The role of exercise in brain DNA damage
Thais Ceresér Vilela, Vanessa Moraes de Andrade, Zsolt Radak, Ricardo Aurino de Pinho
1 Laboratory of Translational Biomedicine, Graduate Program of Health Sciences, University of Southern Santa Catarina - UNESC, Criciúma, SC,Brazil
2 Research Institute of Sport Science, University of Physical Education, Budapest, Hungary
3 Laboratory of Exercise Biochemistry in Health, Graduate Program in Health Sciences, School of Medicine, Pontifícia Universidade Católica do Paraná, Curitiba, PR, Brazil
Abstract Cells are constantly subjected to cytotoxic and genotoxic insults resulting in the accumulation of unrepaired damaged DNA, which leads to neuronal death. In this way, DNA damage has been implicated in the pathogenesis of neurological disorders, cancer, and aging. Lifestyle factors, such as physical exercise, are neuroprotective and increase brain function by improving cognition, learning, and memory, in addition to regulating the cellular redox milieu. Several mechanisms are associated with the effects of exercise in the brain, such as reduced production of oxidants, up-regulation of antioxidant capacity, and a consequent decrease in nuclear DNA damage. Furthermore, physical exercise is a potential strategy for further DNA damage repair. However, the neuroplasticity molecules that respond to different aspects of physical exercise remain unknown. In this review, we discuss the influence of exercise on DNA damage and adjacent mechanisms in the brain. We discuss the results of several studies that focus on the effects of physical exercise on brain DNA damage.
Key Words: aerobic exercise; apoptosis; brain-derived neurotrophic factor; brain; DNA damage; DNA repair;neurodegenerative disease; oxidative stress; physical exercise; strength exercise
Introduction
Cells are often subject to endogenous (metabolic intermediate products), and exogenous (like radiation and chemical substances), insults that result in genetic and epigenetic alterations (Gluckman et al., 2009; Hoeijmakers, 2009) and the accumulation of unrepaired damaged DNA. In this way, the epigenetic changes include aberrant DNA methylation, microRNA (miRNA) and noncoding RNA deregulation and alterations in histone modification states (Dumitrescu, 2018).The common forms of DNA damage, in turn, include single and double-stranded breaks, interstrand DNA crosslink,DNA protein-crosslink, single or multiple base damage and chromosomal damage (Klages and Li, 2017). The DNA damage is a threat to cell viability, compromising the integrity of the genome and the epigenome (Dabin et al., 2016).
The DNA damage, also, leads to neuronal death and is associated with memory impairments and neural deterioration(Bird, 2002). Significant DNA damage activates specific signaling pathways, such as pro-apoptotic or DNA repair proteins. Cells either die or survive depending on the dimension of the damage and the outcome of repair (Rass et al., 2007;Yang et al., 2014). Therefore, DNA damage is associated with the pathogenesis of neurological disorders (like Parkinson and Alzheimer’s diseases), cancer, and aging (Hoeijmakers 2009; Lovell and Markesbery 2017; Poewe et al., 2017).
Exogenous stimuli have been implicated in the protection and/or delay of DNA damage, including physical exercise(Soares et al., 2015). Several studies have reported that physical exercise is neuroprotective and increases brain function by improving plasticity, cognition, learning, and memory(Van Praag et al., 2005; Tuon et al., 2015; Baek, 2016; Vilela et al., 2016), in addition to regulating the cellular redox milieu (Pinho et al., 2019). This regulation induces antioxidant production, which is associated with a reduction in reactive oxygen species and damage to lipids, protein, and DNA (Radak et al., 2013, 2016). Physical exercise alters the cerebral redox state in a type-dependent manner; acute and regular exercise exerts different effects on redox state (Shahandeh et al., 2013). For example, the adaptive response is impaired following acute exercise, whereas regular exercise induces an adaptation on redox signaling that is associated with a wide range of physiological processes (Radak et al., 2016).
Genetic mechanisms, such as DNA methylation, oxidation, and damage are important in the development of different brain diseases and neurodegeneration (Madabhushi et al., 2014). Studies have shown that this can be minimized with adequate doses of lifelong physical exercise (Yang et al., 2014; Fernandes et al., 2017); however, the mechanisms underlying the long-lasting effects of regular exercise on the brain DNA are not fully understood. Most studies have focused on cellular and molecular changes in skeletal muscle. In this review, we discuss the influence of the exercise on DNA damage and their adjacent mechanisms in the brain.
