Reliable cell purification and determination of cell purity: crucial aspects of olfactory ensheathing cell transplantation for spinal cord repair
Ronak Reshamwala , Megha Shah , Lucy Belt , Jenny A. K. Ekberg , , James A. St John ,
1 Griffith Institute for Drug Discovery, Griffith University, Brisbane, QLD, Australia
2 Menzies Health Institute Queensland, Griffith University, Southport, QLD, Australia
3 Clem Jones Centre for Neurobiology and Stem Cell Research, Griffith University, Brisbane, QLD, Australia
Abstract Transplantation of olfactory ensheathing cells, the glia of the primary olfactory nervous system, has been trialed for spinal cord injury repair with promising but variable outcomes in animals and humans. Olfactory ensheathing cells can be harvested either from the lamina propria beneath the neuroepithelium in the nasal cavity, or from the olfactory bulb in the brain. As these areas contain several other cell types, isolating and purifying olfactory ensheathing cells is a critical part of the process. It is largely unknown how contaminating cells such as fibroblasts, other glial cell types and supporting cells affect olfactory ensheathing cell function post-transplantation; these cells may also cause unwanted side-effects. It is also, however, possible that the presence of some of the contaminant cells can improve outcomes. Here, we reviewed the last decade of olfactory ensheathing cell transplantation studies in rodents, with a focus on olfactory ensheathing cell purity. We analyzed how purification methods and resultant cell purity differed between olfactory mucosa- and olfactory bulb-derived cell preparations. We analyzed how the studies reported on olfactory ensheathing cell purity and which criteria were used to define cells as olfactory ensheathing cells. Finally,we analyzed the correlation between cell purity and transplantation outcomes. We found that olfactory bulb-derived olfactory ensheathing cell preparations are typically purer than mucosa-derived preparations.We concluded that there is an association between high olfactory ensheathing cell purity and favourable outcomes, but the lack of olfactory ensheathing cell-specific markers severely hampers the field.
Key Words: antibody; astrocyte; fibroblast; glia; glial cell; injury; nerve; neuron trauma; surgery
Introduction
Olfactory ensheathing cells (OECs) are considered crucial for the constant regeneration of the primary olfactory nervous system that occurs throughout life (Graziadei and Graziadei, 1979; Graziadei and Monti Graziadei, 1980,1985). For this reason, transplantation of OECs to repair the injured nervous system, in particular the spinal cord, has been the focus of research efforts over the last two decades(Munoz-Quiles et al., 2009; Granger et al., 2012; Tabakow et al., 2013, 2014). In their natural environment, OECs support and guide olfactory axons as they continuously extend from the olfactory mucosa (OM) to their target synaptic regions in the olfactory bulb (OB). The concept of OEC transplantation is to replicate the same process in the injured spinal cord,resulting in axonal regeneration through the injury site and re-establishment of conductive pathways. The mechanisms by which OECs promote neural regeneration, both in their natural location and after transplantation, include direct interaction with axons and structural support (Doucette, 1989,1990), secretion of neurotrophic and guidance factors (Barnett and Riddell, 2004; Barton et al., 2017), phagocytosis of axonal debris as well as effective migration and integration with other cell types such as astrocytes and microglia (Lakatos et al., 2000, 2003; Vincent et al., 2007; Leung et al., 2008;Panni et al., 2013).
Conceptually, the use of OEC transplantation to repair damaged axons following a spinal cord injury is quite simple and robust; OECs are harvested from the olfactory nerve,expandedin vitrofor several weeks, and then transplanted(usually autologously in humans) into the injured spinal cord. The method is, however, complicated by a great variability in anatomical cell source, purification methods, and inconsistencies in reporting on issues such as OEC purity.Purification here implies the process to increase the proportion of OECs present in the total cell population, with purity referring to the proportion of OECs present in a transplantation cell population.
Several studies have shown that OECs can restore lost function and structure of an injured spinal cord (Deumens et al., 2006; Toft et al., 2007) but outcomes are variable. One potential reason for this variability is that the purity of OEC transplants is highly variable between studies. Furthermore,OECs can be harvested from either the OM or the OB, where different cell populations are present (Yao et al., 2018). Thus,the anatomical source from which the OECs are harvested and the resultant cell purity are likely to have significant impacts on outcomes after transplantation into the injured spinal cord. While it is often suggested that poorly purified OEC transplants are associated with poor outcomes and side-effects, several studies have found that the presence of fibroblasts in the OEC transplants can be beneficial (Li et al.,1997; Raisman and Li, 2007). One possible reason for this may be that fibroblasts together with OECs maintain channel-like structures through which regenerating axons can extend, which happens in the natural environment of the olfactory nerve (Li et al., 2005).
We have conducted a review of the recent literature to identify and evaluate different factors affecting the purity of the transplanted OECs, discuss the different established ways to identify and quantify the OECs and highlight the impact of the purity on the transplantation outcomes to help guide the future works into the OEC mediated SCI repair.
In this review, covering the last decade of OEC transplantation studies in rodent models of spinal cord injury, we assessed how the anatomical site of isolation (OMversusOB)affected OEC purification methods and resultant cell purity.We analyzed how many studies reported on OEC purity, and which criteria (markers) were used to define cells as OECs.Finally, we attempted to correlate transplantation outcomes with OEC purity and anatomical source. We found that high OEC purity appears associated with better outcomes, but also that purifying OECs and reporting on cell purity is very difficult due to the lack of cell-specific markers. We found that a panel of markers, along with the identification of novel markers, is crucial for improving the therapeutic potential of OEC transplantation for neural repair. The findings are summarized in Figure 1.
