Should mast cells be considered therapeutic targets in multiple sclerosis?
Karen Henriette Pinke , Sofia Fernanda Gonçalves Zorzella-Pezavento Vanessa Soares Lara, Alexandrina Sartori
1 Institute of Biosciences, Department of Microbiology and Immunology, São Paulo State University (UNESP), Botucatu, São Paulo, Brazil
2 Bauru School of Dentistry, Department of Surgery, Stomatology, Pathology and Radiology, University of São Paulo, Bauru, São Paulo, Brazil
Abstract Mast cells are immune cells of the myeloid lineage that are found throughout the body, including the central nervous system. They perform many functions associated with innate and specific immunity, angiogenesis,and vascular homeostasis. Moreover, they have been implicated in a series of pathologies (e.g., hypersensitivity reactions, tumors, and inflammatory disorders). In this review, we propose that this cell could be a relevant therapeutic target in multiple sclerosis, which is a central nervous system degenerative disease. To support this proposition, we describe the general biological properties of mast cells, their contribution to innate and specific immunity, and the participation of mast cells in the various stages of multiple sclerosis and experimental autoimmune encephalomyelitis development. The final part of this review is dedicated to an overview of the available mast cells immunomodulatory drugs and their activity on multiple sclerosis and experimental autoimmune encephalomyelitis, including our own experience related to the effect of ketotifen fumarate on experimental autoimmune encephalomyelitis evolution.
Key Words: central nervous system; degenerative disease; experimental autoimmune encephalomyelitis;immunity; immunomodulatory drugs; inflammatory disease; ketotifen fumarate; mast cells; multiple sclerosis;therapeutic target
Introduction
Mast cells (MCs) are highly plastic innate immune cells that are capable of joining numerous inflammatory processes and classically referred to as “the allergy villains”. The participation of MCs in type 2 immune response is the better-understood immune mechanism involving these cells due to their classical and central role in allergic reactions. However, their considerable physiological impact in homeostasis and host immune defense cannot be ruled out. MCs undergo degranulation, releasing preformed mediators that modulate blood vessel dynamics and orchestrate inflammation and tissue remodeling. They produce a wide range of mediators, including cytokines, chemokines, and growth factors that interplay with numerous tissue-resident or recruited cells.
This high versatility contributes to their participation in many pathological conditions. As such, MCs may take part in various stages of inflammatory and autoimmune diseases. Regarding multiple sclerosis (MS), an autoimmune and inflammatory disorder of the central nervous system (CNS),the contribution of MCs has been investigated since the mid-1970s (Ansari et al., 1976) and gained further insight through studies using the major animal model for this disease, experimental autoimmune encephalomyelitis (EAE). Recently,our research group demonstrated that the administration of ketotifen fumarate, a well-known MC stabilizer drug, to EAE mice has the potential to overcome CNS-blood barrier permeability alterations, thus inhibiting the local inflammatory infiltration and subsequent neurodegenerative inflammatory response. This review aims to discuss why MCs deserve to be envisioned as a relevant target in MS therapy.
Literature review was electronically performed using PubMed database. The following combinations of Key words were used to initially selected the articles to be evaluated:mast cells ontogeny and differentiation; mast cells properties;multiple sclerosis and experimental autoimmune encephalomyelitis immunopathogenesis; mast cells and multiple sclerosis and experimental autoimmune encephalomyelitis; mast cells and modulating drugs. Most of the elected studies (80%of all references) were published form 2010 to 2019. An ancient publication from 1976 was included in consideration to its relevance in the mast cell field.
Mast Cell Ontogeny and Differentiation
The discovery of MCs as tissue-resident cells occurred in mid-1980 when Paul Ehrlich brilliantly described a class of cells filled with aniline-positive cytoplasmatic metachromatic granules (Figure 1). Since then, solving the origin of these cells has remained a challenge. The current consensus indicates that MCs develop from a hematopoietic granulocyte/monocyte progenitor cell that gives rise to macrophages,neutrophils, eosinophils, and basophil-mast cell progenitors.These progenitor cells enter nearly all vascularized tissues,where they complete their differentiation. MC differentiation and survival are classically dependent upon the interaction of c-kit (CD117) (a surface receptor tyrosine kinase) and the stem cell factor (SCF; a soluble and membrane-bound cytokine expressed by stromal cells). This interaction is considered necessary for efficient MC development since it guarantees MC migration, proliferation, maturation and survival(Ribatti and Crivellato, 2014; DeBruin et al., 2015; Shefler et al., 2019).