Search Strategy and Selection Criteria
Systematic database searches of PubMed, Web of Science,Scopus, and MEDLINE were performed to identify peer-reviewed studies from the 2000s. Combinations of keywords related to brain disease, aerobic/resistance or strength physical exercise, and DNA damage were used. Other variables were not addressed in this review but should be considered to gain a complete understanding of the effects of physical exercise on brain DNA damage.
Effect of Different Models of Physical Exercise on Brain
There is little evidence addressing the influence of exercise training itself on the brain and the findings are conflicting due to the use of different exercise models (aerobic, strength,combined) and protocols (duration and intensity; Kim et al.,2005; Ogonovszky et al., 2005; Alghadir et al., 2015; Aguiar et al., 2016). Most research assessing the neuroprotective effects of the exercise focuses on aerobic training alone; therefore, the benefits of other exercise protocols, such as strength training, remain unknown. Most experimental studies have used an treadmill- (Speck et al., 2011; Chen et al., 2012; Yang et al., 2014; Vilela et al., 2016) or swimming pool- (Souza et al., 2009; Nonato et al., 2016) based aerobic training program. However, recent studies have revealed some positive effects of resistance training (Vilela et al., 2016).
Regardless of the type or method of exercise, it is clear that physical exercise improves oxidative status and memory, and prevents neurodegenerative disease progression (Patki et al., 2014; Yau et al., 2015; Vilela et al., 2016). This may occur because physical training increases the level of neurotrophic factors in the brain, which induce neurogenesis, synaptogenesis, neuroplasticity, and angiogenesis (Aguiar et al., 2011;Portugal et al., 2015; Kim et al., 2016; Radak et al., 2016).We have observed that treadmill exercise ameliorates spatial memory in aging rats through brain-derived neurotrophic factor (BDNF) signaling, which regulates proteins of the glutamatergic system (Vilela et al., 2016). Previous studies have reported that swimming training attenuates oxidative damage, increases enzymatic antioxidants in the rat brain, and improves depressive-like behavior in chronically stressed rats (Liu et al., 2013; Nonato et al., 2016). Hsu et al. (2018)have shown that aerobic training in older adults with mild subcortical ischemia can improve executive function and the neural efficiency of associated brain areas. Furthermore,treadmill exercise improves motor function via the preservation of nigrostriatal dopaminergic neurons and fibers and in rats with rotenone-induced Parkinson’s disease (Manoharan et al., 2016; Shin et al., 2017). Aerobic training improves brain energy metabolism by increasing ketone uptake and utilization while maintaining brain glucose uptake in Alzheimer’s disease. This may be associated with some cognitive improvement (Castellano et al., 2017).
Resistance exercise confers cognitive and mental health benefits in humans (Mavros et al., 2017; Gordon et al., 2018);therefore, it is critical that animal models are developed and evaluated to examine the underlying neurobiological systems. Hornberger and Farrar (2004) have described an experimental model of progressive resistance exercise in the rat. This is a practical application of the overload principle and forms the basis of most human resistance training programs. Previously, we have reported that this experimental model improved spatial memory possibly via protein kinase C activation, which activates signaling pathways that are crucial for the formation of many distinct types of memory(Vilela et al., 2016). Furthermore, Tuon et al. (2014) have demonstrated that treadmill and strength training prevent depressive-like behavior and promote neuroprotection. This may, in turn, regulate both mitochondrial function and neuroinflammation in an experimental model of Parkinson’s disease. Another study from our group has shown that strength and endurance training attenuates the progression and pathological hallmarks of experimental autoimmune encephalomyelitis, thereby representing an important non-pharmacological intervention for the improvement of immune-mediated diseases, such as multiple sclerosis (Souza et al., 2017). Interestingly, Özbeyli et al. (2017) have shown that aerobic, resistance and combined exercise is protective against the development of Alzheimer’s Disease by reducing oxidative stress and improving the antioxidant system and brain plasticity. However, the specific molecular mechanisms of neuroplasticity that respond to strength training or other models remain undetermined.
Effect of Physical Exercise on DNA Damage
The DNA damage is dependent on cellular repair capacity,which can reverse DNA failure. Previous research has suggested that it may be possible to increase the DNA repair ability of neurons via BDNF regulation (Yang et al., 2014).The neuroprotective effects of exercise are mediated by BDNF (Marais et al., 2009; Aguiar et al., 2011), which binds to the tropomyosin receptor kinase B receptor and activates intracellular signaling pathways (Yang et al., 2014). BDNF increases neuronal DNA repair via activation of cyclic AMP response element-binding protein (CREB), which mediates expression of APE1, a key enzyme for base excision in the DNA repair pathway (Marais et al., 2009).