Since surface markers are key to determining purity, this review was limited to studies that used rodents as the source of OECs. As most pre-clinical studies to date have been conducted in rodents, and because the expression of many markers varies between rodents and humans, this review was limited to rodent studies. We do, however, comment on the applicability of using certain markers for transplantation of human OECs. A literature search was conducted using the search terms “spinal cord injury”, “olfactory ensheathing cells”, “OECs”, “olfactory ensheathing glia”, “OEGs”and “olfactory glia”. The search was restricted to the studies published since 2008. Only studies using primary OECs for transplantation were included and review articles were excluded. Studies limited toin vitroexperiments, peripheral or cranial nerve repair or focusing on brain injury were also excluded, as this review focuses on the OEC purification and quantification methods employed for spinal cord injury repair only. A total of 67 studies that met the inclusion criteria were included. For each study, details regarding anatomical sources of cells, methods of purification (including method of harvesting cells and enrichments/supplements used), reported purity, method to determine purity, presence of other cell types and structural/functional outcomes of each study are outlined in Additional Table 1.
Anatomical Source of Cells Affects Purity
The primary olfactory nervous system is comprised of the olfactory neuroepithelium, olfactory nerve and outer layer of the OB, known as nerve fibre layer (NFL). The olfactory nerve consists of numerous fascicles which extend from the lamina propria beneath the neuroepithelium to the NFL.Within this system, OECs are found in the olfactory nerve and the NFL of the OB In the olfactory nerve, OECs ensheathe axon fascicles, and in the NFL, they contribute to the complex sorting and organization of axons to their correct glomeruli (synapse regions) in the OB (Doucette, 1984,1989, 1990). For transplantation purposes, OECs can therefore be harvested either from the OM (which contains lamina propria-derived OECs) or from the OB (which contains NFL-derived OECs). OECs harvested from the OM are typically termed OM-OECs, and OB-derived OECs are termed OB-OECs (Mayeur et al., 2013; Yao et al., 2018); these terms will be used throughout this article.
In humans, harvesting OB-OECs from live patients requires invasive brain surgery (Tabakow et al., 2014), and, as the OB is part of the central nervous system, this invasive approach results in permanent neurological injury. Therefore, the OM is the more preferable source of OECs from a clinically viable point-of-view (Bianco et al., 2004; Lima et al., 2006; Gorrie et al., 2010; Ekberg and St John, 2015).
It is, however, important to note that OECs are not a homologous cell population; sub-populations with distinct anatomical location and behaviors exist (Ekberg and St John,2015). For example, OM-OECs primarily adhere to each other, resulting in contact-mediated migration, and they mediate axon fasciculation. In contrast, OB-OECs display a mixed behaviour of interaction and repulsion, and axons cultured with OB-OECs display a disorganized extension pattern (Windus et al., 2007, 2010, 2011). Another important difference between the OM and OB is the other cell types that are present with OECs in the two areas. In the OB, astrocytes, oligodendrocytes, microglia, neuronal cell bodies and meningeal fibroblasts along with endothelial cells are the most common cells. Most of these cells can be avoided if only the NFL is harvested to obtain OECs (Doucette, 1984).Even then, however, astrocytes are still present along with occasional neuronal bodies. Conversely, in the mucosal biopsies, mesenchymal stem cells, fibroblasts, Schwann cells from the trigeminal nerve and olfactory or respiratory epithelium may be found with OECs (Ekberg and St John, 2014;Yao et al., 2018). Whilst other cell contaminating cells can be found in glial cultures, neurons typically do not survive and are not present in the cultures (Windus et al., 2007, 2010);olfactory neurons typically require a monolayer of glia/specific glia-conditioned medium and stringent culture conditions to survive (Ekberg et al., 2011; Gong, 2012).
Instances have been recorded where patients have suffered serious adverse effects of receiving OEC transplants without proper purification (Yao et al., 2018). In recent years, case reports have discussed particularly alarming cases with patients who developed intramedullary masses in the cervical spine many years following transplantation of autografts from mucosal biopsies. These cysts have been found to contain respiratory epithelium and submucosal glands with goblet cells; thus, it appears that the transplants have mistakenly contained respiratory epithelial mucosa (Dlouhy et al.,2014; Woodworth et al., 2019). The first recorded incidence was in a young female who developed the mass 8 years after the transplantation (Dlouhy et al., 2014), and the most recent published case report described a 38-year-old male who presented with the mass 12 years after transplantation(Woodworth et al., 2019). In both these cases, the patients suffered from significant back pain and had to undergo spinal surgery for removal of the mass. Incidences such as these highlight the importance of purification of OECs prior to transplantation. On the other hand, several studies conducted over the years have also suggested that OECs may be more effective if they are mixed with olfactory nerve fibroblasts (Keyvan-Fouladi et al., 2003; Li et al., 2003; Deumens et al., 2006). The importance of purification is conceptualized in the Figure 2. These findings suggest that although purification is essential to avoid any adverse effects following transplantation, certain cell types may provide beneficial effects if co-transplanted with OECs.
Out of the 67 included studies, 5 studies obtained OECs from mice and 62 from rats. Out of the 62 studies that used rats as their source of OECs, the OB was the most commonly used source of OECs, with 43 studies using OB-OECs and 22 studies using OM-OECs; one study compared OB-OECs and OM-OECs (Mayeur et al., 2013) and one study did not specify the anatomical source of OECs (Luo et al., 2013). The information regarding sources of cells as used by the reviewed studies is summarized in Additional Table 2.
