) increase of total proteins in the serum of lead-intoxicated and M.oleifera extract-treated groups compared with the normal control group.Moreover, total proteins and globulins persisted at higher levels in the M.oleifera extract-treated group compared with both the normal control and lead-intoxicated groups at the 12th week of the experiment (Table 3).
Lead acetate significantly (P<0.01) elevated ALT, AST and ALP activities, which were higher than those of the normal control and M.oleifera extract-treated groups (Figure 2).
At the 8th and 10th week, blood glucose level was significantly(P<0.01) increased in the lead-exposed rabbits compared with the normal control and M.oleifera extract-treated groups.A significant increase (P<0.01) was observed in the blood glucose level in M.oleifera extract-treated rabbits at the 12th week (Figure 2).
In addition, lead acetate caused a significant (P<0.05) increase in lipid profile including TC, TG, and LDL-C at the 4th and the 8th week of the exposure.The lipid profile was significantly improved after treatment with ethanol extract of M.oleifera leaf (Figure 3).
The oxidant/antioxidant results in liver tissues revealed that MDA levels were markedly (P<0.01) increased with a significant (P<0.01)decrease of GSH level in the lead-intoxicated group after the 4th and the 8th week of the experiment, which continued at the 10th and 12th week of the experiment after cessation of lead exposure.After treatment with M.oleifera extract, MDA was still at a relatively high level at the 10th week of the experiment, but returned to its normal level at the 12th week, while GSH level was restored at the 10th week (Figure 3).
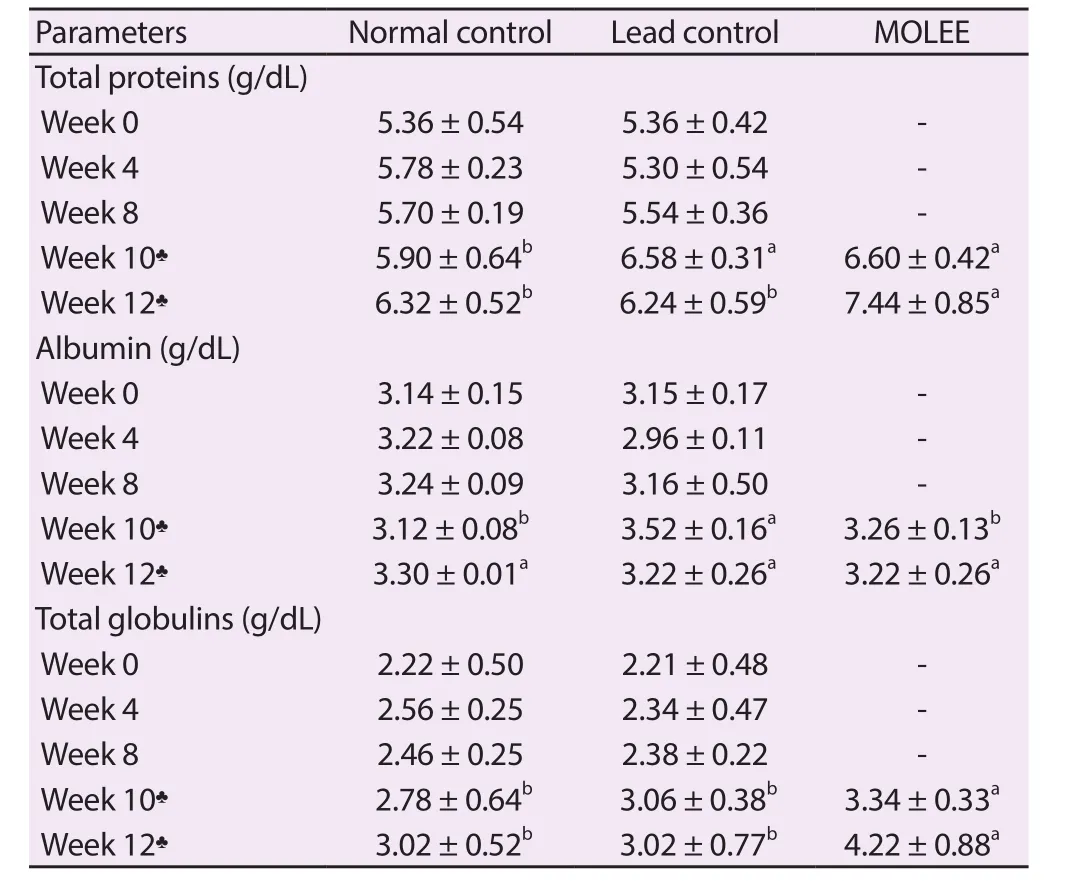
Table 3.Serum protein profile of male rabbits (Mean ± SD).