A great deal of information has been obtained from studies employingin vitrosystems where progenitor cells of diverse origins (e.g., bone marrow, peripheral blood, fetal liver,and cord blood) can generate these cells in the presence of SCF. However, a recentin vitrostudy revealed that MCs progenitors from human peripheral blood can survive, mature,and proliferate independently of SCF and c-kit signaling,thus suggesting that these factors are dispensable for early MC development and that their importance for this process may be overestimated (Dahlin et al., 2017).
Upon leaving the bone marrow, MC precursors populate the peripheral vascularized tissues, where they differentiate according to the locally present cytokines and activating factors. Thus, phenotypically distinct populations of MCs are found in different body tissues. Human MCs differ in terms of the surface expression of chemokine receptors and the content of their granules, thereby allowing the categorization of these cells into MCT (containing tryptase only), MCC(chymase only), and MCTC (both tryptase and chymase).In rats and mice, MCs are classified as mucosal and connective tissue MCs based on tissue localization (DeBruin et al.,2015; Cruse and Bradding, 2016). Additionally, MCs display dynamic plasticity according to their microenvironment,resulting in location- and function-dependent alterations that have important implications in disease clinical manifestations. Notably, tissue-specific foreign microorganisms also contribute to the complexity of MC diversity, suggesting a considerable impact of the microbiome on their functions(Cildir et al., 2017). Once established in the tissue and differentiated into mature cells, they can trigger an inflammatory response associated with innate and/or adaptive immunity whose general characteristics will be addressed below. Briefly, by releasing preformed and newly produced mediators,MCs are considered sentinels that warn the immune system of the presence of various exogenous intruders or endogenous deleterious factors (Agier et al., 2018).
Notably, MCs have a broad set of pattern recognition receptors that are able to detect and bind both pathogen- and damage-associated molecular patterns from a variety of infectious or inflammatory conditions (Agier et al., 2018). For this reason, these cells are strategically located at interface areas such as the skin, conjunctiva, genitourinary/gastrointestinal tracts, pulmonary epithelial lining, and especially around the blood/lymph vessels and nerves in a manner similar to other tissue-resident immune cells (Cruse and Bradding, 2016). In the CNS, they are specifically found in the dura mater/meninges of both the spinal cord and brain on the abluminal side of the blood-brain barrier (BBB) and,interestingly, in close proximity to astrocytes and neurons.In addition, disrupted BBB can also lead to MCs crossing into the CNS (Silverman et al., 2000; Khalil et al., 2007).
The Main Immunological Properties of Mast Cells
The most well-known activation pathway of MCs is the cross-linking of high-affinity receptors for IgE (FcεRI), which are expressed on their surface, by specific antigens. This binding triggers the release of pre-stored bioactive mediators such as histamine, serotonin, heparin, proteolytic enzymes(e.g., tryptase and chymase), proteoglycans, arachidonic acid products, growth factors, cytokines/chemokines, and antimicrobial peptides into the extracellular microenvironment. Upon activation, they also release newly synthesized mediators with inflammatory action such as prostaglandin D2, leukotriene C4, cytokines/chemokines, as well as growth factors such as platelet-activating factor, interleukins, granulocyte macrophage-colony stimulating factor (GM-CSF) in addition to macrophage inflammatory protein-1β, and -1α,and tumor necrosis factor alpha (TNF-α). An inflammatory reaction characterized by vasodilation, the recruitment of eosinophils and neutrophils, and the production of cytokines and chemokines is the primary outcome of this interaction and has been classically described during type I hypersensitivity reactions (Metcalfe, 2008).