The OB was the most frequently used source of OECs for animal experiments. However, from a clinical viewpoint, it would be more desirable to focus on OM-OECs rather than OB-OECs as obtaining OECs from the OB in humans is an invasive procedure that would lead to damage to the olfactory sense. In the one study that specifically compared OMOECs and OB-OECs, there was no significant difference in functional outcome between the two OEC types. This suggests that, due to the difference in harvesting method, OMOECs have a better risk to benefit ratio than OB-OECs as harvesting OM-OECs avoids the need for an invasive brain biopsy (Mayeur et al., 2013).
We found that the purification methods used in the reviewed studies differ between OM-OECs and OB-OECs(Additional Table 2). Enrichment and complement lysis were the principal methods used for OM-OECs while differential attachment and immunopurification were more common for purification of OB-OECs (these purification methods are discussed in detail in the next section). Reported purity was highly variable for OM-OECs (5-98%) compared to OB-OECs (60-100%) (Additional Table 3). This also indicates that further optimization of the purification process is warranted for the mucosal OECs, to establish a purification method that consistently improves the purity of OECs.
From the reviewed studies discussed here, it appears that in an experimental setting (animal model), OB-OECs are preferred primarily because of their higher and more consistent purity than OM-OECs. However, due to the harvesting method, OM-OECs are better from a clinical point-of-view.
Purification Methods Differ between Olfactory Mucosa- and Bulb-Derived Olfactory Ensheathing Cell Preparations
Purification of OECs constitutes one of the most significant challenges in establishing OEC transplantation as a treatment for spinal cord injury (Yao et al., 2018). OEC purification can be viewed as a biphasic approach with the first phase being the dissociation of cells from the tissue biopsy, and the second phase being the isolation of pure OEC population from these dissociated cells. Dissociation of cells from the tissue biopsy aims to remove the extracellular substance and adherent structures such as epithelium or meninges, thus releasing cells from their natural scaffolding. For example, separating the epithelium from the lamina propria is involved in the mucosal biopsies, whereas removal of meninges needs to be performed on OB biopsies. Generally, the dissociation also involves enzymatic digestion and/or mechanical dissociation. The strategies used for the next step (establishment of a pure OEC population) include immune-based purification based on cell-specific surface markers, isolation due to differential adhesion properties, chemical enrichment of OECs over other cells in culture, or removal of contaminating cells.In the reviewed papers, the dissociation part of purification process was not mentioned in 14 studies, whereas five further studies reportedly only used mechanical dissociation without the use of any enzymes to dissociate the cells from tissue biopsies (Negredo et al., 2008; Wang et al., 2010; Lang et al., 2013; Tang et al., 2017; Zhang et al., 2017); all five of these studies used OB-OECs. The studies that reported the use of enzymatic dissociation used trypsin, dispase (I and II), collagenase (A, D, I and II), papain or hyaluronidase but with variations in the protocol. For example, trypsin was used for dissociation in 24 of the studies, out of which only two studies harvested cells from mucosal biopsies (Tharion et al., 2011; Muniswami and Tharion, 2018a). In the remaining 22 studies that used trypsin for OB tissue dissociation,18 studies used trypsin alone, with four studies using trypsin followed by collagenase (Ma et al., 2010; Yazdani et al., 2012;Torres-Espin et al., 2013, 2014).
For the OEC purification protocol, the exact method was not described in 24 of the reviewed studies. Two of these 24 studies reported using pieces of the un-dissociated OM(Iwatsuki et al., 2008; Aoki et al., 2010) which explains the lack of a cell purification step. In contrast, 43 studies reported using several different purification techniques. These techniques can be summarized into the following categories:(1) immunopurification, (2) differential adhesion, (3) OEC enrichment and (4) removal of contaminant cells. The details of different methods of purification are summarized in Additional Table 3.
Immunopurification was the most commonly used purification technique amongst the reviewed papers (20 studies).This method is based on selective expression of key markers by OECs, such as the p75 neurotrophin receptor (p75NTR,also known as the low-affinity nerve growth factor receptor or CD 271), S100β, glial fibrillary acidic protein (GFAP)(Gong et al., 1994; Franceschini and Barnett, 1996; Bianco et al., 2004), SRY-related HMG-box 10 protein (SOX-10)(Barraud et al., 2010; Oprych et al., 2017) and oligodendrocyte marker 4 (O4) (Barnett et al., 1993; Franceschini and Barnett, 1996). One study employed fluorescence-activated cell sorting to sort OECs based on surface expression of O4(positive selection marker) and galactocerobroside C (as a negative selection marker) (Toft et al., 2013). The remaining 19 studies purified OECs based on expression of p75NTRwith five out of these 19 studies using magnetic cell sorting technique (Novikova et al., 2011; Toft et al., 2012; Deumens et al., 2013; Torres-Espin et al., 2013, 2014).