3.4.2.Effect of lead intoxication on kidney functions
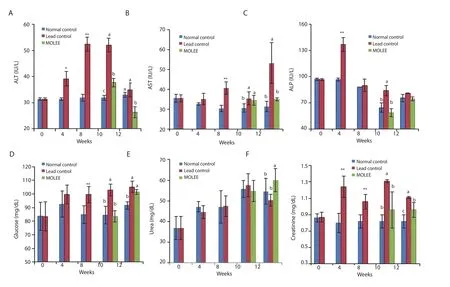
Figure 2.Alanine aminotransferase (ALT) (A), aspartate aminotransferase (AST) (B), alkaline phosphatase (ALP) (C), glucose (D), urea (E), and creatinine (F)levels in the serum of male rabbits (Mean±SD).*P<0.05, **P<0.01 compared with the normal control group.Means with different letters (a, b, c) at the same period are significant at P<0.05.
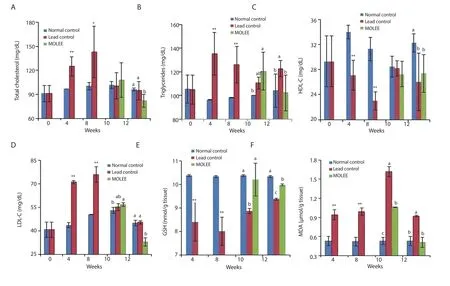
Figure 3.Lipid profile (A-D) [total cholesterol, triglycerides, high density lipoprotein-cholesterol (HDL-C) and low density lipoprotein-cholesterol (LDL-C)],reduced glutathione (GSH) (E), and malondialdehyde (MDA) (F) levels in the serum of male rabbits (Mean±SD).*P<0.05, **P<0.01 compared with the normal control group.Means with different letters (a, b, c) at the same period are significant at P<0.05.
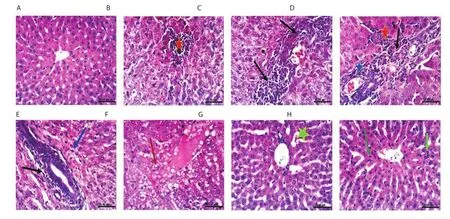
Figure 4.Histopathological photographs of liver sections.The normal control group (A); the lead-intoxicated group at the 4th (B & C), 8th (D), 10th (E)and 12th week (F); Moringa oleifera extract-treated group at the 10th (G) and 12th week (H) (H&E, scale bar: 25 μm).Focal hepatic necrosis (red asterisk);fi broplasia in the portal triad (black arrow); cholangitis (blue asterisk); slight hydropic degeneration of hepatocytes (blue arrow); cytoplasmic vacuolization of hepatocytes (red arrow); slight congestion of hepatic sinusoids (light green asterisk); a slight increase of Kupffer cells (green arrow); binucleation of hepatocytes (light green arrow).
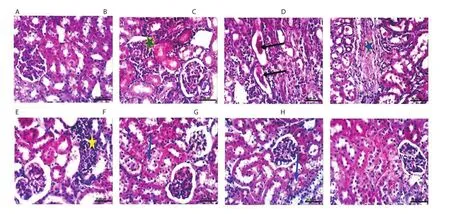
Figure 5.Histopathological photographs of kidney sections.The normal control group (A); the lead-intoxicated group at the 4th (B & C), 8th (D), 10th (E) and 12th week (F); Moringa oleifera extract-treated group at the 10th (G), and 12th week (H) (H&E, scale bar: 25 μm).Interstitial nephritis (green asterisk); cystic dilatation of renal tubules with renal casts in their lumen (black arrow); chronic interstitial nephritis and interstitial collagen fiber deposition (blue asterisk);focal interstitial nephritis (yellow asterisk); slight vacuolation of epithelial lining some renal tubules (blue arrow).
