Research progress on chemical composition, pharmacological effects of Forsythia suspensa (Thunb.) Vahl and predictive analysis on Q-marker
Hui Peng, Peng Lu, Yiting Liu, Chuanxin Liu, Yanquan Gao, EbukaOlisaemeka Nwafor, Yuelin Zhang, Zhidong Liu
REVIEW
Research progress on chemical composition, pharmacological effects ofand predictive analysis on Q-marker
Hui Peng1,2#, Peng Lu1,2#, Yiting Liu1,2, Chuanxin Liu3, Yanquan Gao1,2, EbukaOlisaemeka Nwafor1,2, Yuelin Zhang1,2, Zhidong Liu1,2
1Tianjin State Key Laboratory of Modern Chinese Medicine, Tianjin University of Traditional Chinese Medicine, Tianjin, China;2Engineering Research Center of Modern Chinese Medicine Discovery and Preparation Technique, Ministry of Education, Tianjin, China;3School of Chinese Materia Medica, Beijing University of Chinese Medicine, Beijing, China.
Lianqiao (, Abbreviated as “below) is traditional Chinese medicine in China, widely distributed all over the country and also highly rich in resources. The Northwest Territories is a genuine producing area. There are many chemical components in, including lignans, phenylethanolic glycosides, flavonoids, C6-C2 natural alcohols and their glycosides, phenolic acids, terpenes and volatile oils. Among them, lignans and phenylethanolic glycosides are the main active components. Based on the review of chemical components or constituents and main pharmacological activities, according to the definition of quality marker, the components of quality marker ofwere predicted and analyzed from the aspects of plant genetics and chemical composition, traditional efficacy, traditional medicine, new efficacy, blood entering composition, measurable composition, effective composition in different compatibility, and the influence of storage conditions of extract. Further research on its medicinal active ingredients will provide scientific basis for the evaluation ofquality.
, Phenylethanol glycosides, Lignans, Quality control, Q-marker
Background
Lianqiao (, Abbreviated as “” below) is one of the traditional Chinese medicines (TCMs) in China, a plant of the genus Forsythia and its fruits widely used, well known in TCM as “Forsythiae Fructus”. Its fruit signifies TCM, and its shell as the medicinal part specified in the Pharmacopoeia [1].was first recorded in Shengnong Bencao Jing [2], a classic work of TCM in the Qin and Han Dynasties (100BC-200AD). In 2015 edition of the Chinese Pharmacopoeia,has the functions of clearing heat and detoxification, reducing swelling, dispersing and evacuating wind and heat [1] (also known as fever and common cold). It has also been used as an effective remedy for the treatment of ulcer, gonorrhea, flu, erysipelas and nephritis for thousands of years. Modern pharmacology [3, 4] showed thathad potential medicinal value in protecting nerves [5], protecting liver and choleretic, regulating immunity, antioxidant effects [6], diuretic and anti-hypertensive, anti-aging, anti-tumor, anti-viral [7], anti-bacterial, anti-inflammatory [8], etc.
The quality of TCM includes little or no adverse effect or reactions, safe and guaranteed efficacy or effectiveness in modern clinical applications. In this paper, the resources, chemical components and main pharmacological activities ofwere reviewed. On this basis, the quality standards and quality markers (Q-marker) ofwere studied potentially, providing a scientific basis for the establishment of quantitative standards of material components.
Resource distribution
In China,is mainly produced in Hebei, Shanxi, Shaanxi, Shandong, Western Anhui, Henan, Hubei, Sichuan and other provinces (regions). In addition, it also grows in Korea, Japan and Europe [9].
Chemical composition
More than 200 compounds have been isolated and identified from[10]Studies on the chemical constituents ofare mainly focused on phenylethanolic glycosides and lignans, followed by C6-C2 natural alcohols and their glycosides, terpenes, volatile oils, flavonoids, phenolic acids, sterols and alkaloids.Among them, phenylethanol glycosides and lignans, the main medicinal ingredients, have the highest content.
Phenylethanoid glycosides
Phenylethanoside is a kind of phenolic glycoside compound [11], which is composed of phenylethanol and sugar. The content of this component is high in, and it is one of the important Q-marker inIt mainly includes forsythiaside (forsythoside A) [12], forsythoside B [13], forsythoside D [14], forsythoside E [12, 15], forsythoside M [16], isoforsythiaside [17], forsythiaside G [12], forsythiaside I [14], forsythiaside J [12], lianqiaoxinside B [18], calceolarioside C [19], forsythenside K [20], plantainoside [21], calceolarioside A [22], calceolarioside B [12], etc. The specific compound names were shown in Supplemental data and the chemical structures of phenylethanolic glycosides 1-30 were shown in Figure 1.
Lignans
Lignans are a class of natural compounds polymerized by two molecules of phenylpropanoid derivatives, most of which are in free state, and a few of which are in the wood and resin of plants due to the synthesis of glycosides with sugar. There are three types of monomers that make up lignans: 1. Cinnamolic acid or cinnamaldehyde, 2. Cinyl alcohol, 3. Allylbenzene. Lignans are an important active component of, mainly including (+)-lariciresinol[28], isolariciresinol[29], (+)-phillygenin[27], (+)-phillyrin[27], (-)-arctiin[30], (-)-arctigenin[30], (-)-matairesinol[31], (-)-matairesinoside[31], (+)-pinoresinol[32], (+)-epipinoresinol [30, 33], (+)-pinoresinol-4-O-β-D-glucoside[34], (-)-dimethylmatairesinol[31], etc. The specific compound names were shown in Supplemental data and the chemical structures of lignans 31-75 were shown in Figure 2 and Figure 3.
C6-C2 natural alcohols and their glycosides
Currently, the separation products of C6-C2 natural alcohol and its glycosides are isorengyol [40], rengyol [47], rengyolone [47], rengyoxide [47], rengyoside A [48], rengyoside B [48], rengyoside C [48], suspenol [49], rengynic acid [52], forsythenside A [54], forsythenside B [54], etc. The specific compound names were shown in Supplemental data and the chemical structures of C6-C2 natural alcohol and their glycosides 76-99 were shown in Figure 4 and Figure 5.
Flavonoids
There are more than 20 flavonoids in, mainly including quercetin [25, 57], isoquercetin [30], astragalin [30], rutin [57], wogonin-7-O-β-D- glucuronide [30], cymaroside [43], luteolin [43], kaempferol [24], isorhamnetin [57], hesperidin [59], isorhamnetin-3-O-α-L-ranorhamnoside-(1→2)-β-D- glucopyranoside [43], potengriffioside A [43], hyperoside [59], etc. The specific compound names were shown in Supplemental dataand the chemical structures of flavonoids 100-120 were shown in Figure 6 and Figure 7.
Phenolic acids
So far, the phenolic acids isolated frommainly include caffeic acid [63], ferulic acid [25], p-hydroxyphenylacetic acid [25], palmitic acid [64, 65], vanillic acid [63], succinic acid [63], etc. The specific compound names were shown in Supplemental data and the chemical structures of phenolic acids 121-162 were shown in Figure 8and Figure 9.
Terpenoids and Volatile oils
The volatile oils inare monoterpenes, monoterpenols, sesquiterpenes, triterpenes, and cycloterpenoids. Among them, α-pinene [70], β-pinene [70], α-phellandrene[70],camphene [70], limonene [70], sabinene [70], p-cymene [70], linalool [70], terpinen-4-ol [70], (+)-carene[71], oleanolic acid [72], ursolic acid [27, 73], 2,3-hydroxyursolic acid[74, 75], etc. The specific compound names were shown in Supplemental data and the chemical structures of terpenoids and volatile oils 163-217 were shown in Figure 10, Figure 11 and Figure 12.
Other compounds
Other compounds were isolated and identified as well.Mainly including sterols and alkaloids such as suspensine A[76],(–)-7ʹ-O-methylegenine [76], (–)-egenine [76], (–)-bicuculline [76], taraxasterol [25], β-sitosterol [56], etc. The specific compound names were shown in Supplemental data and the chemical structures of other compounds 218-225 were shown in Figure 13.
Pharmaceutical effects
In recent years, there are more and more studies on the activity screening of some compounds in. Their pharmacological effects are also obvious, with antibacterial, anti-inflammatory, antiviral, anti-tumor, liver protection, nerve protection, anti-allergic, anti-oxidative effects and regulating body immunity, etc.
Antibacterial effects
Antibacterial activities ofcontribute to its detoxification effects in terms of traditional efficacy.is a kind of broad-spectrum antibacterial drug, which can inhibit many kinds of bacteria. It is mainly used in the treatment of upper respiratory tract infection and acute nephritis [78]. The results [38] showed that caffeoyl phenylethanoid glycosides, forsythiaside and isoforsythiaside were the main antibacterial components of. In addition, the volatile oil inhad a good antibacterial effect onStaphylococcus aureus, Bacillus subtilis, Pseudomonas aeruginosa, Candida albicans, Aspergillus niger and other sensitive microorganisms in vitro [70]. Shuanghuanglian Oral Liquid is a proprietary Chinese medicine, which consists of Rendongteng (), Huangqin (), and, and it is mainly used to treat colds and fevers, coughs and sore throats.The study of Bai YE [79] showed that compared with Shuanghuanglian Oral Liquid, water extract ofwas more sensitive to Staphylococcus aureus and Streptococcus pneumoniae, but it is not sensitive to Escherichia coli (E. coli) and Candida albicans. Han X and others [80]tested the antibacterial activity of 80% methanol extract ofon E. coli K88 (MIC=25.00mg/ml), Staphylococcus aureus (MIC=12.50mg/ml) and Salmonella (MIC=1.56mg/ml) in vitro by a well-diffusion assay procedure and a 2-fold dilution method. And in-vivo tests on 252 broilers for 42 days showed that the extract (100 mg/kg) could reduce the level of E. coli and was a potential antibacterial agent. Some researchers have pointed out that forsythiaside [81] could significantly reduce the mortality of model animals infected with Staphylococcus aureus, Pseudomonas aeruginosa, and E. coli through experiments. Meanwhile, the body temperature of the rabbit model of fever caused by endotoxin and the rat model of yeast fever were significantly reduced. Therefore, it can be confirmed that forsythiaside has antipyretic and anti-infective effects.
Anti-inflammatory effects
The anti-inflammatory activity ofranks first among many TCMs, and it is the most effective and promising anti-inflammatory drug for inhibiting inflammation [82]. The main anti-inflammatory components ofare phenylethanolic glycosides and lignans. Wang TT [83] have done the related in vitro bacteriostatic test and anti-inflammatory activity research, which showed that both forsythoside A and phillyrin have significant bacteriostatic effect. Modern pharmacology showed that the anti-inflammatory activity ofsupported traditional effects of heat-clearing and detoxifying, reducing swelling, dispersing, and evacuating wind and heat.
The anti-inflammatory effect ofhas been proved to play a role in various disease models, including edema [84], colitis [85] and peritonitis [86].Studies by Lee JJ and other studies [87] have shown that water extract of(mainly including forsythiaside A, lariciresinol, arctigenin, etc.) activated Nrf2 (Nrf2, important transcription factors that regulate cellular oxidative stress) and inhibited NF-κB activity (NF-κB, a transcription factor that regulates the expression of key cytokine genes that promote inflammation) and induced A20 [88](A20, a negative regulator of NF-κB signaling pathway) expression to play an anti-inflammatory effect. Ethanol extract of(such as phillyrin, phillygenin, forsythoside A, pinoresinol, suspensaside, caffeic acid, etc.) could reduce the production of inducible nitric oxide synthetase (iNOS), prostaglandin E2 (PGE2) and cyclooxygenase-2 [89]. It has been reported that the anti-inflammatory effect of forsythiaside A on BV-2 microglia (mouse microglioma cells) and primary microglia in vitro was studied at different concentrations. And the inhibitory effect [90] of was recognized to inhibit NF-κB signaling pathway and higher levels of Nrf2 and heme oxygenase 1 (HO-1). From the number of neutrophils and the levels of tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and monocyte chemoattractant protein-1 (MCP-1) in the peritoneal cavity, it was confirmed that forsythiaside A [86] could significantly reduce peritonitis. In a mouse model of acute lung injury [91], phillyrin could inhibit the pathological changes of lung tissue, alveolar hemorrhage and neutrophil changes in mice. It protected lung injury by inhibiting TNF-α, interleukin-1β (IL-1β), IL-6 and peroxidase.
Lee S [92] has shown that arctiine could reduce the production of NO and pro-inflammatory cytokines such as IL-1β, IL-6, TNF-α, and PGE2, and reduce gene and protein levels detected by reverse transcription-polymerase chain reaction (RT-PCR) and western blot. In addition, arctiin [93] could inhibit the expression of costimulatory molecules (such as B7-1, B7-2), and the NF-κB signaling pathway in macrophages to achieve anti-inflammatory purposes. Arctiin has a good anti-inflammatory biological activity.
Antiviral effects
Antiviral activities ofalso contribute to its detoxification effects[94]. Studies [95] have shown that in mice inoculated with H1N1 in vivo, the administration of phillyrin (20 mg/kg/day) for three consecutive days significantly prolonged the average survival time, and it was determined that the virus titer, IL-6 and influenza hemagglutinin production were significantly reduced, and lung tissue damage was reduced. Forsythiaside A [96] could reduce the content of influenza A virus M1 protein in model mice infected with influenza virus, prevent the generation and spread of new virions, and improve the survival rate of mice infected with influenza virus in vivo. More importantly, forsythiaside A [97] could control influenza A virus infection by inhibiting influenza A virus replication and improve the prognosis of influenza A virus infection. Yang Y [98] evaluated the anti-swine influenza virus effect of. Experiments have shown that it had antiviral effects, mainly by resisting virus adsorption, interfering with virus replication, and direct killing.