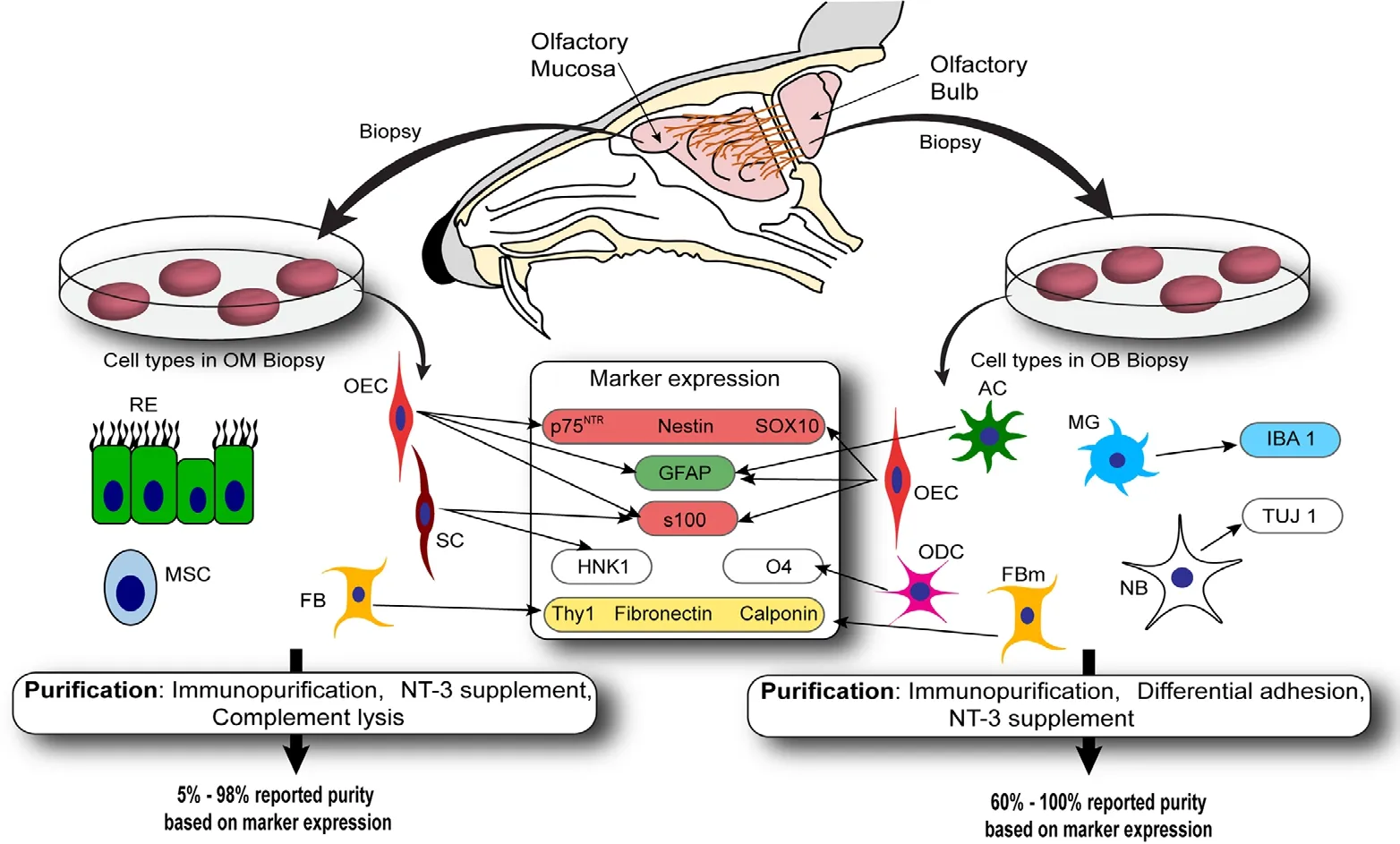
Figure 1 Overview of OEC collection,characteristics and purification.
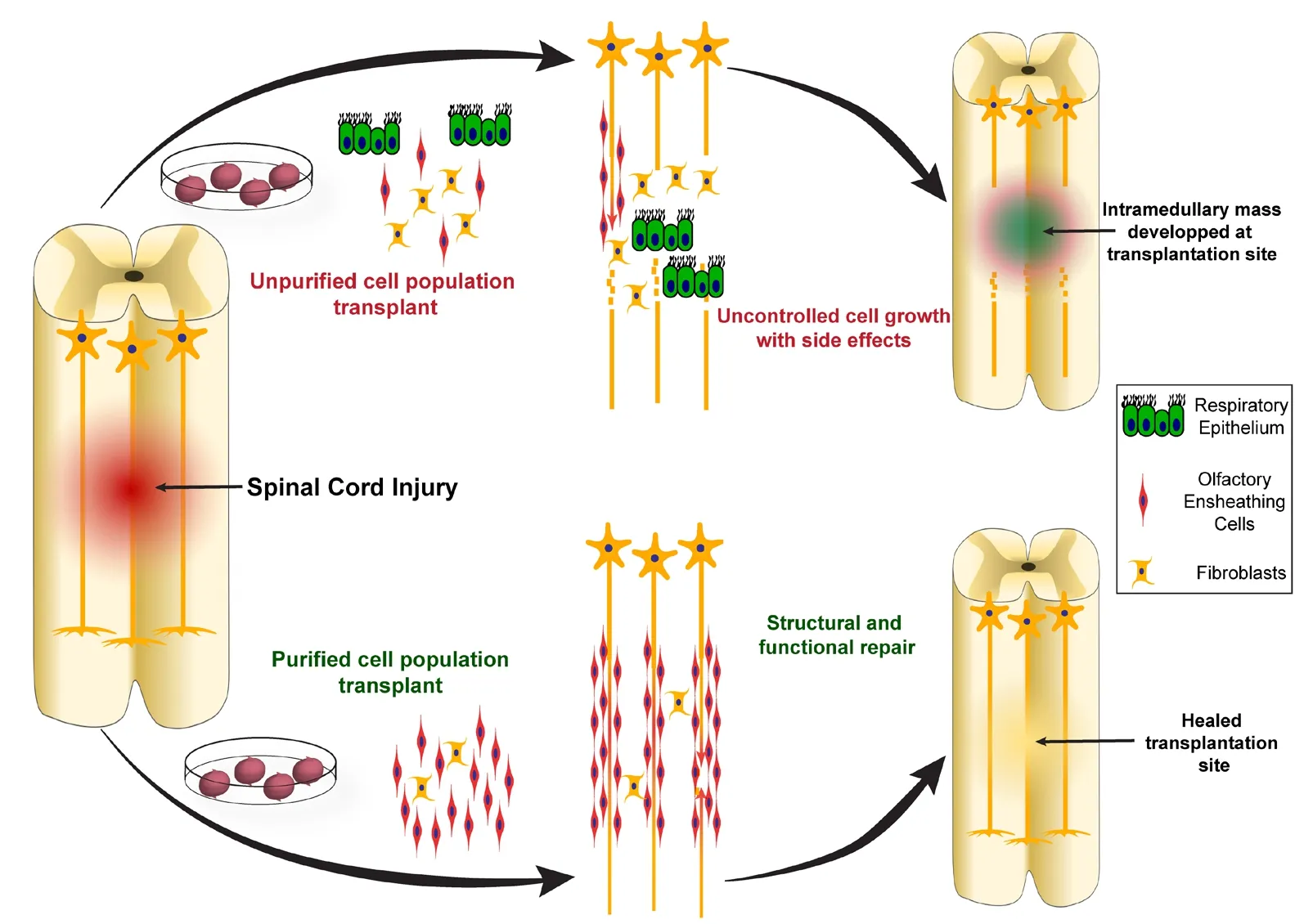
Figure 2 Consequences of unpurified and purified OEC transplantation.