Lead intoxication also increased creatinine level which still persisted at high levels even at the 10th and 12th week.Treatment with M.oleifera extract did not significantly affect the creatinine level as compared with normal control animals at the 10th week(Figure 2).In addition, at the 12th week, serum urea levels were significantly (P<0.05) elevated in the M.oleifera extract-treated group in comparison with both the control and lead-intoxicated groups (Figure 2).
3.5.Pathological findings
3.5.1.Liver
Macroscopic examination revealed that after 12 weeks of the experiment, macroscopic photographs of livers of lead intoxicated rabbits showed enlargement and damage in architecture.In M.oleifera extract-treated group, the livers showed intact and normal architecture.Normal histological structure was also observed in the liver of the normal control group (Figure 4A).At the 4th week (Figure 4B and C), 8th week (Figure 4D) and 10th week (Figure 4E), focal hepatic necrosis (red asterisk) associated with inflammatory cells infiltration, fibroplasia in the portal triad (black arrow), cholangitis(blue asterisk), and slight hydropic degeneration of hepatocytes (blue arrow) were observed in the livers of lead-intoxicated group.At the 12th week (Figure 4F), cytoplasmic vacuolization of hepatocytes (red arrow) was seen in the livers of lead-intoxicated group.M.oleifera extract-treated group showed slight congestion of hepatic sinusoids(light green asterisk) at the 10th week (Figure 4G).At the 12th week,a slight increase of Kupffer cells (green arrow) and binucleation of hepatocytes (light green arrow) were seen in the liver of M.oleifera extract-treated rabbits (Figure 4H).
3.5.2.Kidney
Macroscopic examination revealed that after 12 weeks of the experiment, the kidneys showed enlargement and pale cortex.In M.oleifera extract-treated group, the kidneys showed slight enlargement.Normal structure was observed in the kidney of the normal control group (Figure 5A).At the 4th week, kidneys of the leadintoxicated group showed interstitial nephritis (green asterisk)(Figure 5B), and cystic dilatation of renal tubules with renal casts in their lumen (black arrow) (Figure 5C).At the 8th week, chronic interstitial nephritis, and interstitial collagen fiber deposition (blue asterisk) were noticed in the kidneys of the lead-intoxicated group(Figure 5D).At the 10th week, focal interstitial nephritis (yellow asterisk) was observed in the kidneys of the lead-intoxicated group(Figure 5E).Kidneys of lead intoxicated and M.oleifera extracttreated groups showed slight vacuolation of epithelial lining some renal tubules (blue arrow) at the 12th and 10th week, respectively(Figure 5F and G).No histopathological alterations were observed in kidneys of M.oleifera extract-treated rabbits at the 12th week (Figure 5H).
4.Discussion
The present study assessed the effect of M.oleifera ethanol extract on lead-induced toxicity in rabbits.In this study, the decreased daily body weight gain in rabbits exposed to lead acetate may be due to loss of appetite, detrimental effect on the nervous, hematopoietic and cardiovascular system, and liver and kidney functions, as well as the loss of minerals (Ca, Zn, Cu, Se, and Mg) from the body[3].Decreased body weight was recorded in male rats administered 0.1% lead acetate for 11 months[17].The decrease in body weight gain may be correlated with imbalanced metabolism produced by reducing zinc-dependent enzymes which are important for metabolic activities; and/or decreased level of erythropoietin hormone which has a negative anabolic effect by lead[18].The decreased body weight may also be due to mal-absorption of nutrients by the direct toxic effect of lead on the gastrointestinal tract or by impairing protein synthesis[19].