Anti-cancer effects
Anti-cancer activity ofalso supports its detoxification and antioxidant effects.has multiple therapeutic effects [99] on lung cancer, colon cancer, liver cancer, leukemia, oral epidermoid cancer, gastric cancer, breast cancer, colorectal cancer, melanoma, esophageal cancer, lung adenocarcinoma, melanoma, etc. Bao J et al [100] tested the anti-proliferative effect ofwater extract on mouse melanoma B16-F10 cells in vitro. In vivo transplantation of B16-F10 melanoma in mice, the extract (10000 mg/kg) significantly inhibited tumor growth, cell proliferation and angiogenesis. Its anti-tumor effect was related to the anti-inflammatory and anti-oxidation properties regulated by mitogen-activated protein kinase (MAPKs)/Nrf2/ HO-1.
Hepatoprotective effect
Pan CW [101] reported that forsythiaside A (15, 30, 60 mg/kg) at a certain concentration could be alleviated inflammatory response and had hepatoprotective effect in a model of lipopolysaccharide (LPS)/D-galactosamine (GalN) induced acute liver injury. It could reduce the production of malondialdehyde (MDA) and the levels of alanine aminotransferase in serum and aspartate aminotransferase. And it could also activate Nrf2 and inhibit the NF-κB signaling pathway. Similarly, forsythiaside A, forsythiaside B, forsythiaside N, and forsythiaside O all showed survival rates with strong liver cells.
Antioxidant effect and regulating body immunity
Isoforsythiaside was isolated fromby Qu H [102] and its pharmacological test showed that isoforsythiaside had high antioxidant and antibacterial activity. In vitro, isoforsythiaside and forsythiaside had a strong scavenging effect on DPPH free radicals. And the strong antioxidative effect of isoforsythiaside was related to the ortho substitution of the hydroxyl group in the phenol structure. Forsythiaside B, forsythiaside H, and calceolarioside B [18] also showed free radical scavenging activity in vitro. Lu T [103] used DPPH and ABTS free radical scavenging experiments to confirm the antioxidant capacity of 80% ethanol extract ofin vitro.
Phillygenin, 8-hydroxypinoresinol, forsythialan and forsythialan B obviously protected the renal tubular epithelial cells LLC-PK1 in vitro [104]. Forsythiaside could enhance the expression of antioxidant enzymes in H2O2-treated neurons cultured in vitro [105]. It has been reported that phenolic constituents also had strong antioxidant properties and were stronger than ascorbic acid, such as methyl caffeate, rutin and quercetin [106].
Anti-allergic effects
Sung YY [107] studied that forsythiaside, phillyrin, lariciresinol, and phillygenin can reduce the production of thymus activation regulating chemokine, macrophage-derived chemokine and rantes. In a mouse in vivo model, the ethanol extract ofcould alleviate the symptoms of allergic dermatitis induced by the crude extract of dust mites. The methanol extract ofcould reduce the allergic symptoms caused by β-companion soybean globulin in weaned piglets. Hao Y [108] believed that the mechanism may be related to inhibiting allergic antibodies, hindering mast cell degranulation, T lymphocyte proliferation, histamine production and interleukin-4 synthesis.
Neuroprotective effects
It was reported that forsythiaside and phillyrin [109] were considered as promising drugs for the treatment of cognitive impairment and Parkinson's disease, and were candidates for the treatment of amnesia. Zhang S [110] studied the inhibitory effect of 75% ethanol extract of(mainly including forsythoside A, suspensaside, rutin, etc.) on rotenone induced neurotoxicity in SD male rats. It could improve the behavior of rats, protect dopamine neurons from loss and restore dopamine level. The results showed that its potential function is by relieving pro-inflammatory molecules of Parkinson's disease and regulating phosphatidylinositol 3-kinase, protein kinase B, NF-κB and MAPK pathways.
Kim JM [111] found that phillygenin could shorten the escape latency, reduce the level of thiobarbituric acid reactive substances assay (TBARS) in rats and protect the nerves in the scopolamine-induced mouse memory impairment model. Its mechanism was that phillygenin could express its anti-inflammatory activity through forsythia lipoproteins by inducing a decrease in glial cell activation and expression levels of IL-1β and tumor necrosis factor-α, thereby achieving a neuroprotective effect.
By processing the mouse brain homogenate in the Wang’s model group [109], the levels of IL-1β, NO, MDA and norepinephrine (NE) were significantly decreased, and the activities of total superoxide dismutase and glutathione peroxidase and the levels of glutamate and acetylcholine were significantly increased. Therefore, this study indicated that forsythiaside had a herapeutic effect on amnesia and neuroprotection.
Other pharmacological effects
In addition to the above effects,could also promote gastric emptying, lower blood lipids and relax blood vessels. It was reported that phillyrin [112] had vasodilating effect on vasoconstriction caused by NE. The mechanism was that it protected the aorta of isolated rats by limiting the inflow of calcium ions in vitro. Hao FF [113] studied that compared with the blank group, the water extract ofadministration group had no effect on gastric emptying and small intestinal advancing of mice under normal physiological conditions. However, it had a good two-way regulation and improvement effect on gastric emptying and small intestine models of mice under pathological conditions caused by dopamine, adrenaline, atropine and neostigmine.
Predictive analysis of Q-marker
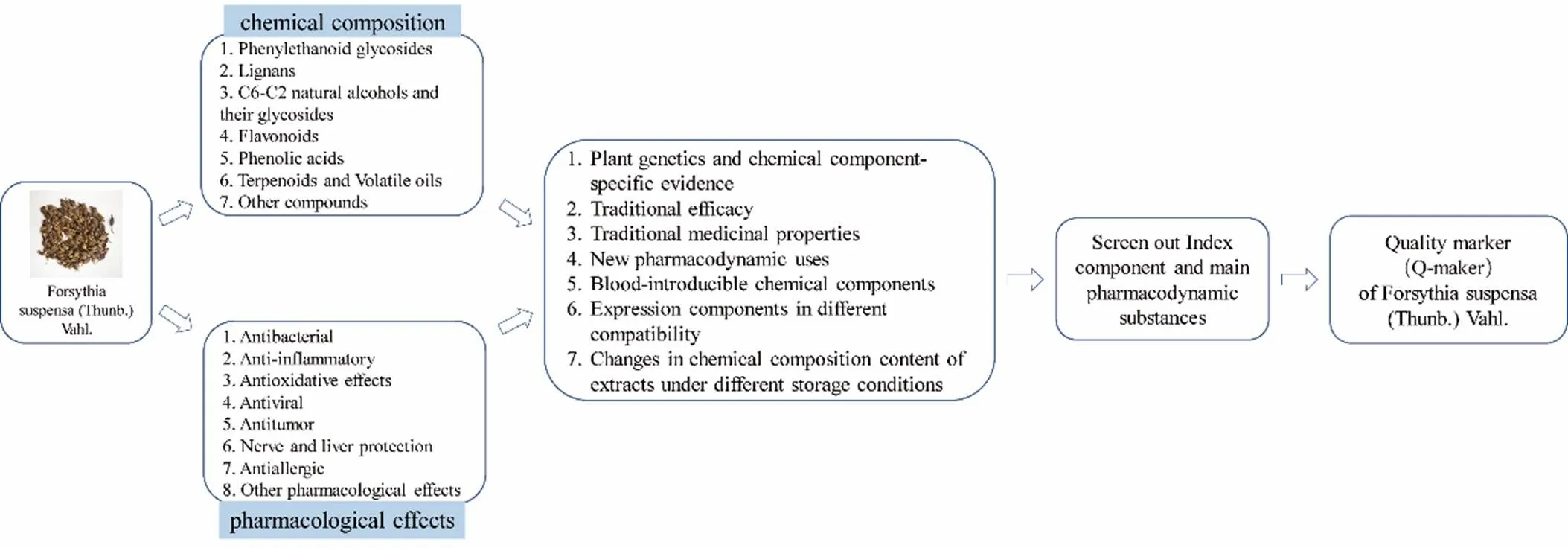
The chemical composition types ofin different provinces, different places of origin, different growth years and different harvest periods are generally similar, but the contents are different. Q-marker [114, 115] was a new concept put forward by academician Liu Changxiao [116, 117] (Researcher of Tianjin Institute of Pharmaceutical Research, academician of Chinese Academy of Engineering) in 2016, which closely linked the “effectiveness – material basis – quality control landmark components” of TCM. Q-marker systematically expounded the relationship between the quality and efficacy of TCM with new perspectives, improved the quality standard of TCM in China, and improved the scientificity, rationality and operability of the standard [118]. It referred to chemical substances that were inherent in TCM and TCM products (Chinese herbal extracts, TCM decoction pieces, TCM preparations, etc.) were formed during processing and were closely related to the functional properties of TCM, as a reflection of the safety of TCM and quality control of effectiveness. Since academician Liu Changxiao put forward, scholars have conducted preliminary discussions on some Chinese medicines [119, 120] and TCM Patent Prescriptions [121, 122]. In order to evaluate the quality ofobjectively and scientifically, according to the definition of Q-marker, the Q-marker ofis predicted, which is helpful to establish a scientific quality control method of.
Predictive analysis of Q-marker based on plant genetics and chemical component-specific evidence
According to herbal research [123, 124], the TCM “Forsythia” mostly used Hyperic ascyron L. as the basis in the Song Dynasty. After the Song Dynasty,was used as the basis of the TCM “Forsythia” and has been used ever since.is derived from Forsythia of Oleaceae. Forsythia species include Lianqiao ()Jinzhonghua(), and Qinqiao() in China. Studies on other breeds have been scarce.is mainly distributed in Hebei, Shanxi, Shaanxi, Shandong, Western Anhui, Henan, Hubei, Sichuan and other provinces in China. There are many chemical constituents isolated from, including 36 phenylethanolic glycosides, 55 lignans, 58 terpenes and volatile oils, 25 C6-C2 natural alcohols and their glycosides, 23 flavonoids, more than 50 sterols, alkaloids and phenolic acids. Among them, phenylethanol glycosides and lignans are the main active ingredients and important chemical markers ofThese two kinds of effective components are selected as the quality indicators of
Predictive analysis of Q-marker based on traditional efficacy
has the functions of clearing heat and detoxification, reducing swelling and dispersing, and evacuating wind and heat. In modern pharmacological studies, the traditional efficacy ofis based on the anti-inflammatory and anti-oxidation properties of phenylethanolic glycosides (forsythiaside A, B, C, D, E, etc.) and lignan compounds (phillygenin, phillyrin, etc.) in. In modern pharmacology, the traditional efficacy of “clearing away heat and detoxification” is mainly reflected in the pharmacological effects of antipyretic, cooling, antibacterial and anti-inflammatory; The traditional effect of “swelling and dispersing the wind and dispersing wind and heat” is mainly reflected in anti-virus, swelling and anti-inflammatory.
Hu NH [125] studied the inflammation model and fibrosis model of human hepatic stellate cells (LX2) induced by different concentrations of lipopolysaccharide (LPS), and then given high, medium and low doses of phillygenin for intervention. The results showed that phillygenin could inhibit LPS-induced LX2 cell activation and inflammation of the TLR4/MyD88/NF-κB signaling pathway. Phillygenin could also inhibit LX2 cell activation and fibrotic cytokine expression by inhibiting the LPS-induced proinflammatory response. Zou SS [126] intravenously and orally administered forsythiaside to rabbit models infected with parainfluenza virus, and tested to significantly reduce the contents of the heating medium protein kinase C and cyclic adenosine monophosphate (cAMP) in the hypothalamus. Experiments showed that forsythia could reduce the body temperature of rabbits after parainfluenza virus infection, and its antipyretic mechanism may be related to the down-regulation of cAMP content in rabbit hypothalamus. Forsythiaside A [127] could prevent cigarette smoke-induced lung tissue damage in mice with chronic obstructive pulmonary disease by activating Nrf2 and inhibiting the NF-κB signaling pathway and this experiment also demonstrates that forsythiaside A can attenuate the penetration of inflammatory cells.
Phenylethanol glycosides and lignans correspond to the traditional efficacy of, which were the main medicinal substance basis of traditional efficacy of, and also the main way and important basis for screening Q-marker of.
(4)提高球化能力 将球化温度控制在1450℃左右,球化剂上覆盖0.2%硅钡孕育剂,球化能力刚好合适,既不会太强也不会太弱。球化完毕后铁液中的渣易于浮出,便于除渣,可保证铁液纯净。
Predictive analysis of Q-marker based on traditional medicinal properties
The classic taste of TCM [128] is the basic attribute of TCM, and it is also an important basis for the treatment of clinical symptoms and formulating prescriptions. It should also be used as one of the bases for determining Q-marker.possesses related properties: it has a bitter taste and cold attribute to lung, heart and small intestine. The important material foundation of “bitter taste” should first have the taste characteristics and functional properties of bitterness. The taste of the medicine has a corresponding relationship with its chemical components or constituents used in formulation. The glycosides and alkaloids are the main sources of the bitter taste possessed by the cold medication [129]. Based on the above, it can be concluded that the chemical components of phenylethanol glycosides and lignans inshould be the main material basis of its “sexual taste” and the main choice of Q-marker.