Differential adhesion (also known as differential attachment) was the second most commonly reported purification method. This method relies on the different adhesion time to the cell culture plate for different cell types; removal of media and non-adherent cells at different times leads to partial purification of the target cells. A total of 14 studies used this method, as originally published (Nash et al., 2001), or using a modified version of the same method to purify OECs taken initially from OB. All studies using differential adhesion used OB-OECs.
OEC enrichment of the cultures using neurotrophin-3(NT-3) was used by six of the studies (Kalincik et al., 2010a, b;Li et al., 2011b; Stamegna et al., 2011; Wu et al., 2011; Cloutier et al., 2016). One of these six studies used this method to purify OB-OECs (Li et al., 2011b), the other five used NT-3 to purify cells from the mucosa. This method originates from an earlier experimental work showing that OECs have receptors for NT-3, BDNF and nerve growth factor, all three of which promote purification and proliferation of OECsin vi-tro(Bianco et al., 2004). In one study, the authors used transforming growth factor α (TGF-α) followed by five repeated passages to enrich mucosal OECs in the culture (Mayeur et al., 2013); TGF-α has been shown to promote mitosis of olfactory epitheliumin vitro(Farbman and Buchholz, 1996).
Removal of contaminant cells was the least common purification method amongst the papers reviewed, being used in only three studies and all for purification of OM-OEC.Complement lysis of fibroblasts by targeting Thy 1.1 surface antigen was reported as a purification method by two studies(Bretzner et al., 2008, 2010), whereas a third study mentioned “removal of fibroblasts” as their purification method(Zhang et al., 2011) which was originally described as a method to purify astrocytes in an older publication (Noble and Murray, 1984).
In summary, out of the 44 studies using OECs from the OB, 11 did not specify the purification method, 18 used immunopurification, 14 used differential adhesion, and one study used NT-3 supplementation. Similarly, out of the 23 studies that reported the use of OM-OECs, seven did not mention any purification methods, six did not purify the OECs, five studies used NT-3 supplement, three used complement lysis method, and only two purified the cells by p75NTRmediated immuno-purification. The summary of the differential adhesion protocols used by all 14 studies is given in Additional Table 4.
Out of the 20 studies using immunopurification, most studies sorted out OECs from other cells based on p75NTRexpression. p75NTRis probably the most well characterized OEC marker, however, it is important to note that OECs of the inner NFL do not express p75NTR(the NFL has an inner and an outer part, both populated by OECs) (Vickland et al., 1991; Franceschini and Barnett, 1996; Au et al., 2002).Furthermore, Schwann cells also express p75NTR, and trigeminal nerve Schwann cells can contaminate OEC preparations as branches of the trigeminal nerve are present in the OM and OB (Ziege et al., 2013). Only one study selected OECs(OB-OECs) based on the expression of O4 and galactocerobroside C (Franceschini and Barnett, 1996). However, more recent work suggests that O4 may not be a suitable marker for OEC purification, since O4 has been suggested to be derived from olfactory axons, and only detected in OECs after phagocytosis of axonal fragments (Wewetzer et al.,2005; Oprych et al., 2017). It could, however, be possible that fluorescence-activated cell sorting sorting for O4 can select OECs if the cells exhibit O4-positive axon-derived debris particles attached to the membrane, in process of being internalized; however, OECs that are not phagocytosing axon debris would not be selected.
Reporting on Purity Is Highly Variable between Studies
Reporting purity of the cells to be transplanted is an important detail in order to link outcomes with the composition of cells in the transplant (Raisman and Li, 2007). However, the biggest challenge in reporting on purity is the lack of clearly established OEC-specific markers (Oprych et al., 2017). This makes it difficult to determine the percentage of OECs within mixed population of cells obtained after any purification process. While OECs have been reported to express certain markers, expression of these markers can differin vivoandin vitroas well as change significantly over time in culture(Yao et al., 2018). These difficulties have significantly hampered reporting on OEC purity throughout the literature.
Among the 67 studies reviewed here, 23 studies did not report on the purity of their cell populations. Two further studies (Iwatsuki et al., 2008; Aoki et al., 2010) were not able to report the purity since they did not use any purification methods, but rather they transplanted pieces of OM. Two more studies reported the purity of their cell populations in a qualitative manner by stating that all (Liu et al., 2017) or most (Sun et al., 2013) of the cells expressed p75NTR. Only three studies reported a detailed quantification of their cell cultures expressing a number of surface markers useful for identifying OECs such as p75NTR, S100β, GFAP and others(Coutts et al., 2013; Tsai et al., 2017; Muniswami and Tharion, 2018b).