Previous results from our group have shown that aerobic and strength training improves spatial memory in aging rats via different mechanisms (Vilela et al., 2016). Interestingly,we observed a reduction in DNA damage after treadmill training alone, as previously reported (Yang et al., 2014;Soares et al., 2015; Vilela et al., 2016). We found an increase in BDNF and CREB; however, there were no significant alterations in the expression of APE1. This suggested that other mechanisms are involved in the reduction of DNA damage (Figure 1).
Enzymes such as 8-oxoguanine DNA glycosylase-1, which is primarily responsible for removing 8-hydroxy-2′-deoxyguanosine from DNA, plays a central role in the base excision repair system (Iida et al., 2002; de Souza-Pinto et al., 2008). In addition, the gene expression of 8-oxoguanine DNA glycosylase-1 is upregulated by redox state alterations(Adaikalakoteswari et al., 2007). Several studies have observed that exercise can increase the activity of base excision repair enzymes in human and animal models (Radak et al.,2009; Koltai et al., 2010; Siu et al., 2011). However, few studies have investigated the effects of exercise training on DNA damage and repair in the brain (Table 1).
Soares et al. (2015) have shown that the decrease in oxidative damage associated with exercise training may be explained by an increase in antioxidant and metabolic effi-ciency. Further, Radak et al. (2002) have shown that regular exercise-induced adaptation attenuates the age-associated increase in 8-hydroxy-2′-deoxyguanosine levels and increases DNA repair activity and resistance against oxidative stress in proteins. Interestingly, similar results were observed in a human study using a single-bout of physical exercise; they found increases in the activity of the 8-oxoG repair enzyme in skeletal muscle (Radák et al., 2003). Human models have argued that a reduction in DNA damage after training is related to a decrease in prooxidant cellular conditions (Siu et al., 2011; Pittaluga et al., 2015; Soares et al., 2015). However,strenuous endurance exercise can cause muscle and lymphocyte DNA damage and the extent of this damage increases as running distance increases (Ryu et al., 2016).
Results are scarce and conflicting for the effects of exercise on DNA in the brain. Ogonovszky et al. (2005) have shown that exercise training, even with a very severe protocol, is unlikely to cause significant oxidative damage to brain DNA.Other studies have shown that regular aerobic exercise decreases the accumulation of DNA damage (Kim et al., 2010;Yang et al., 2014; Vilela et al., 2016). Importantly, the brain is susceptible to the damaging effects of reactive oxygen species due to a very high rate of metabolism and oxygen consumption, and specific biochemical reactions that generate free radicals (Manoharan et al., 2016). Therefore, several mechanisms may be related to the effects of exercise in the brain, such as reduced production of oxidants, up-regulation of antioxidant capacity, and consequent decrease of nuclear DNA damage (Soares et al., 2015; Figure 2). Furthermore,chronic exercise has a positive response to the brain, which is a different effect when compared with other organs (Liu et al., 2000). Therefore, we can understand its role in novel therapeutic and rehabilitation strategies by further elucidating exercise-induced mechanisms in the brain.
Conclusion
The DNA damage is a common feature of neurodegenerativeillnesses and the ability to repair this damage is crucial for neuronal survival. Data from previous studies have revealed clear evidence that physical exercise induces profound genetic alterations that regulate synaptic and brain plasticity.Furthermore, exercise may be fundamental in the control of the neurological disease; however, a better understanding of the effects of different types of exercise is required. Future studies in animals and humans may address these gaps in knowledge and contribute to understanding the effects of physical exercise on the brain.