Other starters can trigger the activation of some MCs populations, including complement activation components,direct injury, pathogens, microbial products, venoms, cytokines, interaction with activated T cells, and neural mediators (Yu and Akin, 2016; Cildir et al., 2017; Shefler et al.,2019). Such additional activation routes are mediated by the so-called pattern recognition receptors, including Toll-like receptors (TLR), C-type lectins, NOD-like receptors (NLR),and scavenger receptors that may be present on their surface,endosomal vesicles, and cytoplasm (Agier et al., 2018). Activation routes are also mediated by specific G protein-coupled receptors that include endothelin receptor type A, complement component 5a receptor, adenosine A3 receptor, and MAS-related G protein-coupled receptor family member X2(Cildir et al., 2017). After recognition of soluble or cell-anchored ligands, these receptors trigger the release of a plethora of mediators that have the ability to activate different cell types and mediate many functions. For example, MCs can use TLRs and C-type lectins to directly recognize and eliminate microorganisms by phagocytosis with the concomitant production of microbicidal substances (Lima et al., 2013;Trevisan et al., 2014; Pinke et al., 2016). MCs also participate in the clearance ability of macrophages (Jachetti et al., 2019)and other mechanisms related to homeostasis and tissue repair (Ramirez-GarciaLuna et al., 2017), such as limitation of vasoconstrictor activity of endothelin-1 released after injury via protease degradation (Maurer et al., 2004). In addition,MCs can also activate other cells by direct contact or even by the transgranulation process, which is a phenomenon observed between MCs and neurons (Wilhelm et al., 2005;Hong et al., 2015).
The complex field of MCs mediators was recently updated by Mukai et al. (2018). Briefly, histamine, cytokines such as TNF-α, chemokines (CCL3, CCL4, CCL5, and others), and proteases can recruit innate and adaptive immune cells in addition to other proinflammatory effects such as cell activation or direct antimicrobial activity. Also, interleukin (IL)-2,IL-6, interferon (IFN)-γ, IL-4, IL-5, IL-33, and transforming growth factor-β (TGF-β) can act in the polarization of T helper responses or even in the downregulation of lymphocyte activity as determined by IL-10 (Mion et al., 2014; Mekori et al., 2016). A plethora of angiogenesis-related factors also produced and spontaneously released by MCs can modulate blood vessel formation and act as pro- or anti-tumorigenic mediators (Ribatti, 2015; McHale et al., 2019).
Regarding innate immunity, MCs can recruit neutrophils through the production of chemokines (De Filippo et al., 2013). This is especially true for TNF-α, as previously demonstrated in bacterial infection sites or during delayed-type hypersensitivity reactions when cytokines determine the pattern of T cell infiltration (Biedermann et al., 2000). Indeed, a prominent role of MCs in cell recruitment has been claimed in various contexts (Abraham and St. John, 2010; Elieh Ali Komi and Ribatti, 2019; Succar et al., 2019). In inflamed lymph nodes, MC-derived histamine was associated with the influx of a plasmacytoid CD8+dendritic cell (DC) subset following intradermal injection ofStaphylococcus aureus-derived peptidoglycan (Dawicki et al., 2010). Also, the production of TNF-α by these cells has been strongly associated with the massive migration of DCs into draining lymph nodes (Suto et al., 2006) and injured peripheral tissue (Shelburne et al., 2009). Notably, Kunder et al.(2009) demonstrated that this interplay between peripheral inflammatory sites and draining lymph nodes can be driven by the release of stable submicrometer heparin-based particles containing TNF-α and other proteins that rapidly enter lymphatic vessels. Furthermore, during direct crosstalk, MCs induce the expression of maturation markers, the production of IFN-γ, IL-2, IL-6, and TGF-β, and an increased lipopolysaccharide-induced secretion of IL-12p70, IFN-γ, IL-6,and TGF-β by DCs (Dudeck et al., 2011). Further regarding TNF-α production, McLachlan et al. (2003) demonstrated that MCs can regulate the hypertrophy of draining lymph nodes duringEscherichia coliinfection by recruiting circulating T effector cells that were threefold greater after the local injection of 48/80 compound, which is an MC secretagogue. Such data reinforce the hypothesis that MCs could be involved in T lymphocytes accumulation in the draining lymph nodes of autoimmune-injured mice (Pinke et al.,2019), as we will discuss later in this review. On the other hand, under tolerizing conditions, production of TNF-α and GM-CSF by MCs contributes to suppressing T cell proliferation by inducing the survival of tolerogenic DCs (de Vries et al., 2011). Additionally, IL-10 and TGF-β production have been associated with both the amplification of T regulatory function and the attenuation of T effector responses(Chacon-Salinas et al., 2011; Jungraithmayr, 2015).