The reported purity of OECs before transplantation ranged from 5% (Yamamoto et al., 2009; Ibrahim et al., 2014) to 99.7% (Roet et al., 2012). Most of the studies (27 studies),however, reported the cell purity to be 90% or more. A study that compared two different purification methods reported that differential adhesion yielded 93-95% pure cells and p75NTR-assisted magnetic bead separation yielded 80% pure cells (Novikova et al., 2011). A separate study that compared OB-OECs with OM-OECs, reported that purity of the OBOECs increased from 70% to 97% following purification by differential adhesion, and the purity of the OM-OECs increased from 15% to 98% following TGF-α induced purification (Mayeur et al., 2013).
As discussed briefly in the previous section, OB tissue appears to yield OEC cultures with a higher purity than mucosa-derived biopsies. In addition, the purity of bulb-derived preparations also appears more consistent than OM-OEC preparations. Out of the 32 studies that reported on purity in the bulb-derived cell preparations, the average purity was 91.2 ± 3.4%. However, among the 12 studies that reported on purity of OM-OECs, the average purity was 74.2 ± 21.1%.
It is important to note that reported purity may not always be an accurate indicator of the actual purity of the cells present in the culture. For example, four of the studies which used p75NTR-targeted immunopurification method, also used p75NTRimmunolabelling to quantify the purified cell population (Negredo et al., 2008; Ma et al., 2010; Roet et al., 2012;Yazdani et al., 2012), and reported 99.7% (Roet et al., 2012)and > 95% (Ma et al., 2010; Yazdani et al., 2012) purity. The problem with this approach is that an independent marker is not used between the purification and identification processes. Using one or more additional markers to quantify the cell purity based on co-localization of the markers can help eliminate bias.
Identification of Olfactory Ensheathing Cells Is Difficult Due to Lack of Specific Markers
Using the optimal method of estimating OEC purity is critical for correlating OEC purity with functional outcomes after transplantation. Since OECs are known to express different markersin vitroandin vivo(Au and Roskams,2003; Oprych et al., 2017), assessment of OEC purity prior to transplantation should use markers expressed by OECs which have been culturedin vitro. Determination of OEC purity is complicated by the fact that the markers known to be expressed by OECs are not exclusively expressed by OECs and other cell types such as fibroblasts can express the same markers (Yao et al., 2018).
As discussed in the previous section, of the 67 studies reviewed here 19 studies did not describe their method of quantification of OEC purity. The remaining 48 studies used immunolabelling for cell surface markers such as p75NTR,GFAP, S100β and nestin (to label OECs) combined with“negative selection markers” to identify cells that are not OECs, such as fibronectin and Thy-1 (which label fibroblasts). These markers were used alone or in combination.In a few studies, markers other than these were also used,which included, SOX10 (Khankan et al., 2016), TUJ-1 (Toftet al., 2013) and cytokeratin (Toft et al., 2012) to label OECs,and calponin (Coutts et al., 2013) and human natural killer antigen-1 to label cells that are not OECs (HNK-1, also known as CD57 or LEU7, thought to label Schwann cells but not OECs) (Stamegna et al., 2011; Wu et al., 2011).
Nineteen studies used only a single marker for this quantification, with p75NTRused in 18 of these 19 studies. The remaining one study (Su et al., 2009) used S100. The details regarding methods of quantification of purity are summarized in Additional Table 5.
In the reviewed literature, 29 out of 67 studies reported using a combination of markers, out of which 17 studies used combination of two markers, eight studies used three and four studies used combination of four or more markers. The combination of p75NTRand S100β was used in 16 studies, and in eight out of the 16 studies, those were the only two markers used. Secondly, the combination of p75NTRand GFAP was reported 15 times, out of which seven studies used only these two markers. The remaining two studies used p75NTRwith fibronectin to quantify the purity. While some studies used several markers, the study using the most detailed panel to characterize OECs used six markers: p75NTR, S100β,GFAP, fibronectin, Thy-1 and calponin (Coutts et al., 2013).This study reported that most of the OB-OECs (> 90%) were positive for p75NTR, S100β and GFAP; and cells staining positively for fibronectin, Thy 1.1 or calponin (< 1-5%) did not stain for p75NTR.
As there are no specific OEC markers, it is clearly important to use a panel of markers to identify the cells. For example, it is well established that GFAP is a glial cell marker,strongly expressed by astrocytes but weakly expressed by the OECs (Oprych et al., 2017). Using GFAP as a marker for OECs, therefore, can lead to an overestimation of the cell population, especially if the cells originated from the OB, where astrocytes are found in abundance. In five of the reviewed studies, OB-derived cultures contained significant amounts of GFAP-expressing astrocytes (Novikova et al.,2011; Takeoka et al., 2011; Coutts et al., 2013; Tsai et al.,2017; Zheng et al., 2017). It may also be a good idea to use specific markers that are not expressed by OECs but are expressed by the cells that are likely to be present in the tissue biopsy, such as fibronectin, Thy 1, calponin, SMA, HNK-1 and TUJ-1, to identify cells that are not OECs. This option has an added advantage of allowing determination of contaminant cell identity as well. This can be especially relevant when the cells are harvested from the mucosa when purification seems to be more complicated and less consistent. In the future, mass analysis such as RNA sequencing and proteomics, comparing expression patterns between OECs and other cell types, may reveal more cell-specific markers.
Which Cells Accompany Olfactory Ensheathing Cells in Transplants?