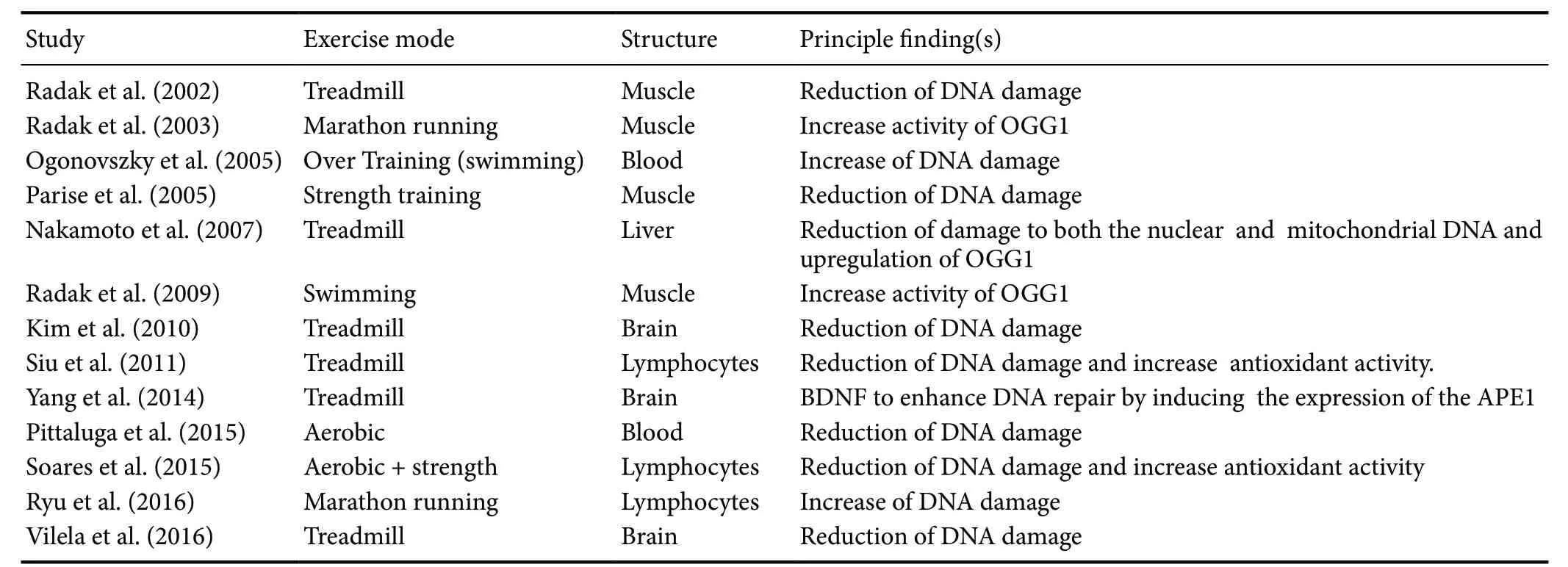
Table 1 Effects of exercise training on DNA damage and repair in human and animal models
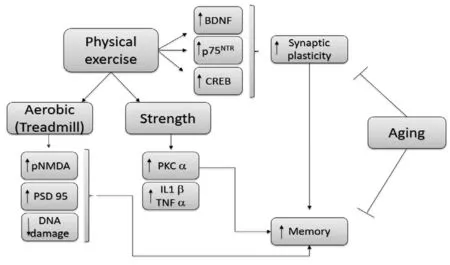
Figure 1 Explanatory scheme of the effect of two physical training models on neurochemical parameters in the hippocampus of old rats.
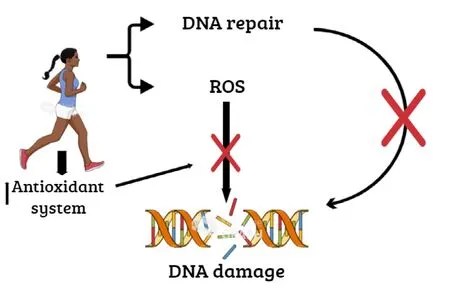
Figure 2 Explanatory scheme of the effect of exercise on DNA damage.
Acknowledgments:We thank the National Counsel of Technological and Scientific Development-CNPq/Brazil, Coordination for the Improvement of Higher Education Personnel-CAPES/Brazil, and Research Fund of the Pontifícia Universidade Católica do Paraná for contributing to research activities.
Author contributions:TCV, VMA, ZR and RAP contributed to literature searching and the manuscript writing. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Conflicts of interest:The authors declare no conflicts of interest.
Financial support:None.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- The role of the TrkB-T1 receptor in the neurotrophin-4/5 antagonism of brain-derived neurotrophic factor on corticostriatal synaptic transmission
- Could non-invasive brain-stimulation prevent neuronal degeneration upon ion channel re-distribution and ion accumulation after demyelination?
- Combined effect of repetitive transcranial magnetic stimulation and physical exercise on cortical plasticity
- Should mast cells be considered therapeutic targets in multiple sclerosis?
- Neuroprotection mediated by natural products and their chemical derivatives
- Reliable cell purification and determination of cell purity: crucial aspects of olfactory ensheathing cell transplantation for spinal cord repair