Predictive analysis of Q-marker based on new pharmacodynamic uses
The phenylethanolic glycosides contained inhave an effect of protecting the liver and eliminating jaundice, and the aglycone caffeic acid and its structural analog chlorogenic acid also have the effect of protecting the liver and promoting gall [101]. Cheng Y [130] reported that phillyrin and forsythiaside A had different induction effects on cytochrome P450 in rat liver microsomal incubation system. Both phillyrin and forsythiaside A can potentially induce cytochrome oxidase activity. Zhang YY [131] studied that the ethyl acetate extract of(EAF) could reduce the content of serum triglyceride and total cholesterol in streptozotocin induced diabetic mice model. And it also increased the content of high-density lipoprotein, and significantly reduced blood glucose levels in diabetic mice treated with EAF. In addition, EAF may have anti-diabetic effects by regulating gene expression and pancreatic function. Leng W [132] explored the effects of phillyrin on renal function, inflammatory response and oxidative stress in diabetic nephropathy rats by establishing a diabetic nephropathy rat model, and the results showed that phillyrin can protect renal damage in diabetic nephropathy.
It can be concluded that phenylethanolic glycosides and lignans are the main material foundations forto protect the liver and lower blood sugar, which should be taken as the Q-marker of
Predictive analysis of Q-marker based on blood-introducible chemical components
The chemical composition of TCM is very complicated, and its complexity is manifested in the fact that TCM contains a large number of different structural types of chemical components, and each type has a large number of chemical components. The complexity of TCM ingredients is the basis for its multiple functions and multiple pharmacological effects. Although the chemical composition of TCM is complex, the chemical ingredients must be absorbed into the blood and reach a certain blood concentration in the body before they can play a pharmacological effect directly or indirectly (except for a few components that directly affect the intestine). By analyzing the components of the prototype and their metabolites after administration, analyzing the markedly effective ways based on the compound-target-pathway, and combining the tissue distribution of the blood-injecting components with the pathological site of the disease and the drug intervention method, the effective ingredients ofwere screened and used as an important basis for predicting the Q-marker of