If there are cells present in transplants other than the OECs,they can be a significant confounder for the outcome of the experiment, as well as interpretation of the outcome. Especially when the OEC purity is low, knowing the type of contaminating cells becomes more important. Different cells have different proliferation rates and different responses to stressful stimuli, which is why the dynamics of the cell-cell interaction may change significantly upon transplantation into a hostile milieu such as a spinal cord injury site. This affects OECs more since they are known to require intercellular connections to survive in cultures. If the cells such as fibroblasts are present along with OECs, they may have some beneficial effect on the outcome after transplantation, but any immune cells such as microglia or monocytes may lead to an exacerbated immune response following transplant which may even lead to graft rejection. Thus, it is important to identify which cells are present in transplants along with OECs.
A limited number of the reviewed studies discussed contaminating cell types in their purified OECs populations. 50 out of the 67 studies did not mention other cell types. The 17 studies that did discuss the contaminating cell types, usually mentioned fibroblasts or fibroblasts-like cells and Schwann cells (non-myelinating phenotype or negative for HNK-1 expression) as the contaminating cells. Additionally, in some cases, the authors also identified astrocytes (Coutts et al.,2013) and endothelial cells (Novikova et al., 2011) based on their surface marker expression. One particular study (Toft et al., 2012) compared two distinct populations derived from OM and labeled them OM-I and OM-II, which were, in fact,mesenchymal cells of OM, and olfactory epithelial basal cells,respectively. In this study, the authors report that after purification, OM-I cells had a mixture of MSCs and OECs, but do not describe the contaminating cells in OM-II population.
Out of all the studies that used cells from OM, 43.5% (10 out of 23) studies discussed other cell types in the transplantation population. One of these ten studies compared two distinct populations from the OM (Toft et al., 2012). Two studies specified contaminating cells as Schwann cells (Kalincik et al., 2010a; Stamegna et al., 2011), however, only one of those two studies stained for HNK-1, a marker expressed by Schwann cells (Stamegna et al., 2011). The authors in this study also noted that HNK-1 staining does not identify non-myelinating or sensory myelinating Schwann cells. Six more studies discussed fibroblasts as contaminants on the pure OEC population, three of which (Tharion et al., 2011;Ibrahim et al., 2014; Muniswami and Tharion, 2018b), investigated fibronectin expression.
Only seven studies using OB-OECs discussed other cells present with OECs. One study included the possibility of Schwann cell contamination as the authors only described using p75NTRand S100 markers (Zhang et al., 2017). Another study simply mentioned having equal proportions of OECs and olfactory fibroblasts in their cultures, without discussing the markers used for quantification (Collins et al., 2017).Two more studies assumed the presence of fibroblasts (Nategh et al., 2016; Feng et al., 2017), and did not mention the markers used for characterizing OECs after purification.Another study mentioned fibroblasts and endothelial cells as contaminating population (Novikova et al., 2011), although,their marker panel included p75NTR, S100 and GFAP only.One further study confirmed the presence of fibroblasts with fibronectin (Li et al., 2016), and the last study performed a detailed characterization of all cell types present in their cultures by using a six markers panel and defined the contaminant cells as astrocytes and fibroblast-like cells (Coutts et al.,2013).
Most OEC transplantation studies to date (~75%) have not focused on identifying the cells that accompany OECs in transplants, most likely because, as discussed earlier, cell identification is complicated since no specific OEC markers exist. Characterization of the contaminant cells in transplants, however, is important to correlate OEC purity and cell composition with outcomes, and the use of a panel of markers is to date the best strategy to achieve this. Identification of the cells transplanted is also critical to avoid serious side effects (such as accidental transplantation of respiratory epithelium).
Correlation of Olfactory Ensheathing Cell Purity with Transplantation Outcomes
Outcomes after OEC transplantation are typically assessed on a structural (histological) and functional (behavioral) level, taking into account the known behaviors and functions of transplanted OECs. OECs can migrate considerable distances into the injury site where they integrate with the host tissue and interact with damaged axons, as well as with reactive astrocytes and microglia (Barakat et al., 2005; Windus et al., 2007; Chehrehasa et al., 2012). They have been found to repair the damaged axonal tracts, promote axonal sprouting, form a structural “bridge” across injury sites to guide the newly formed axonal branches, limit secondary damage following an injury, preserve spared tissues, and mitigate reactive astroglial scarring (Sasaki et al., 2004; Barakat et al.,2005; Windus et al., 2007, 2011). Hence, studies assessing structural repair in spinal cord injuries following OECs mediated treatments, commonly address (1) cell survival, (2)cell migration, (3) reparative changes in the cord parenchyma, (4) degenerative changes at or near the injury site, and(5) immune or inflammatory reactions associated with the injury as well as OEC transplant.
Similarly, the functional outcomes are generally assessed based on (1) recovery of motor functions, gait (or stepping)pattern, (2) ability to perceive noxious stimuli such as pain or heat, or (3) general sensory perception recovery, and by studying (4) electrophysiological changes of the nerve-muscle tissues.
For the sake of consistency in this review, structural repair is defined as reporting of (1) cell survival, (2) migration (into the cord parenchyma or formation of cell bridges across injury), (3) axonal repair/regeneration, (4) decrease in the defect size, and (5) reduction or regulation of immune and/or inflammatory reaction. Similarly, functional regain is defined as motor recovery, sensory recovery and electrophysiological recordings.
Out of the 56 studies that performed histological analysis,23 studies addressed (1) cell survival, 15 discussed (2) cell migration, 40 studies commented on (3) axonal regeneration and/or repair, 22 studies investigated effects of OECs in (4)reducing degenerative changes (cell death, cavitation after injury, secondary injury) and 16 studies investigated (5)OEC interactions with immune/inflammatory cells (macrophages, microglia, and/or astrocytes). The majority of the studies analyzed more than one of the structural repair parameters, however, 22 studies only commented on any one of these parameters. Other studies reported bladder and bowel autonomic functions, or other specific functional measures but there were too few to make comparisons.