The reported decreases in HCT and MCV values as a result of lead intoxication may be attributed to decreases in copper metabolism and iron consumption[3].These effects were reversible after cessation of lead exposure, similarly as it has been previously reported after the duration of recovery in rabbits[20].After treatment with M.oleifera ethanol extract, improvement occurred.Disturbance of intestinal absorption of some essential trace elements involved in RBCs activities by lead has been previously suggested[21], and cessation of lead exposure may lead to improvement of affected parameters by improving the absorption of these trace elements.The present results referred that anemia induced by lead is of the microcytic type which might be due to decreased Hb production.Lead inhibits ferrochelatase, the enzyme that catalyzes the integration of iron into the porphyrin ring leading to decreased iron integration in Hb[22].Treatment with M.oleifera ethanol extract improved HCT and MCV values, but we could not confirm if the improvement in HCT and MCV values is due to cessation of administration of lead or M.oleifera ethanol extract.
There was no change in leukocyte count which agrees with that of Alqayim and Asis[23].Lead intoxication required more phagocytic functions which accelerate the damaging effects of the reactive oxygen species on living systems[24].The decrease of lymphocytes may be attributed to lead toxicity which causes the depletion of lymphoid tissue and consequently decreases the production of lymphocytes[25].After treatment with M.oleifera ethanol extract, the lymphocytes and monocytes counts were normalized.This effect may be due to the presence of arginine, which is one of the nonessential amino acids in Moringa that is known to escalate cellular immunity and boost lymphocyte and macrophage proliferations[26].The platelet count was significantly increased by lead intoxication,which could be due to hyperstimulation of thrombopoiesis in the bone marrow responding to the loss of peripheral platelets caused by increased platelet aggregation and adhesion[3].After treatment with the extract, the platelet count was still high.The increased platelets in M.oleifera ethanol extract-treated rabbits may be ascribed to its high content of amino acids, vitamins, minerals (such as iron and copper) and antioxidants (such as vitamin C)[27].
In this study, levels of total proteins and globulins in the serum of M.oleifera ethanol extract-treated rabbits were increased.It may be due to the presence of high content of protein, amino acids, minerals,and vitamins in the M.oleifera ethanol extract or increased protein synthesis[28,29].
Significant elevation of ALT, AST and ALP activities and creatinine concentration may be caused by the toxic effect of lead[30].Abdel-Moneim et al.[4] reported that lead poisoning causes hepatotoxicity and nephropathy.The results of the present study revealed a protective effect of M.oleifera leaves against lead toxicity.This effect could be attributed to its content of vitamins, carotenoids, and other phytocompounds including polyphenols, phenolic acids,flavonoids,alkaloids, glucosinolates, isothiocyanates, tannins and saponins[9,31]which have been proved to have potent antioxidant properties and other biological activities.These results were confirmed by the histopathological photographs of the liver and kidney after 12 weeks of the experiment.The protective effect of the extract could be also due to its content of β-carotene which has been proved to be of significant hepato- and nephroprotective effects and can be efficiently converted into vitamin A.The present results agree with those of Mohamed et al.[25] who reported histopathological alterations from lead acetate-induced injury in the different organs.Meanwhile, in the present study, improvement in histopathological changes has been revealed after treatment with M.oleifera ethanol extract which is in conformity with previous studies[11,12].
In lead intoxicated group, creatinine concentration was still higher after stopping lead exposure compared to the normal control and M.oleifera ethanol extract treated groups.This increase in creatinine may be due to chronic renal injury resulted from lead acetate exposure[32].M.oleifera ethanol extract attenuated the toxicity induced by lead.Similar result reported that the M.oleifera ethanol extract ameliorates renal injury in rabbits with gentamicininduced nephrotoxicity[33].The slight elevation of urea level may be attributed to the high source of protein (27.51%), and amino acids as dominant components of high nutritive value in M.oleifera leaves[34]and renal function impairment by lead toxicity[3].
The significant increase in blood glucose was observed after lead exposure due to altered DNA methylation.DNA methylation alterations might be involved in its pathogenesis by affecting insulin secretion of pancreatic β cells and the body’s resistance to insulin across multiple tissues[35].Lead (0.05%) concentration in drinking water could cause glucose intolerance and insulin resistance in rats[36].