The oral bioavailability of phenylethanolic glycosides is low, but the efficacy is significant, which means that the mixture of prototypes and metabolites may be the group of active ingredients that play the role of efficacy. The main reactions of phenylethanolic glycosides in vivo are hydrolysis, reduction, dehydrogenation, methylation, sulfation, etc. Bai Y [133] was used to study the pharmacokinetics ofmethanol extract ofin rats. The liquid chromatography, electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS) was established and validated for the determination of 9 selected components (forsythiaside A, forsythiaside B, rutin, astragaloside, phillyrin, kaempferol, quercetin, iaorhamnetin and arctiin) in rat plasma. The experimental results showed that only three forsythiasides A, rutin and phillyrin are detected in the plasma after oral administration ofmethanol extract in rats. Li C studied the antiviral evaluation of phillyrin and its metabolites [134]. Phillyrin is converted to aglycone forsythiaside by hydrolysis, and then converted to other metabolites mainly through a series of metabolic processes in the blood such as methylation, demethylation, dehydroxylation, and ring opening. The results showed that phillyrin and four metabolites had inhibitory effects on the H3N2 influenza virus, indicating that phillygenin were the main components of phillyrin in rat plasma.
Based on the above analysis, the blood components ofare mainly phenylethanol glycosides and lignans, which are closely related to medicinal effects, and are likely to be the basis of the main pharmacodynamic substances, which serve as an important reference for screeningQ-marker.
Predictive analysis of Q-marker based on changes in chemical composition content of extracts under different storage conditions
The clinical efficacy of Chinese medicine is related to its quality, which is related to the effective active ingredients. After the TCM is harvested and processed for clinical applications, it is also extracted and stored. At this time, temperature, humidity, and light will affect the effective active ingredient content in it. Selecting a suitable storage method is of great significance to ensure the quality and stability of the TCM. During the packaging and storage of, the contents of forsythiasides A,phillyrin and phillygenin showed a decreasing trend [135]. In addition, different morphological conditions have different effects on their chemical composition content.