We here aimed to correlate OEC purity with structural and functional repair after spinal cord injury. Out of all the studies included in this review, 50 (~75%) reported that OEC transplantation resulted in structural and/or functional repair (positive outcomes). The remaining (17) studies reported either no significant improvement or adverse effects such as pain (Additional Table 1).
Poor outcomes - Out of all the reviewed studies, two studies (from the same research group) reported that the structural repair was comparable between acute and delayed transplants (Centenaro et al., 2013), and that the axonal repair could not be linked with the OEC treatments (Centenaro et al., 2011), respectively. Neither of the studies mentioned cell purity. Five more studies reported negative or insignificant outcomes from a structural perspective. One of the five studies (Li et al., 2016) mentioned that the cells failed to survive beyond one week despite the continued evidence of axonal repair and motor function regain; the purity reported here was 60-70%. The remaining majority of studies included here reported positive or desirable histological outcomes following OEC treatments for one or more of the above-mentioned parameters. While fifteen of the 48 studies did not mention the purity of their OEC treatments, the average OEC purity of all the studies that reported a positive outcome was ~91%.
Similarly, from a functional regain viewpoint, 12 studies out of 52, reported non-significant or negative outcomes,however cell purity was not reported for most studies. Of those studies that reported OEC purity, one study sought to use 95% pure OECs (source not specified) to treat neuropathic pain (Luo et al., 2013), but found that OEC treatment resulted in hyperalgesia. A similar study used 95% pure OBOECs to treat neuropathic pain following a hemi-section injury (Lang et al., 2013) and reported that OECs caused hyperalgesia. Conversely, a study using 90% pure OM-OECs(Wu et al., 2011) found that the treatment alleviated neuropathic pain but did not improve complex goal-oriented skilled locomotion.
Favourable outcomes - Of the 40 studies that reported positive functional outcomes of OEC treatments, 14 studies did not comment on the OEC purity. The lowest reported purity among the remaining 26 studies were 5% (Yamamoto et al., 2009), 50% (Collins et al., 2017) and 60-70% (Li et al.,2016). Two studies reported 75% (Bretzner et al., 2008, 2010)and one study reported > 80% (Amemori et al., 2010) purity of the transplanted OECs, the rest 20 studies had 90-100%pure cultures, with an average purity of ~86.5% including all 26 studies.
Overall, it appears high OEC purity correlates with favourable outcomes, whereas transplanting OECs with a purity of 75% or less are frequently associated with failed recoveries or undesirable outcomes. In addition, using OB-OECs is in some studies correlated with worsened neuropathic pain,whereas OM-OECs may instead alleviate pain. Due to lack of a sufficient number of studies, and the inconsistencies and difficulties in reporting on OEC purity, a concise conclusion regarding OEC purity and structural/functional outcomes of OEC transplantation cannot be made.
Conclusion
We have here reviewed all OEC transplantation studies in rodent models of spinal cord injury over the last decade with a focus on OEC purity. We found that OB-OECs have been more frequently used than the OM-OECs although the use of OB-OECs in humans is problematic due to the invasive and destructive surgery required to obtain them.Immune-based purification methods, often based on expression of p75NTR, were the most common purification techniques for both OB-OECs and OM-OECs. Out of the studies which assessed OEC purity, the majority of studies reported an OEC purity of over 90%, with OB-OEC cultures typically having a higher purity than OM-OECs. However, the lack of cell-specific markers makes both OEC purification and determination of OEC purity challenging tasks, particularly for transplantation of OM-OECs. Robust quantification methods are essential to reliably estimate OEC purity; a panel of several markers is better than the use of a single marker. As per the reviewed literature, the most commonly used markers to identify OECs are p75NTR, S100β and GFAP either by themselves or a combination of any two of the three. Transplantation of OECs that resulted in favourable outcomes had high reported purity whereas studies reporting low purity tended to have poorer outcomes. However, OEC purification is a complex process and thus, reliable purification and quantification methods are essential to improve the therapeutic potential of OEC transplantation for spinal cord injury repair.
Author contributions:RR, MS and LB drafted the manuscript, JASJ and JAKE edited and revised the manuscript. All authors approved the final manuscript.
Conflicts of interest:The authors declare no conflicts of interest.
Financial support:None.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Ashraf S. Gorgey, Virginia Commonwealth University, USA.
Additional files:Additional file 1:Open peer review report 1.Additional Table 1:Summary of source of cells, method of purification,reported purity, method of estimating/ quantification, cells in the mix, key structural and functional outcomes.Additional Table 2:Summary of the numbers of studies using OB and OM from rodents as their OEC sources.Additional Table 3:Summary of studies using different purification methods for OB and OM derived OECs.Additional Table 4:Summary of the studies using differential adhesion as their purification method, and the details of their protocols.Additional Table 5:Summary of the different markers used by the studies for quantification of purity of the OECs.
- 中国神经再生研究(英文版)的其它文章
- The role of the TrkB-T1 receptor in the neurotrophin-4/5 antagonism of brain-derived neurotrophic factor on corticostriatal synaptic transmission
- Could non-invasive brain-stimulation prevent neuronal degeneration upon ion channel re-distribution and ion accumulation after demyelination?
- The role of exercise in brain DNA damage
- Combined effect of repetitive transcranial magnetic stimulation and physical exercise on cortical plasticity
- Should mast cells be considered therapeutic targets in multiple sclerosis?
- Neuroprotection mediated by natural products and their chemical derivatives