The elevation of LDL-C in rabbits exposed to lead demonstrated an increase in the hepatic cholesterol biosynthesis or a decrease in the hepatic re-uptake of these molecules from the circulation by a receptor-mediated endocytosis[37].Lead exposure had a direct effect on the activity of enzymes, regulating the biosynthesis of cholesterol that results in the enhancement of hepatic cholesterogenesis and hypertriglyceridemia[38].The reduced HDL-C of the lead-intoxicated group could be referred to interrupted intrahepatic cholesterol metabolism.Alterations of the concentration of major lipids such as serum TC, HDL-C, LDL-C, and TG could be helpful in management of atherosclerosis and coronary heart diseases[39].The hypolipidemic activity of several medicinal plants has been associated with bioactive agents like alkaloids, tannins, and cardiac glycosides.Alkaloids may mediate hypolipidemic action either by upregulation of activities of lipolytic enzymes or by stimulating faecal bile acid excretion[40].In addition, other phytoconstituents such as β-sitosterol could be the major cholesterol-reducing components of M.oleifera ethanol extract as it may work singly or in synergy with other bioactive agents[41], which may improve lipid profile in this study.
Lead-induced nephrotoxicity was mainly associated with overproduction of reactive oxygen species and loss of endogenous antioxidant activity.MDA, considered as one of the end-products of polyunsaturated fatty acids peroxidation, is an important indicator of the degree of lipid peroxidation, which is associated with leadinduced tissue damage[42].The present results indicated the ability of M.oleifera ethanol extract to break the chain reaction of lipid peroxidation and enhance the level of GSH.It is suggested that the therapeutic potential of M.oleifera ethanol extract is dependent on an antioxidant mechanism due to the presence of various antioxidant bioactive compounds[11,12].The importance of phenolic compounds from the extract and their antioxidant properties has been strongly suggested.
In conclusion, the present study showed that lead acetate induced marked changes in hemato-biochemical parameters and histopathological structure.M.oleifera ethanol extract had the potential to prevent the alterations caused by lead acetate induced oxidative injuries and restore normal hepatic and renal functions.The mechanism of action might be due to its antioxidant, free radical scavenging abilities and anti-inflammatory effects.Further studies are required to explore the mechanism of action and the future clinical application of M.oleifera leaves.
Conflict of interest statement
The authors declare no conflict of interest.
Acknowledgments
The authors are grateful to Prof.Dr.Attia H.Atta, Professor of Pharmacology, Faculty of Veterinary Medicine, Cairo University,Egypt, for his valuable support during this work.The authors are also greatly indebted to Prof.Dr.Aboelfetoh M.Abdalla, Horticulture and Crops Technology Department, National Research Centre, Egypt for identification and supplying the plant material.
Authors’ contributions
AHM designed and supervised this study.NBM, NAA, SAN and SMN performed the extraction of M.oleifera and experiment,analysis of the blood and biochemistry, as well as collection,analysis and interpretation of data.KAA examined and wrote the results of the histopathological samples.AHM, NBM, NAA, and SMN implemented writing the manuscript.All authors revised the manuscript for publication.All authors read and approved the final version of the manuscript.
 Asian Pacific Journal of Tropical Biomedicine2020年6期
Asian Pacific Journal of Tropical Biomedicine2020年6期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Enzyme-treated date plum leave extract ameliorates atopic dermatitis-like skin lesion in hairless mice
- Leishmania tropica: The comparison of two frequently-used methods of parasite load assay in vaccinated mice
- Formononetin alleviates diabetic cardiomyopathy by inhibiting oxidative stress and upregulating SIRT1 in rats
- Standardized extract of Centella asiatica ECa 233 inhibits lipopolysaccharide-induced cytokine release in skin keratinocytes by suppressing ERK1/2 pathways
- Response surface methodology-based optimization of ultrasound-assisted extraction of β-sitosterol and lupeol from Astragalus atropilosus (roots) and validation by HPTLC method