Conclusions and future perspectives
is a TCM commonly used in China. It has a wide range of physiological activities and has a long history of extensive application, accurate curative effects, and abundant sources of medicine. There are many new clinical applications, such as reducing blood sugar, lowering blood pressure, regulating blood lipids, and protecting the liver. The composition system ofis complex, and the single index component has been used as the standard to meet the entire forsythia industry chain. However, to establish a scientific and reasonable quality evaluation method, the quality ofis evaluated comprehensively and accurately, to guide the rational utilization of forsythia species, is of great practical significance for healthy development ofindustry.
On the basis of summarizing the research status ofchemical composition and modern pharmacological effects, this paper takes the Q-marker of TCM as the core concept. As shown in Figure 14, the relationship between traditional efficacy and modern pharmacological effects need be fully understood. According to the chemical components and pharmacological effect of, relevant literature of sexual taste, its measurable components, blood composition, etc., and combined with chemical composition analysis and some related researches on effectiveness, the literature analysis and demonstration of the selection and determination of. Q-markers were carried out, and a feasible path and a reference model method for Q-marker analysis of TCM were put forward. It will lay a foundation for the prediction and analysis of the Q-marker ofin the future, so as to establish the quality control and quality traceability system of.

Figure 1 Chemical structures of phenylethanoid glycosides (No. 1-30)
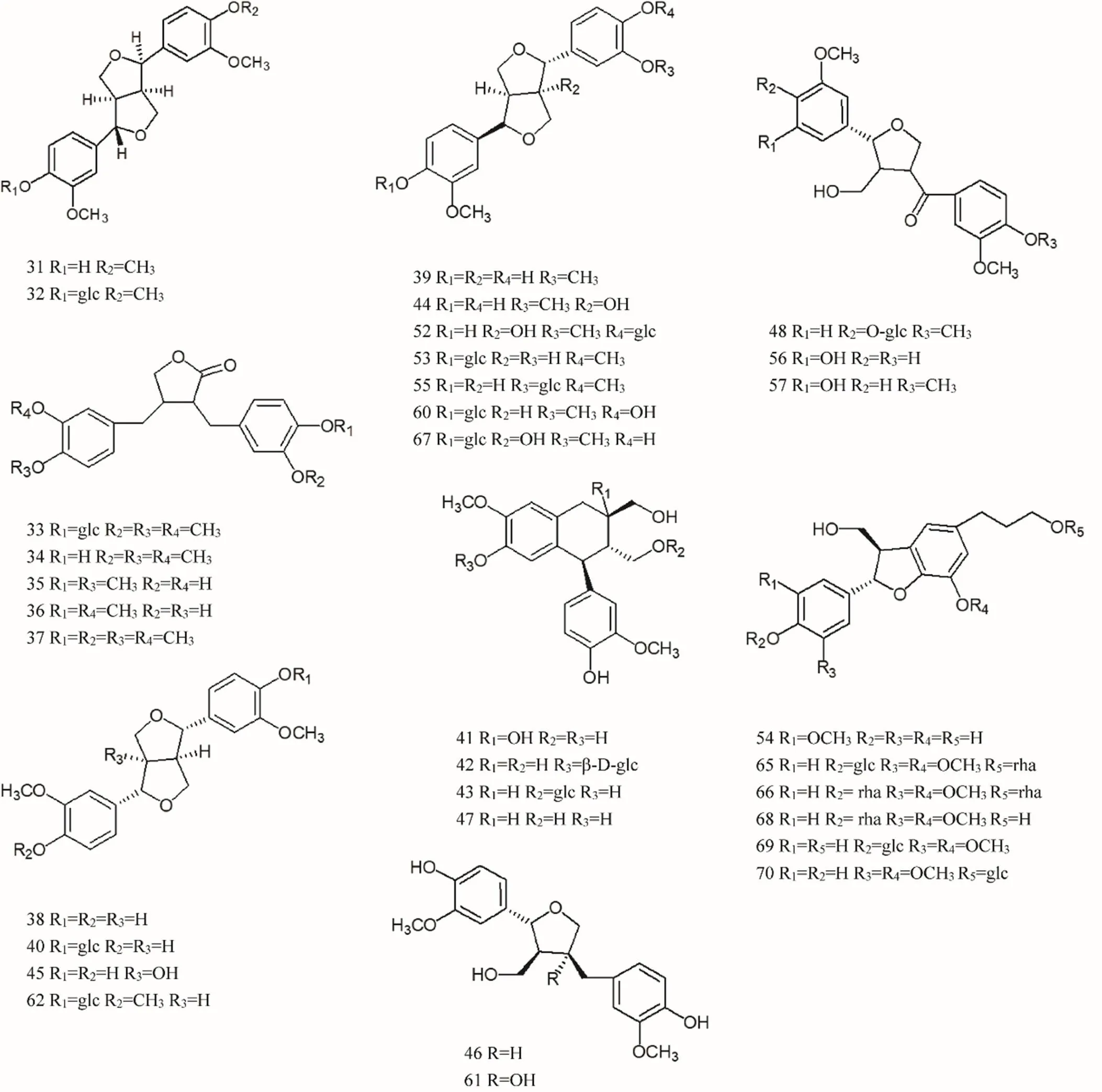
Figure 2 Chemical structures of lignans (No. 31-75)
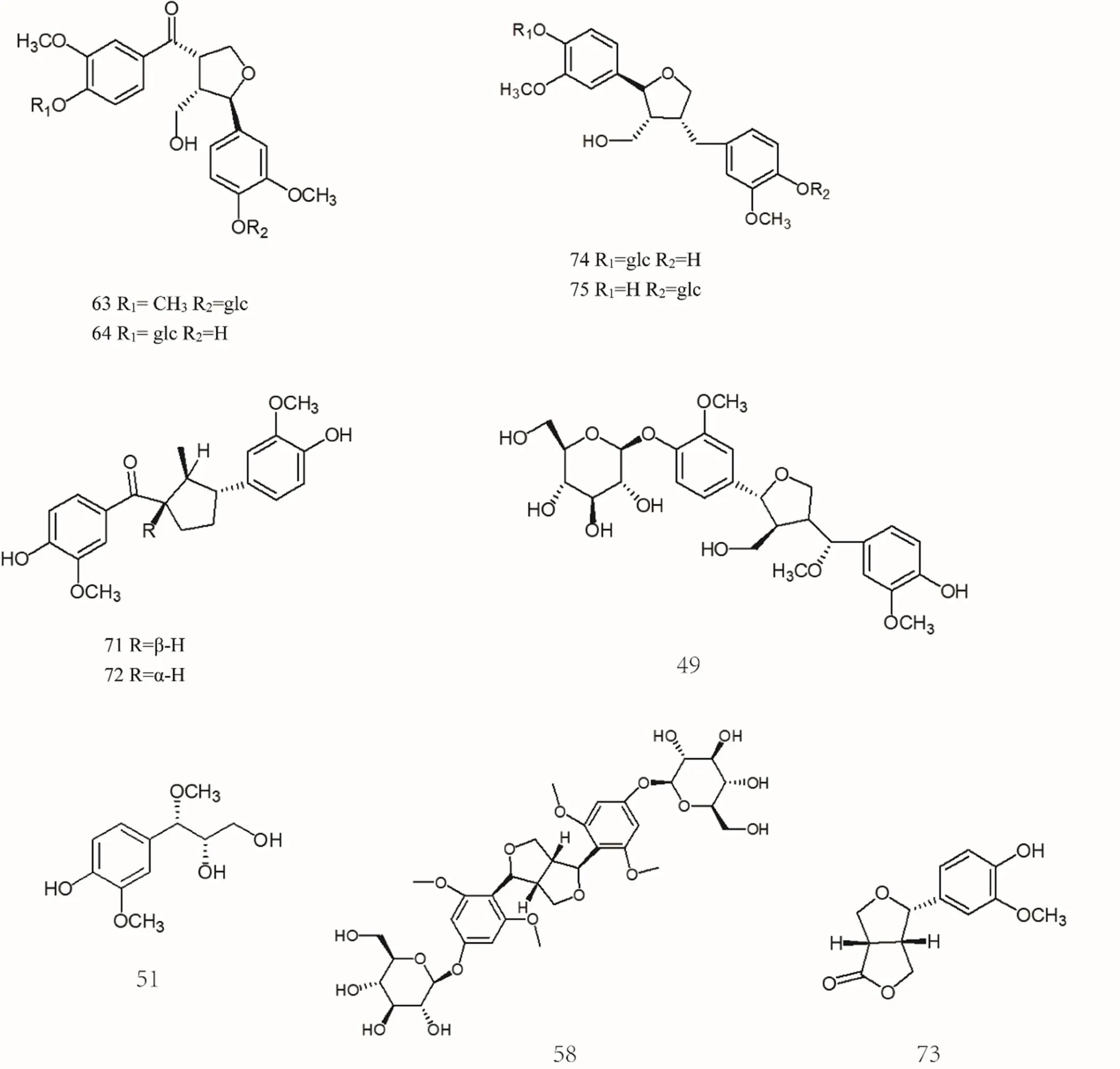
Figure 3 Chemical structures of lignans (No. 31-75)
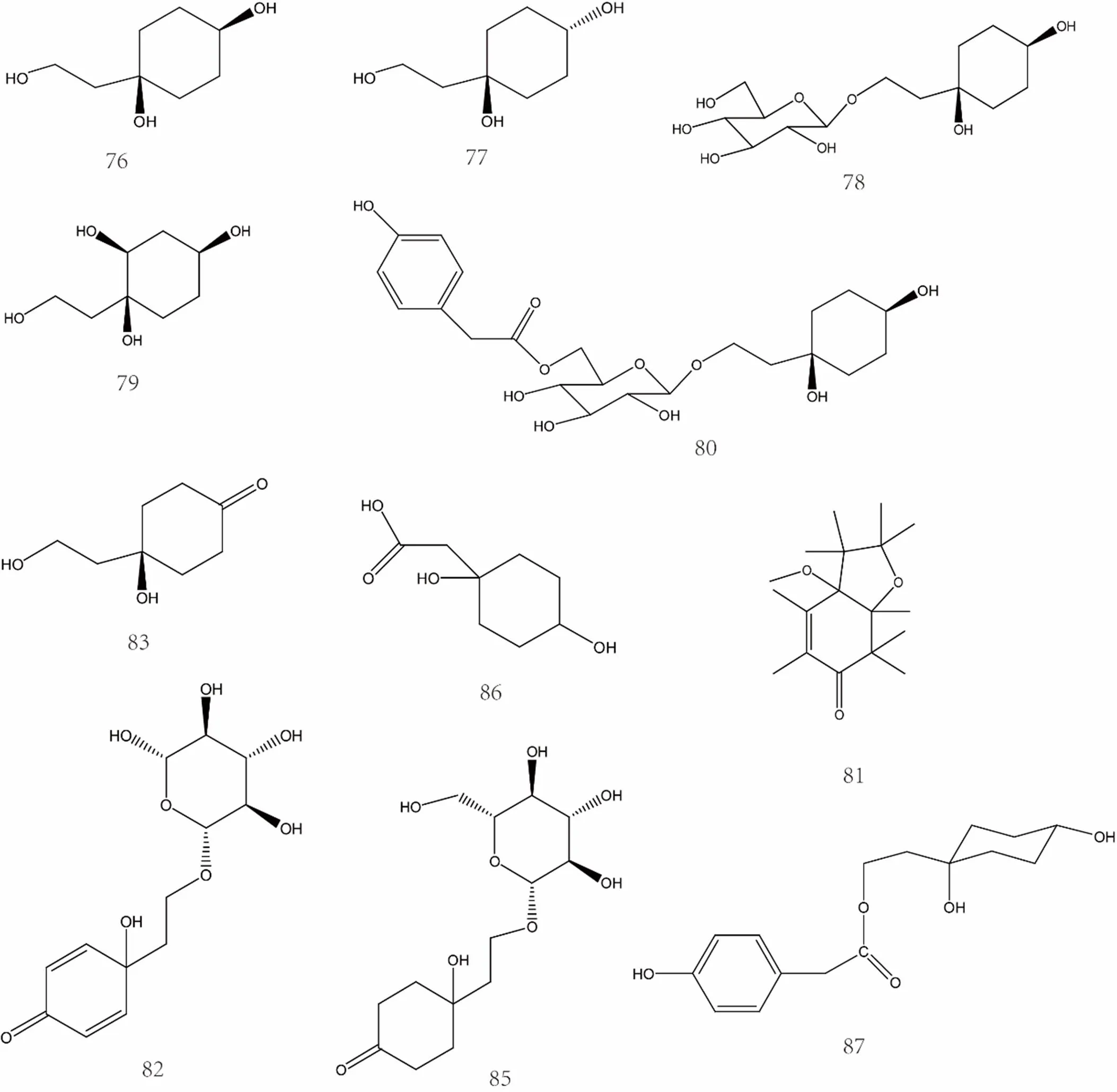
Figure 4 Chemical structures of C6-C2 natural alcohol and their glycosides (No. 76-99)
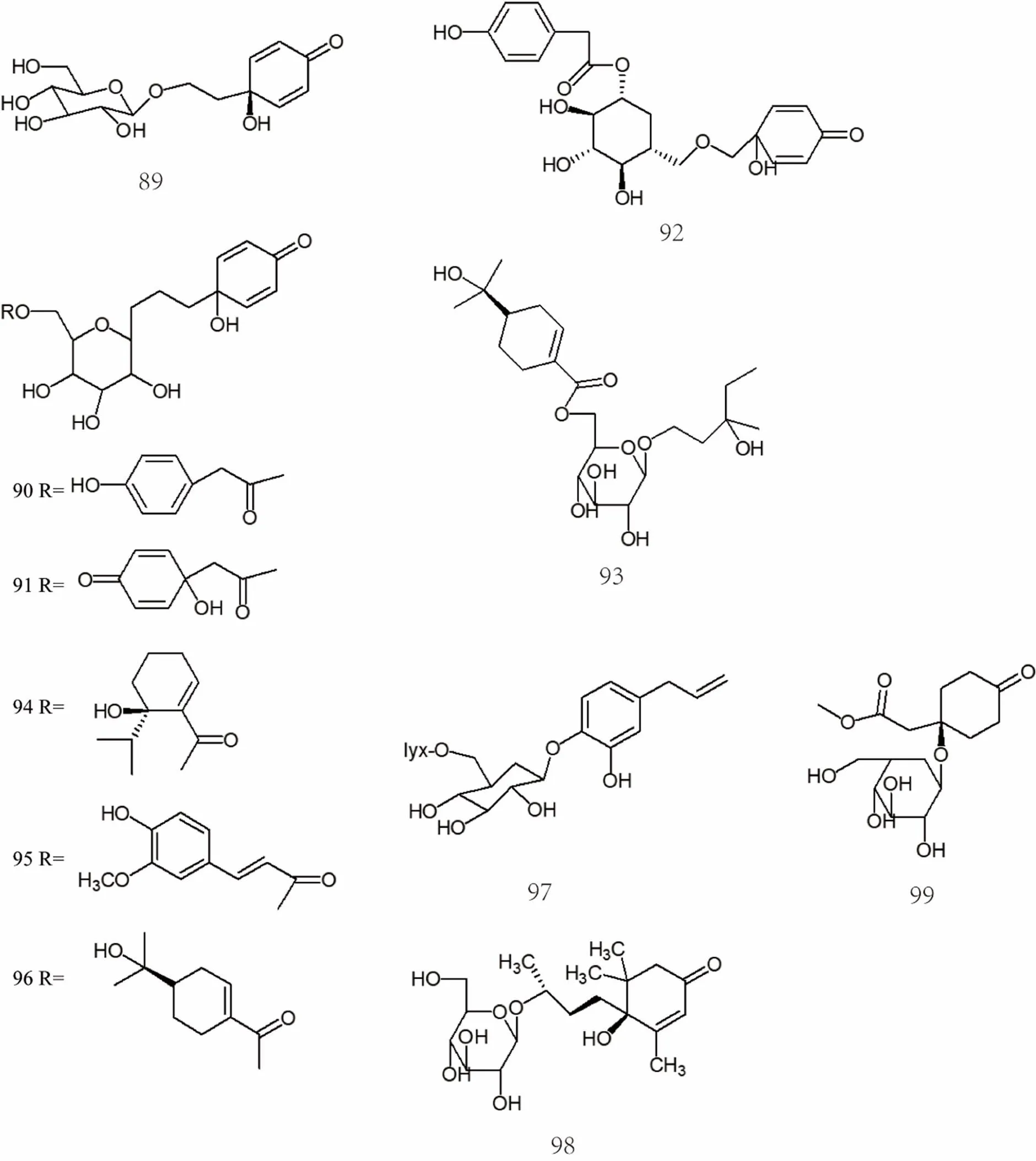
Figure 5 Chemical structures of C6-C2 natural alcohol and their glycosides (No. 76-99)
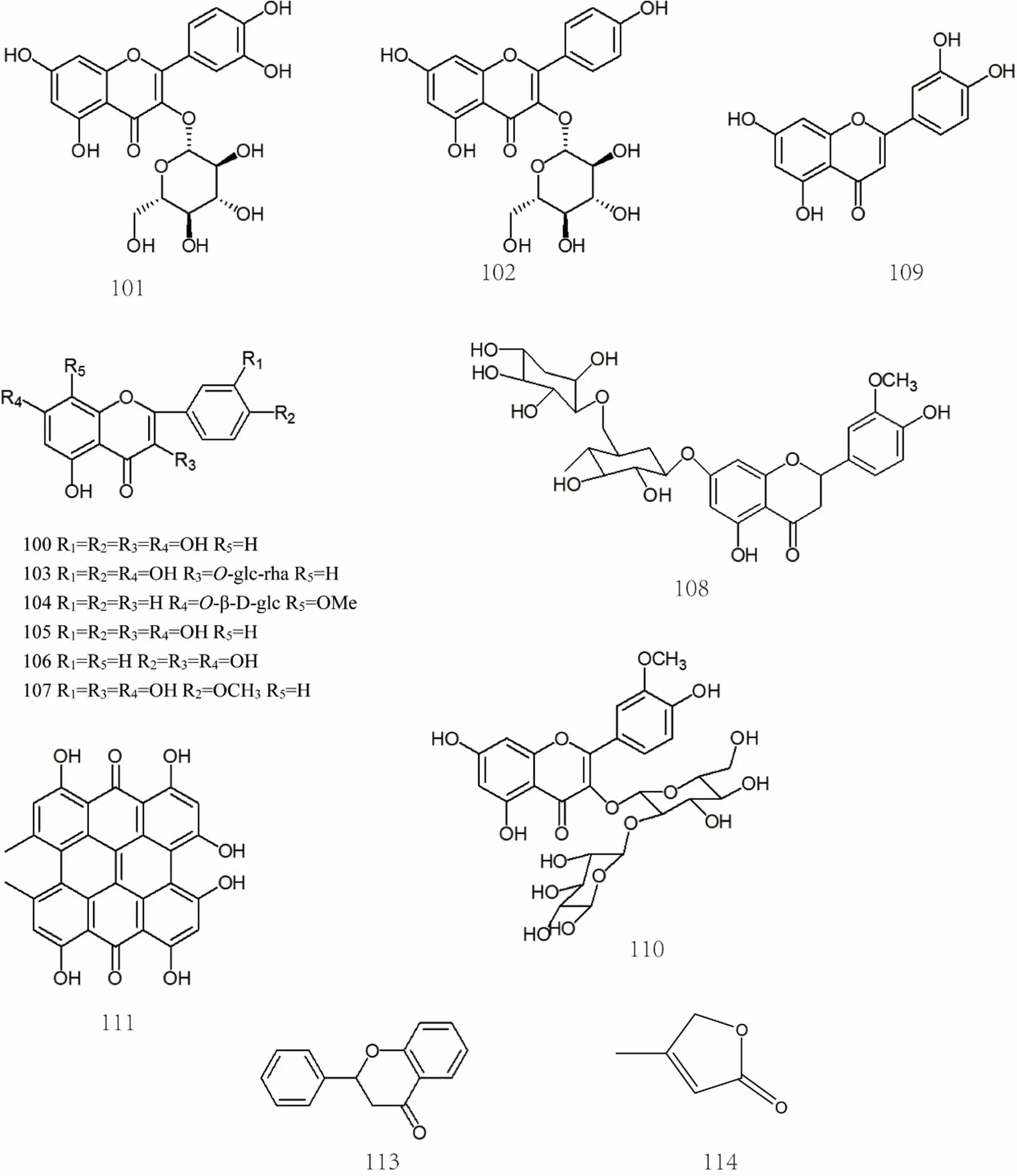
Figure 6 Chemical structures of flavonoids (No. 100-120)
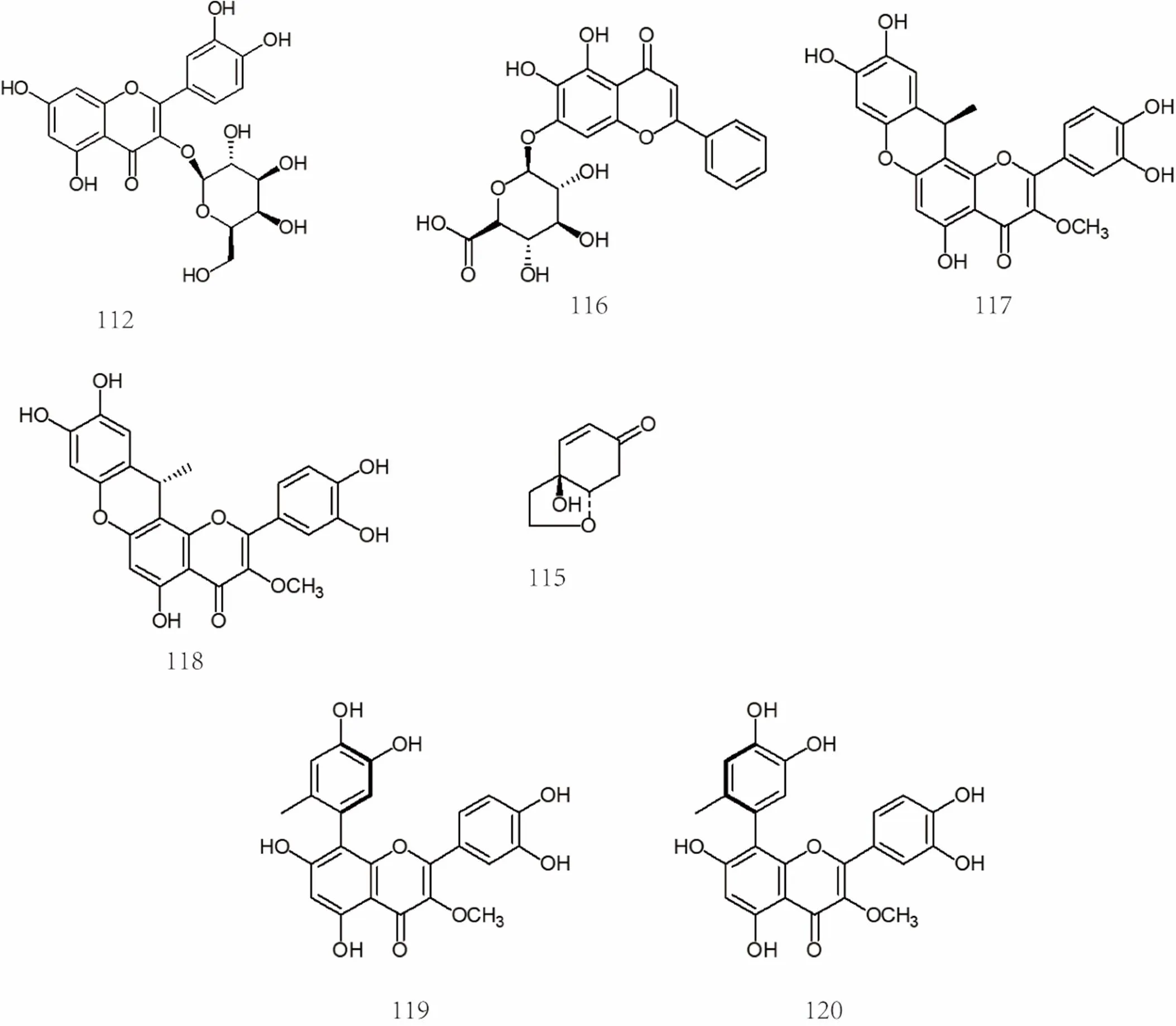
Figure 7 Chemical structures of flavonoids (No. 100-120)
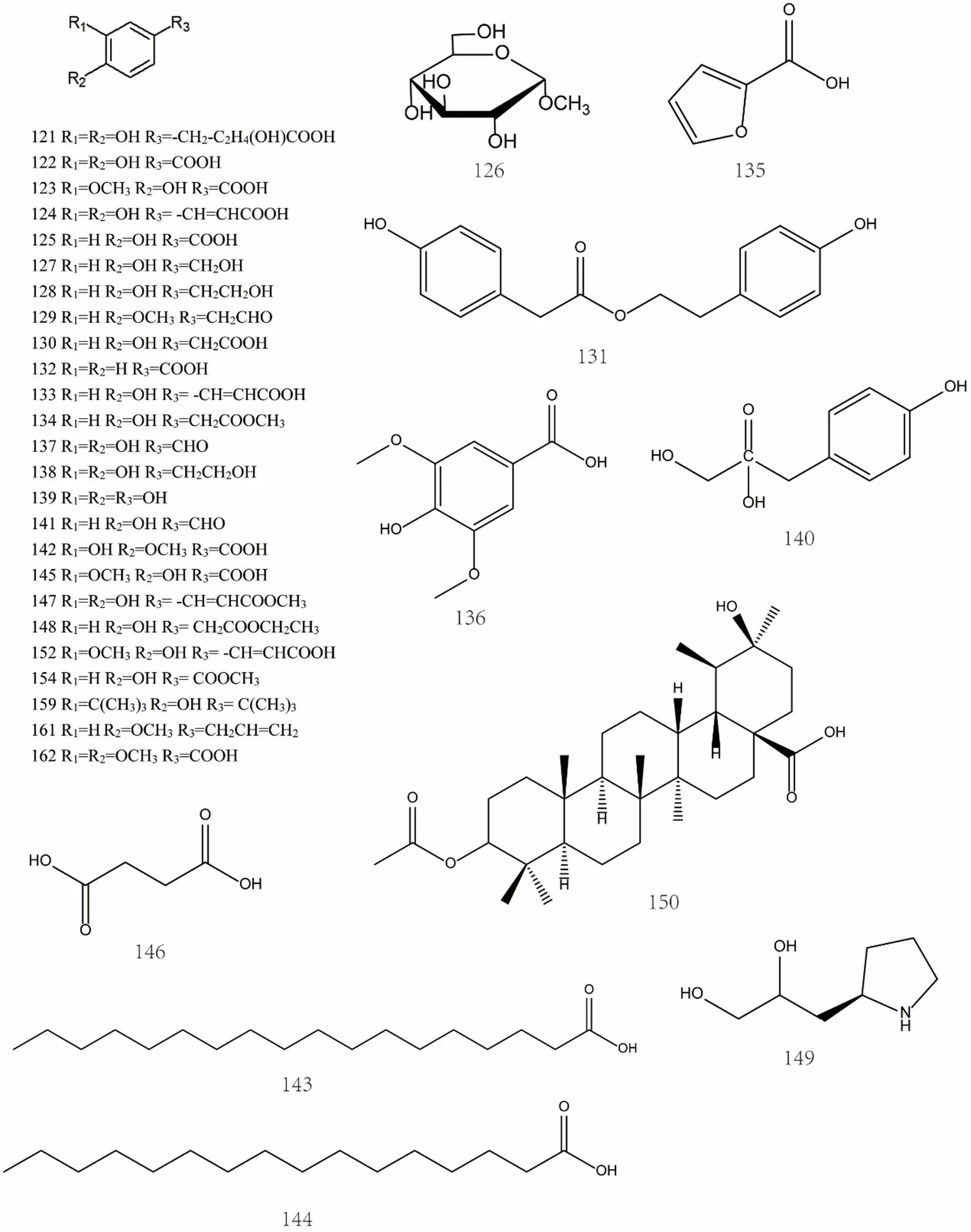
Figure 8 Chemical structures of phenolic acids (No. 121-162)
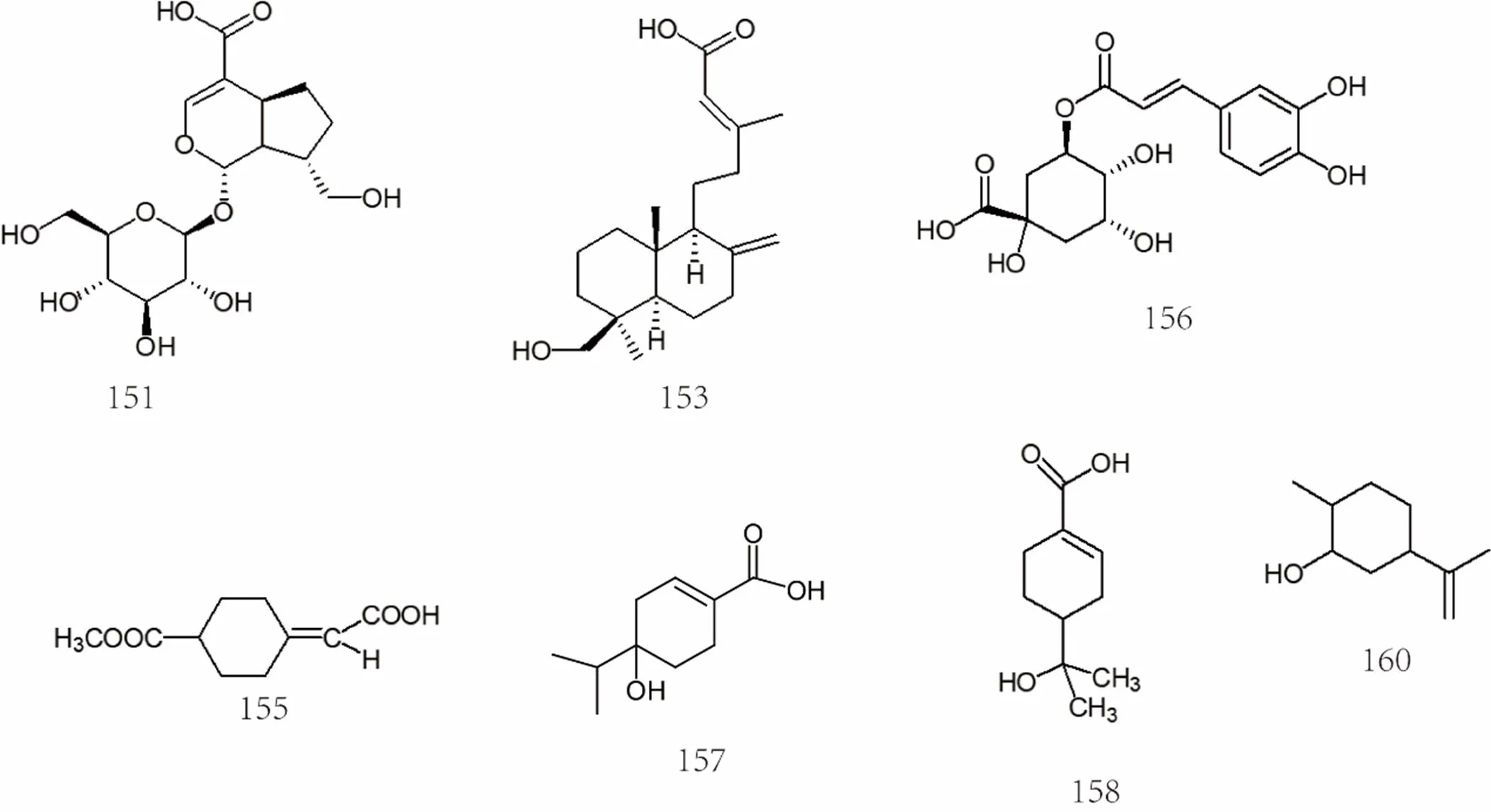
Figure 9 Chemical structures of phenolic acids (No. 121-162)
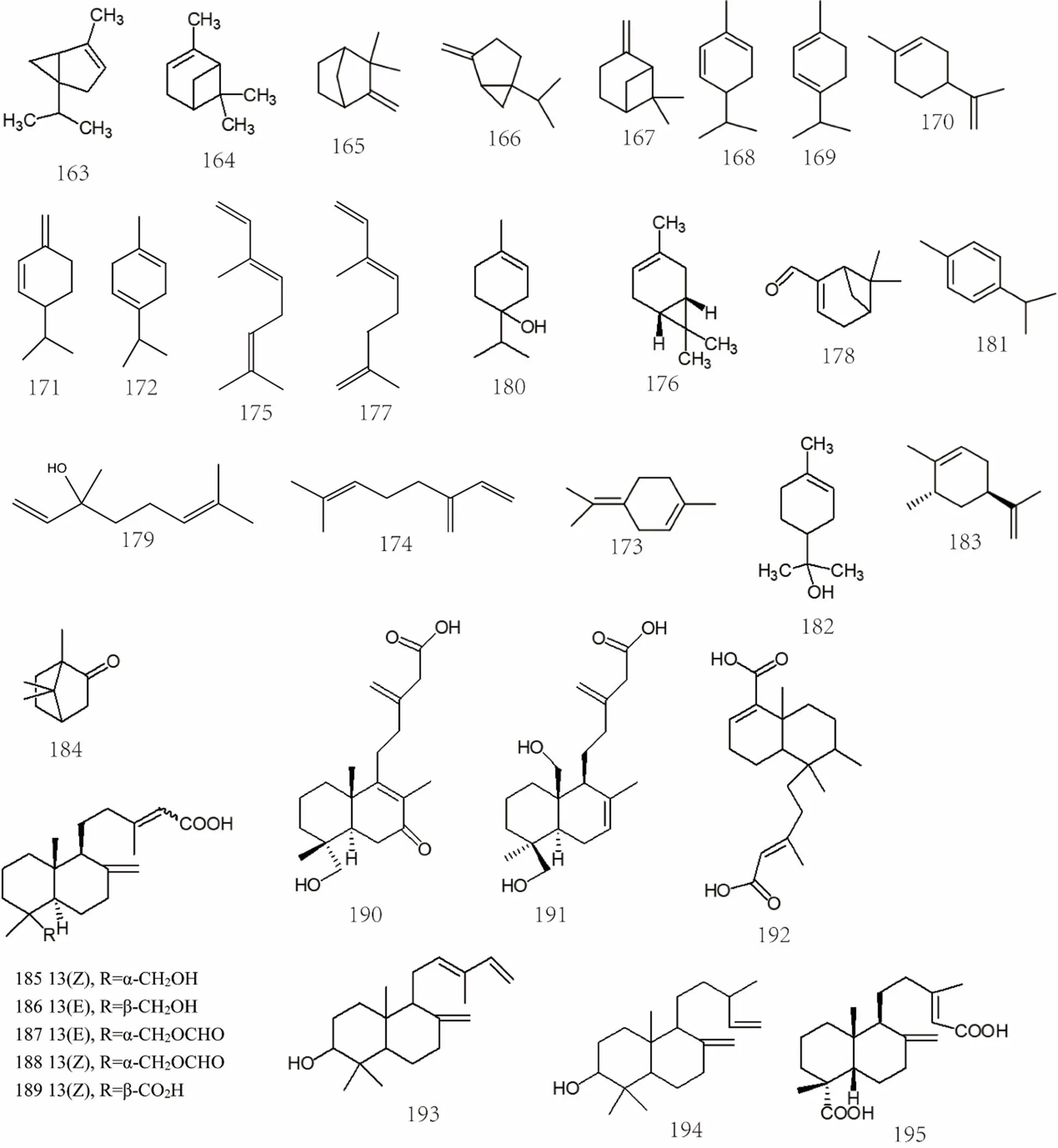
Figure 10 Chemical structures of terpenoids and volatile oils (No. 163-217)
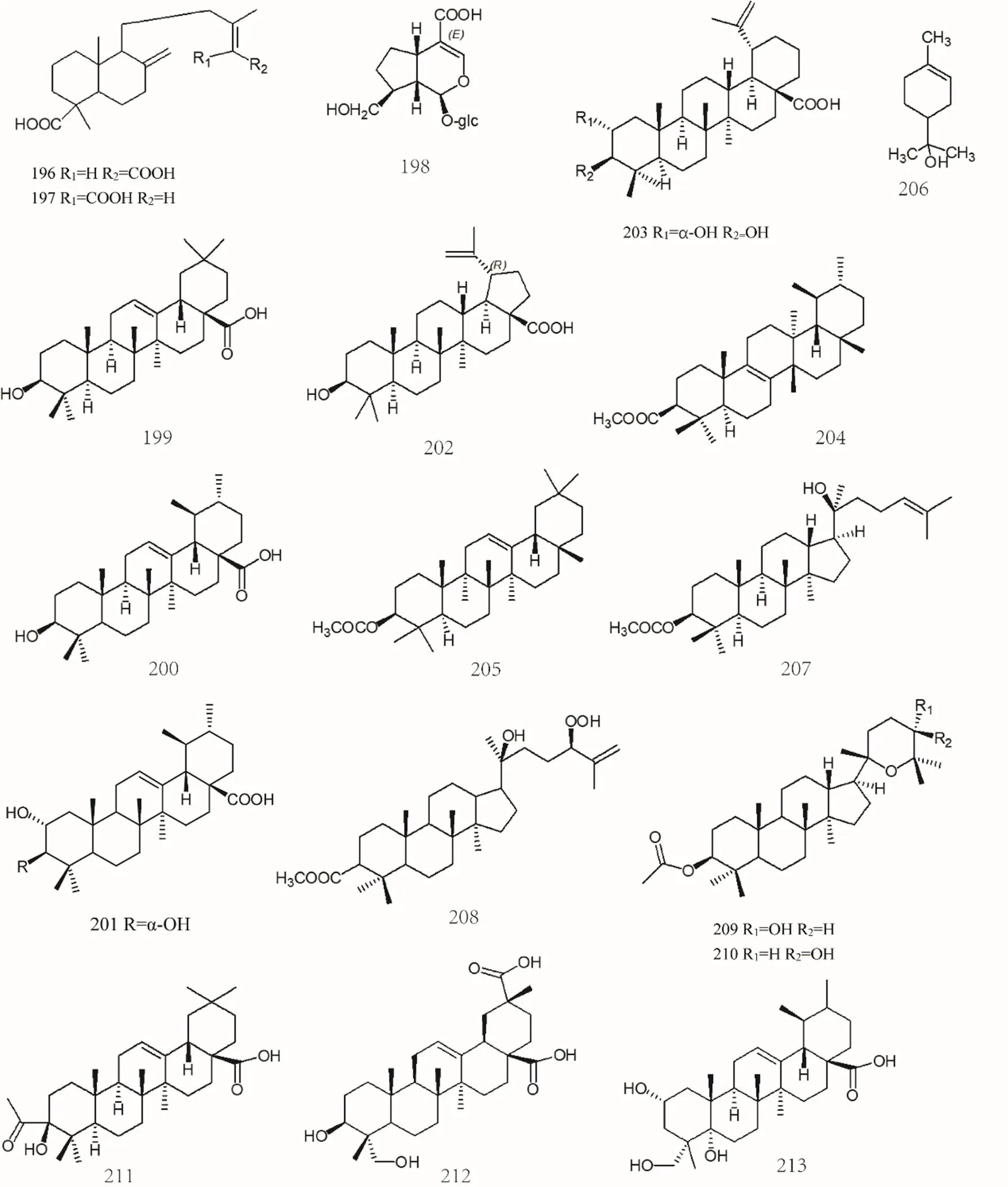
Figure 11 Chemical structures of terpenoids and volatile oils (No. 163-217)
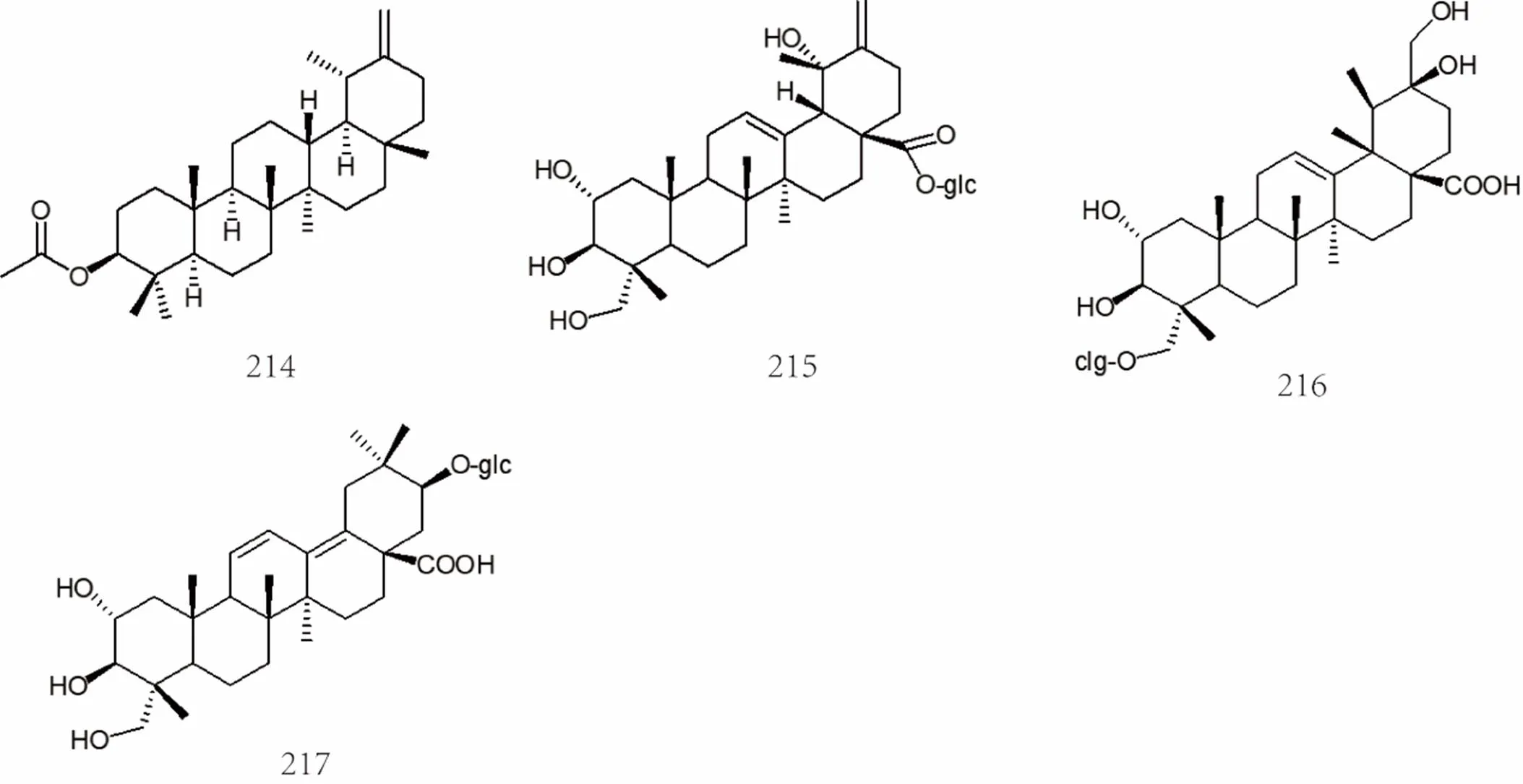
Figure 12 Chemical structures of terpenoids and volatile oils (No. 163-217)
Figure 13 Chemical structures of other compounds (No. 218-225)
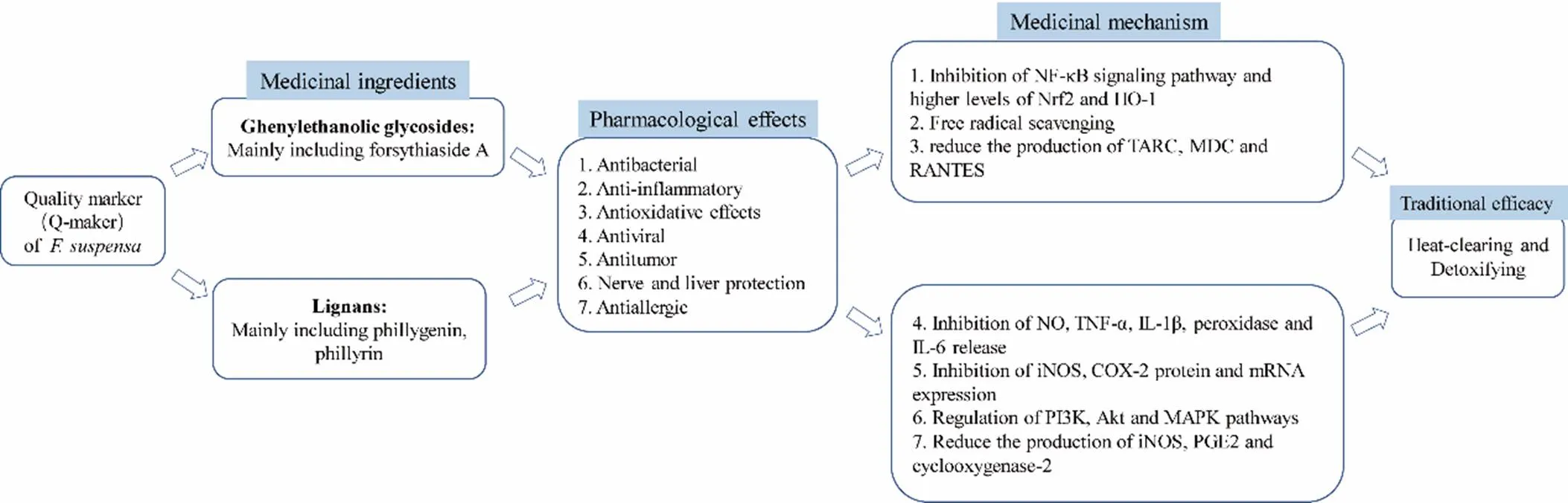
Figure 14 Inductive summary of the mechanism of drug effect
1.National Pharmacopoeia Commission. Pharmacopoeia of the People's Republic of China. China Medl Sci and Tech Press, 2015. (Chinese)
2.Gu GG(Qing dynasty). Shengnong Bencao Jing. Xueyuan Press 2007.
3.Wang T, Si XQ, Zhou GL, et al. In vivo anti-tumor effect and in vitro anti-angiogenic effect of alcohol extract from Euphorbia prostrata. China J Chin Mater. Med 2017, 42:1722-1729.
4.Han X, Piao XS, Zhang HY, et al.Extract Has the Potential to Substitute Antibiotic in Broiler Chicken. ASIAN AUSTRAL J ANIM 2012, 25: 569-76.
5.Zhang S, Shao SY, Song XY, et al. Protective effects ofextract with antioxidant and anti-inflammatory properties in a model of rotenone induced neurotoxicity. NEUROTOXICOLOGY 2016, 52:72-83.
6.Chen CC, Chen HY, Shiao MS, et al. Inhibition of Low Density Lipoprotein Oxidation by Tetrahydrofurofuran Lignans fromand Magnolia coco. PLANTA MED 1999, 65:709-711.
7.Ko HC, Wei BL, Chiou WF. Dual regulatory effect of plant extracts ofon RANTES and MCP-1 secretion in influenza A virus-infected human bronchial epithelial cells. J ETHNOPHARMACOL 2005, 102: 418-423.
8.Kim MS, Na HJ, Han SW, et al. Forsythia fructus inhibits the Mast-Cell-Mediated Allergic Inflammatory Reactions. INFLAMMATION 2003, 27:129-135.
9.Editorial Board of Flora of China Flora of China, Science Press, Beijing 1978, P 163.
10.Wang Z, Xia Q, Liu X, et al. Phytochemistry, pharmacology, quality control and future research of: A review. J ETHNOPHARMACOL 2018, 210:318-339.
11.Han J, Ye M, Guo H, et al. Analysis of multiple constituents in a Chinese herbal preparation Shuang-Huang-Lian oral liquid by HPLC-DAD-ESI-MSn. J PHARMACEUT BIOMED 2007, 44:430-438.
12.Nan WF, Qiang MZ, Ying L, et al. New phenylethanoid glycosides from the fruits of. MOLECULES 2009, 14:1324-1331.
13.Wang Y, Guo Z, Jin Y, et al. Selective enrichment with "click oligo (ethylene glycol)" column and TOF-MS characterization of simple phenylpropanoids in the fruits ofJ SEP SCI 2009, 32:2958-2966.
14.Li C, Dai Y, Duan YH, et al. A new lignan glycoside from. CHIN J NAT MEDICINES 2014, 12:697-699.
15.Yan XJ, Wen J, Xiang Y, et al. Study on Chemical Composition of Forsythia Suspension. China Tradit Herb Drugs2017,48:644-647. (Chinese)
16.Shao SY, Feng ZM, Yang YN, et al. Eight new phenylethanoid glycoside derivatives possessing potential hepatoprotective activities from the fruits of. FITOTERAPIA 2017, 122:132.
17.Qu H, Zhang Y, Chai X, et al. Isoforsythiaside, an antioxidant and antibacterial phenylethanoid glycoside isolated fromBIOORG CHEM 2012, 40:87-91.
18.Kuang HX, Xia YG, Liang J, et al. Lianqiaoxinoside B, a novel caffeoyl phenylethanoid glycoside from. MOLECULES 2011, 16:5674-5681.
19.Xia YG, Yang BY, Liang J, et al. Caffeoyl phenylethanoid glycosides from unripe fruits of. CHEM NAT COMPD+ 2015, 51:656-659.
20.Li C, Dai Y, Zhang SX, et al. Quinoid glycosides from. PHYTOCHEMISTRY 2014, 104:105-113.
21.Zhao Q, Zhang HL, Zhang X, et al. Studies on the chemical constituents of ethyl acetate in. China J Chin Mater Med 2015, 09:1755-1758.
22.Liu DD, Zhang Y, Xu SX, et al. Phenylethanoid glycosides from. J Chin Pharm Sci 1998, 7:103-105.
23.Yan XJ, Bai XY, Liu QB, et al. Two new glycosides from the fruits of. J ASIAN NAT PROD RES 2014, 16:376-382.
24.Ming DS, Yu DQ, Yu SS. Two new caffeyol glycosides from.J ASIAN NAT PROD RES 1999, 1:327-335.
25.Kuo PC, Chen GF, Yang ML, et al. Chemical constituents from the fruits ofand their antimicrobial activity. BIOMED RES INT 2014, P 1-7.
26.Chen L, Li X, Li Q, et al. Research progress on chemical constituents of Forsythia plants. Modern Medicine and Clinic 2013, 03: 441-445. (Chinese)
27.Nishibe S , Chiba M , Hisada S . Studies on the Chinese Crude Drug "Forsythiae Fructus." I. On the Constituents of Forsythiae Fructus on the Market. YAKUGAKU ZASSHI 1977, 97:1134-1137.
28.Liang J, Gong FQ, Sun HM. Simultaneous separation of eight lignans inby β-cyclodextrin-modified capillary zone electrophoresis. MOLECULES 2018, 23: 1-9.
29.Kuo PC, Hung HY, Nian CW, et al. Chemical constituents and anti-inflammatory principles from the fruits of. J NAT PROD 2017, 4:1055.
30.Song XJ. Research progress on chemical components in different parts of forsythia . Northwest Pharmaceutical Journal 2014, 02:220-222. (Chinese)
31.Li C, Wei Q, Zou ZH, et al. A lignan and a lignan derivative from the fruit of, PHYTOCHEM LETT 2019, 32: 115-118
32.Sun YS, Wang Yan J, Wang JH, et al. Determination of four components inflower from different habitats by high performance liquid chromatography. Journal of Food Safety & Quality 2014:2791-2795.
33.Luan L, Wang GL, Lin RC.Studies on the chemical constituents of extract with water from. J Chin Med Mater 2010,33:220-221.
34.Yan XJ, Peng Y, Liu ZX, et al. Three new lignan glycosides from the fruits of. J ASIAN NAT PROD RES 2014.
35.Zou QY, Deng WL, Jiang SY, et al. Studies on chemical constituents from fruits of. China J Chin Mater Med 2012, 37:57-60.
36.Feng WS, Li KK, Zheng XK. Studies on chemical constituents inChin Pharm J, 2009, 44:490-492. (Chinese)
37.Su W, Liu Q, Yang Q, et al. Separation of Four Compounds fromby Counter-Current Chromatography with Stepwise Elution. SEP SCI TECHNOL 2014, 49:2098-2104.
38.Kuang HX, Xia YG, Yang BY, et al. A New Caffeoyl Phenylethanoid Glycoside from the Unripe Fruits of. Chin J Nat Med 2009, 7:278-282.
39.Yan XJ, Wen J, Xiang Z, et al. Two new phenolic acids from the fruits of. J ASIAN NAT PROD RES 2017, 19: 254-259.
40.Wang Y, Ren LL. Research progress on chemical constituents ofand Yinqiao Jiedu Tablets. Modern Medicine and Health 2014, 09: 1333-1335. (Chinese)
41.Chang MJ, Hung TM, Min BS, et al. Lignans from the Fruits ofProtect High-Density Lipoprotein during Oxidative Stress. BIOSCI BIOTECH BIOCH 2008, 72:2750-2755.
42.Piao XL, Jang MH, Cui J, et al. Lignans from the fruits of. BIOORG MED CHEM LETT 2008, 18:1980-1984.
43.Xia W, Dong CM, Yang CF, et al. Research progress on chemical constituents and pharmacology of. Modern Chinese Medicine 2016, 12:1670-1674. (Chinese)
44.Shi T. Study on active substances of chloroform extract of. Xiamen University 2011. (Chinese)
45.Ming DS. Study on Chemical Composition and Biological Activity of Forsythia and Spider Fragrance. Beijing: China Union Medical College 1998. (Chinese)
46.Li C, Dai Y, Zhang SX, et al. Quinoid glycosides from. PHYTOCHEMISTRY 2014, 104:105-113.
47.Endo K, Hikino H. Structures of rengyol, rengyoxide, and rengyolone, new cyclohexylethane derivatives fromfruits. CAN J CHEM 1984, 62:2011-2014.
48.Seya K, Endo K, Hikino H. Structures of rengyosides A, B and C, three glucosides offruits. PHYTOCHEMISTRY 1989, 28:1495-1498.
49.Endo K, Seya K, Hikino H. Structure and enantioselective synthesis of suspenol, a new polyol of. Tennen Yuki Kagobutsu Toronkai Koen Yoshishu 1987, 29: 660-667.
50.Shao SY, Feng ZM, Yang YN, et al. Eight new phenylethanoid glycoside derivatives possessing potential hepatoprotective activities from the fruits of. FITOTERAPIA 2017, 122:132-137.
51.Wen J, Yan XJ, Liang W, et al. Two new polyhydric alcohols from fruits of. China Tradit Herb Drugs 2018, 49:278-281.
52.Zhang GG, Song SJ, Ren J, et al. A New Compound fromwith Antiviral Effect on RSV. J Herb Pharmacother 2002, 2:35-40.
53.Wang WF, Liu DL, Xu SX, Xiao FH. New compounds in forsythia. Journal of Shenyang Pharmaceutical University 1999, 02:63-70. (Chinese)
54.Kitagawa S, Nishibe S, Baba H. Studies on the Chinese Crude Drug "Forsythiae Fructus." VIII. On Isolation of Phenylpropanoid Glycosides from Fruits of Forsythia koreana and Their Antibacterial Activity. YAKUGAKU ZASSHI 1987, 107:274-278.
55.Wang L, Piao XL, Kim SW, et al. Effects ofExtract on Growth Performance, Nutrient Digestibility, and Antioxidant Activities in Broiler Chickens Under High Ambient Temperature. POULTRY SCI 2008, 87:1287-1294.
56.Wu YF, Wang XS, Yuan YL, et al. Studies on the chemical constituents ofChina Tradit Herb Drugs2013, 44:2052-2054. (Chinese)
57.Bai Y, Li J, Liu W, et al. Pharmacokinetic of 5 components after oral administration of Fructus Forsythiae by HPLC-MS/MS and the effects of harvest time and administration times. J CHROMATOGR B 2015, P:36-46.
58.Liu Y, Song SJ, Xu SX, Fu XH. Study on Chemical Composition ofJournal of Shenyang Pharmaceutical University 2003, 02:101-103. (Chinese)
59.Cui Y, Wang Q, Shi X, et al. Simultaneous Quantification of 14 Bioactive Constituents inby Liquid Chromatography-Electrospray Ionisation-Mass Spectrometry. PHYTOCHEM ANALYSIS 2010, 21:253-260.
60.Zhang F, Yang YN, Song XY, et al. Forsythoneosides A-D, neuroprotective phenethanoid and flavone glycoside heterodimers from the Fruits of. J NAT PROD 2015, 78:2390-2397.
61.Yan XJ, Xiang Y, Wen J, et al. Study on the chemical constituents of phenolic acids in. Chinese Pharmaceutical Journal 2017, 02: 105-108. (Chinese)
62.Ge Y, Wang Y, Chen P, et al. Polyhydroxytriterpenoids and Phenolic Constituents fromLeaves. J AGR FOOD CHEM 2015, 64: 125-131.
63.Ming DS, Yu DQ, Yu SS, New Quinoid Glycosides from. J NAT PROD 1998, 61: 377-9.
64.Xue J, Xie Li, Liu BR, et al. Triterpenoids from the Fruits of. CHIN J NAT MEDICINES 2010,8:414-418.
65.Bai XL. Study on the chemical constituents of traditional Chinese medicine forsythia. Heilongjiang Medicine 2016, 29:601-602. (Chinese)
66.El-Desouky SK, Kim YK. A phenylethanoid glycoside and other constituents from the Fruits of Forsythia koreana.Z NATURFORSCH B 2014, 63:90-94.
67.Ming DS, Yu DQ, Yu SS, et al. A new furofuran mono-lactone from. J ASIAN NAT PROD RES 1999,1: 221-226.
68.Ming DS, Yu DQ, Yu SS. Three new compounds from. CHINESE CHEM LETT 1998, 01:51-55.
69.Yan XJ. Research progress on chemical composition and pharmacological activity ofChinese Commodity Association. Proceedings of the 5th National Congress of Chinese Medicine Commodities of Chinese Commodity Association. Chinese Commodity Association: Chinese Commodity Association 2017, P: 558-580. (Chinese)
70.Jiao J, Fu YJ, Zu YG, et al. Enzyme-assisted microwave hydro-distillation essential oil from Fructus forsythia, chemical constituents, and its antimicrobial and antioxidant activities. FOOD CHEM 2012, 134:235-243.
71.Yang JJ, Wei HM, Teng XN, et al. Dynamic ultrasonic nebulisation extraction coupled with headspace ionic liquid-based single-drop microextraction for the analysis of the essential oil in. PHYTOCHEM ANALYSIS 2014, 25:178-84.
72.Zhang Y, Xue KP, Zhao EY, et al. Determination of oleanolic acid and ursolic acid in Chinese medicinal plants using HPLC with PAH polymeric C18. PHARMACOGN MAG 2013, 9: S19-24.
73.Fang Y, Zou GA, Liu YW. Chemical Composition of.China Natural Medicine 2008, 03: 235-236. (Chinese)
74.Zhang Q, Lu Z, Li X, et al. Triterpenoids and Steroids from the Leaves of. CHEM NAT COMPD+ 2015, 51:178-180.
75.Rouf AS, Ozaki Y, Rashid MA, et al. Dammarane derivatives from the dried fruits of. PHYTOCHEMISTRY 2001, 56:815-818.
76.Dai S, Ren Y, Shen L, et al. New Alkaloids fromand their anti-inflammatory activities. PLANTA MED 2009, 75:375-377.
77.Guo Q, Wang ZM, Lin LM, et al. Research Progress on Chemical Components of Medicinal Plants of Forsythia. China Journal of Experimental Traditional Medical Formulae 2009,15:74-79. (Chinese)
78.Guo YP, Lin LG, Wang YT. Chemistry and pharmacology of the herb pair Flos Lonicerae japonicae-Forsythiae fructus. CHIN MED-UK 2015, 10:16.
79.Bai YE, Qi XM, Yang GH, et al. In vitro antibacterial test of forsythia extract. Journal of Shanxi Medical University 2003, 06:506-507. (Chinese)
80.Han X, Piao XS, Zhang HY, et al.extract has the potential to substitute antibiotic in broiler chicken. ASIAN AUSTRAL J ANIM 2012, 25: 569-576.
81.Feng SY, Li XR, Sun JN. Study on anti-infection and antipyretic effect of forsythiaside. Progress in Modern Biomedicine 2006, 10: 73-75. (Chinese)
82.Chen CL, Zhang DD. Anti-Inflammatory effects of 81 Chinese herb extracts and their correlation with the characteristics of Traditional Chinese Medicine. EVID-BASED COMPL ALT 2014, P:1-8.
83.Wang TT, Zhang Y, Yang ZM, et al. Study on antibacterial and anti-inflammatory activity and its main chemical constituents ofandleaves. Chin Drug Clin Med 2019,19: 2380-2381. (Chinese)
84.Yuan A, Gong L, Luo L, et al. Revealing anti-inflammation mechanism of water-extract and oil of forsythiae fructus on carrageenan-Induced edema rats by serum metabolomics. BIOMED PHARMACOTHER 2017, 95:929-937.
85.Hwang YH, Kim DG, Li W, et al. Anti-inflammatory effects ofin dextran sulfate sodium-induced coliti. J ETHNOPHARMACOL 2017, 206:73-77.
86.Zhang XT, Ding Y, Kang P, et al. Forsythoside A modulates zymosan-induced peritonitis in mice. MOLECULES 2018, 23:593.
87.Lee JJ, Kim KH, Kim EJ, et al. Anti-inflammatory activity of the decoction ofis related to Nrf2 and A20. J ETHNOPHARMACOL 2018, 227:97-104.
88.Rothschild DE, Mcdaniel DK, Ringel-Scaia VM, et al. Modulating inflammation through the negative regulation of NF-κB signaling. J LEUKOCYTE BIOL 2018.
89.Hwang YH, Kim DG, Li W, et al. Anti-inflammatory effects ofin dextran sulfate sodium-induced colitis. J ETHNOPHARMACOL 2017, 206.
90.Wang Y, Zhao H, Lin C, et al. Forsythiaside A exhibits anti-inflammatory effects in LPS-Stimulated BV2 microglia cells through activation of Nrf2/HO-1 signaling pathway. NEUROCHEM RES 2016, 41:659-665.
91.Zhong WT, Wu YC, Xie XX, et al. Phillyrin attenuates LPS-induced pulmonary inflammation via suppression of MAPK and NF-κB activation in acute lung injury mice. FITOTERAPIA 2013, 90:132-139.
92.Lee S, Shin S, Kim H, et al. Anti-inflammatory function of arctiin by inhibiting COX-2 expression via NF-κB pathways. J INFLAMM-LOND 2011, 8: 16.
93.Muluye RA, Bian Y, Alemu PN. Anti-inflammatory and antimicrobial effects of heat-clearing Chinese Herbs: A Current Review. J TRADIT COMPLEM MED 2014, 4:93-98.
94.Guo YP, Lin LG, Wang YT. Chemistry and pharmacology of the herb pair Flos Lonicerae japonicae-Forsythiae fructus. CHIN MED-UK 2015, 10:16.
95.Qu XY, Li QJ, Zhang HM, et al. Protective effects of phillyrin against influenza A virus in vivo. ARCH PHARM RES 2016, 39:998-1005.
96.Law HY, Yang LH, Lau SY, et al. Antiviral effect of forsythoside A fromfruit against influenza A virus through reduction of viral M1 protein. J ETHNOPHARMACOL 2017, 209: 236-247.
97.Li D, Peng P, Ke Z, et al. Forsythoside A Controls Influenza A Virus Infection and Improves the Prognosis by Inhibiting Virus Replication in Mice. MOLECULES 2016, 21:524.
98.Yang Y. Screening of single Chinese medicine against swine influenza virus in vitro. Tai an: Shandong Agricultural University 2013. (Chinese)
99.Yuan A, Zhao MJ, Li Y, et al. A review of the pharmacological effects offorsythia.Traditional Chinese Medicine and Clinical Medicine 2015,6: 56-59. (Chinese)
100.Bao J, Ding R, Zou L, et al. Forsythiae Fructus inhibits B16 melanoma growth involving MAPKs/Nrf2/HO-1 mediated anti-oxidation and anti-Inflammation. AM J CHINESE MED 2016, 44(05):1043-1061.
101.Pan CW, Zhou GY, Chen WL, et al. Protective effect of forsythiaside A on lipopolysaccharide/d-galactosamine-induced liver injury. INT IMMUNOPHARMACOL 2015, 26:80-85.
102.Qu H, Zhang Y, Chai X, et al. Isoforsythiaside, an antioxidant and antibacterial phenylethanoid glycoside isolated fromBIOORG CHEM 2012, 40:87-91.
103.Lu T, Piao XL, Zhang Q, et al. Protective effects ofextract against oxidative stress induced by diquat in rats. Food & Chemical Toxicology An International Journal Published for the British Industrial Biological Research Association 2010, 48:764-770.
104.Piao XL, Cho EJ, Cho EJ, et al. Cytoprotective effect of lignans fromagainst peroxynitrite-induced LLC-PK1 cell damage. PHYTOTHER RES 2009, 23: 938-942.
105.Huang CK, Lin Y, Su H, et al. Forsythiaside Protects Against Hydrogen Peroxide-Induced Oxidative Stress and Apoptosis in PC12 Cell. NEUROCHEM RES 2015, 40:27-35.
106.Ge Y, Wang Y, Chen P, et al. Polyhydroxytriterpenoids and phenolic constituents fromLeaves. J AGR FOOD CHEM 2015, 64125-131.
107.Sung YY, Yoon T, Jang S, et al.suppresses house dust mite extract-induced atopic dermatitis in NC/Nga mice. PLOS ONE 2016, 11, e0167687.
108.Hao Y, Li D, Piao X, et al.extract alleviates hypersensitivity induced by soybean β-conglycinin in weaned piglets. J ETHNOPHARMACOL 2010,128:412-418.
109.Wang HM, Wang LW, Liu XM, et al. Neuroprotective effects of forsythiaside on learning and memory deficits in senescence-accelerated mouse prone (SAMP8) mice. PHARMACOL BIOCHEM BE 2013, 105:134-141.
110.Zhang S, Shao SY, Song XY, et al. Protective effects ofextract with antioxidant and anti-inflammatory properties in a model of rotenone induced neurotoxicity. NEUROTOXICOLOGY 2016, 52:72-83.
111.Kim JM, Kim S , Kim DH, et al. Neuroprotective effect of forsythiaside against transient cerebral global ischemia in gerbil. EUR J PHARMACOL 2011, 660:326-333.
112.Iizuka T, Nagai M. Vasorelaxant effects of forsythiaside from the Fruits ofYAKUGAKU ZASSHI 2005, 125:219-224.
113.Hao FF. Experimental study ofand its effect on gastrointestinal motility in baohe pills. Shandong University of Traditional Chinese Medicine 2018. (Chinese)
114.Liu CX, Chen SL, Xiao XH, et al. Q-Marker: A New Concept of Quality Control of Traditional Chinese Medicine Products. China Tradit Herb Drugs 2016, 47: 1443-1457. (Chinese)
115.Liu CX. Construction of Chinese Medicine Quality Traceability System Based on Chinese Medicine Quality Markers. China Tradit Herb Drugs 2017 48:3669-3676. (Chinese)
116.Liu CX, Chen SL, Xiao XH, et al. Q-Marker of Chinese Medicine: A New Concept of Quality Control of Traditional Chinese Medicine Products. China Tradit Herb Drugs 2016,47:1443-1457. (Chinese)
117.Liu CX. Q-marker of Traditional Chinese Medicine: Improving the Quality Standard of Traditional Chinese Medicine and Quality Control Theory and Promoting the Scientific Development of Traditional Chinese Medicine Industry. China Tradit Herb Drugs 2019,50:4517-4518. (Chinese)
118.Zhang TJ, Bai G, Liu CX. The concept, core theory and research methods of quality markers of traditional Chinese medicine. ACTA PHARM SIN B 2019,54:187-196 + 186. (Chinese)
119.Xue QQ, Li AP, Li K, et al. Summary of Quality Evaluation Research of Astragalus and Preliminary Exploration of Quality Marker Research Strategy. Drug Evaluation Research 2019,42:2459-2463. (Chinese)
120.Zhang HJ, Gong SX, Xu J, et al. Research Progress and Prediction and Analysis of Quality Markers of Alisma orientalis. China Tradit Herb Drugs 2019,50:4741-4751. (Chinese)
121.Hao M, Tong HJ, Zhang J, et al. Study on Quality Markers of Chinese Herbal Pieces: Study on Quality Evaluation of Zedoary Rhizoma Pieces and Discussion on Quality Standards. China Tradit Herb Drugs 2019, 50: 4673-4682. (Chinese)
122.Zhang TJ, Xu J, Han YQ, et al. Research on quality markers of hippocampus tonifying kidney pills. China Tradit Herb Drugs 2019,50:4613-4619. (Chinese)
123.Li YX, Meng QM. Research on Herbal Medicine of Forsythia. J Chin Med Mater 2002, 06:435-437. (Chinese)
124.Wang N. Research on Herbs of. J Chin Med Mater 2013,36:670-674. (Chinese)
125.Hu NH, Wang C, Dai XY, et al. Phillygenin inhibits LPS-induced activation and inflammation of LX2 cells by TLR4/MyD88/NF-κB signaling pathway. J ETHNOPHARMACOL 2020, 248: 112361.
126.Zou SS, Xuan ZY. Effects of different routes of administration of forsythiaside on the fever model induced by parainfluenza virus in rabbits. Pharmacol Clin Chin Mater Med 2015,31:57-59. (Chinese)
127.Zheng X, Fu YJ, Shi SS, et al. Effect of Forsythiaside A on the RLRs Signaling Pathway in the Lungs of Mice Infected with the Influenza A Virus FM1 Strain. MOLECULES 2019,24: 4219.
128.Jiang CX, Zhang TJ, Chen CQ, et al. Research progress of Huang Jing and prediction analysis of its quality markers, China Tradit Herb Drugs 2017, 48:1-16. (Chinese)
129.Zhang JY, Cao H, Xu J, et al. Sexual expression of bitterness of traditional Chinese medicine and its application in clinical compatibility. China Tradit Herb Drugs 2016,47:187-193. (Chinese)
130.Cheng Y, Liang XL, Feng LY, et al. Effects of phillyrin and forsythoside A on rat cytochrome P450 activities in vivo and in vitro. XENOBIOTICA 2016,47:1-7.
131.Zhang YY, Feng F, Chen T, et al. Antidiabetic and antihyperlipidemic activities ofin streptozotocin-induced diabetes mice. J ETHNOPHARMACOL 2016, 192:256-263.
132.Leng W, Liu CY, Shang C, et al. Protective effect and mechanism of forsythin on diabetic nephropathy rats. Chinese Journal of Immunology 2019,35:2604-2608. (Chinese)
133.Bai Y, Li J, Liu W, et al. Pharmacokinetic of 5 components after oral administration of Fructus Forsythiae by HPLC-MS/MS and the effects of harvest time and administration times. J Chromatogr B Analyt Technol Biomed Life Sci 2015, e:36-46.
134.Li C, Yao ZH, Qin ZF, et al. Isolation and identification of phase I metabolites of phillyrin in rats. FITOTERAPIA 2014, 97:92-97.
135.Tang L. Study on packaging and storage of forsythia. Henan Univ 2019. (Chinese)
136.Wang SB, Zheng YJ. Stability of forsythiaside A in forsythia extract and forsythiaside A raw material. China Tradit Herb Drugs 2010,41:909-911. (Chinese)
The new quality evaluation system takes traditional Chinese medicine (TCM) quality markers as its core concept, and lays foundation for the future analysis of the quality markers of Lianqiao (, Abbreviated as “” below), in order to establish aquality control and quality traceability system.
is a common medicinal plant in China, and it grows in hillside shrubs, underwoods, grasses, or in valleys. It can effectively improve immune functions, and have obvious curative effects in protecting nerves, anti-inflammatory, anti-virus, delaying aging, and reduce tumorigenesis.
Abbreviations: Q-marker, Quality marker;,; TCM, traditional Chinese medicine; MIC, minimum inhibitory concentration; cAMP, Cyclic adenosine monophosphate; RT-PCR, Reverse Transcription-Polymerase Chain Reaction; LPS, Lipopolysaccharide; TARC, Thymus activation regulating chemokine; MDC, Macrophage-derived chemokine; MAPK, Mitogen-activated protein kinase; IC50, half maximal inhibitory concentration; iNOS, inducible nitric oxide synthetase; PGE2, prostaglandin E2; HO-1, heme oxygenase 1; TNF-α, tumor necrosis factor-α; IL-6, interleukin-6; MCP-1, monocyte chemoattractant protein-1; IL-1β, interleukin-1β; E. coli, Escherichia coli; MDA, malondialdehyde; GalN, D-galactosamine; DPPH, 1,1-diphenyl-2-picrylhydrazyl; ABTS, 2, 2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid); EAF, the ethyl acetate extract of F. suspensa; MyD88, Myeloid differentiation primary response gene 88; TLR-4, toll like receptor-4; NE, norepinephrine.
Competing interests: The authors declare that there is no conflict of interests regarding the publication of this paper.
Online: 6March 2020.
:Peng H, Lu P, Liu YT, et al. Research progress on chemical composition, pharmacological effects of Forsythia suspensa (Thunb.) Vahl and predictive analysis on Q-marker. TMR Modern Herbal Medicine 2020, 3(2):86-112.
Executive Editor: Chaoyong Wu
Submitted: 16 February 2020,
28 February 2020,
*Correspondence to: Zhidong Liu, Tianjin State Key Laboratory of Modern Chinese Medicine, Tianjin University of Traditional Chinese Medicine, Tianjin 301617, China. Tel: +86-22-59596170; Email: liuzhidong@tjutcm.edu.cn
#These authors contributed equally to this work.
 TMR Modern Herbal Medicine2020年2期
TMR Modern Herbal Medicine2020年2期
- TMR Modern Herbal Medicine的其它文章
- Dendrobium huoshanense improves doxorubicin-induced heart failure in C57BL/6 mice
- The functional components and mechanism of Linderae Radix in treating diabetic nephropathy based on the network pharmacology
- The research idea of Xuebijing injection in influencing severe pneumonia-pulmonary fibrosis with blood stasis syndrome evolution by inhibiting inflammation, endotoxin and dispersing blood stasis
- Effects of theophylline/chitosan/β-cyclodextrin microspheres for oral drug delivery on an asthmatic rat model
- The roles of traditional Chinese herbal medications in regulating mitochondrial activity to reverse cancer