MCs are also key-inducers of adaptive type 2 immune responses (Th2) due to their ability to secrete IL-4, which is a property strongly linked with their role during hypersensibility reactions and defense against parasites (McLeod et al., 2015). Moreover, histamine production by MCs was associated with an unbalanced Th1/Th2 response under specific antigen stimulation, leading to the predomination of IL-4-producing T cells and the accumulation of DCs with a reduced capacity to induce Ag-specific Th1 cells (Mazzoni et al., 2006). Besides directing Th2 polarization, it was recently described that MCs, under the inductive effect of IL-33, also control the dichotomy between RORγt+Treg and Helios+Treg differentiation by producing IL-6 or IL-2, respectively(Andreas et al., 2019).
The MC secretome displays an array of possible therapeutic interventions that can be studied and developed, especially in the context of inflammatory diseases (Varvara et al.,2018). Indeed, their protective or deleterious secretome-related activities have been associated with innate and adaptive responses from homeostasis to host protection against microorganisms(Abraham and St. John, 2010); for example,they have been associated with pathological steps of inflammatory and autoimmune diseases (Xu and Chen, 2015), as will be particularly discussed.
Multiple Sclerosis: Concept and Animal Model
The classical definition of MS describes it as an autoimmune neurodegenerative pathology that is mainly mediated by T cells whose target is the myelin sheath that surrounds and protects the CNS. Its pathological hallmark is the presence of focal demyelinated plaques within the CNS that are characterized by inflammation, reactive gliosis, and axonal and oligodendrocyte loss. Since the lesions can appear anywhere in the CNS, MS patients may present motor, sensory, or cerebellar symptoms, visual disturbances, or sphincter, psychological, or cognitive dysfunctions. Four clinical disease forms based on disease evolution have been widely recognized: relapsing-remitting (the most frequent), primary progressive,secondary progressive, and progressive-relapsing (Filippi et al., 2018).
Notably, some meaningful controversies and questions continue to surround this disease; for example, the possibility that it is a primarily neurodegenerative process followed by further immunological involvement. The exact contribution of environmental and genetic factors implicated in disease start or evolution, as well as therapies to restrain the neurological decline observed in the progressive forms continue to await elucidation. Despite this scenario, it is believed that MS is an immune-mediated pathology that, in most cases, is responsive to treatment with immunomodulatory and immunosuppressive drugs.
A significant portion of the knowledge concerning MS immunopathogenesis is derived from data obtained by using its animal model entitled EAE, which is artificially induced in rodents (mainly in mouse strains) via immunization with CNS-derived antigens emulsified with complete Freund’s adjuvant, usually in the presence of pertussis toxin (Terry et al.,2016). Since these two pathologies resemble each other in many features, this animal model has been broadly used to decipher underlying immunopathogenic mechanisms and to propose new therapies. For example, both primarily display multiple CNS lesions in the brain stem and spinal cord as well as the presence of immunoglobulins in the cerebrospinal fluid. Additionally, their lesions progress from inflammation to demyelination, gliosis, and partial remyelination. The pharmacological or genetic deletion of specific cells or molecules in this model has generated valuable data concerning their contribution to MS immunopathogenesis. For example,mouse strains deficient in MCs or their mediators develop a less severe EAE (Sayed et al., 2011; Desbiens et al., 2016),thereby demonstrating that these cells are relevant players for MS/EAE evolution.
A Brief Overview of Multiple Sclerosis/Experimental Autoimmune Encephalomyelitis Immunopathogenesis
The following understanding of the complex MS/EAE immunopathogenesis has been established on literature grounds as well as through our own perception of the disease and is briefly summarized in Figure 2A.
Peripheral activation of self-reactive lymphocytes
Regarding its beginning, the predominant hypothesis is that MS is initiated by immune activation in peripheral lymphoid organs. The primary inductive signals have been identified as cross-reactive antigens derived from infectious environmental agents that reach the CNS, or from CNS-derived molecules released after preceding aggression or degeneration.These molecules would reach the spleen or the lymph nodes and, similarly to any other immunogen, would be processed and presented by local antigen-presenting cells (APCs) to self-reactive T cells. It is widely accepted that Th1 and Th17 play major roles in the pathogenesis of this disease; notably, both cell subsets and even T cells with Th1/Th17 mixed characteristics have been detected in the periphery and in the CNS of MS patients and EAE mice (Segal, 2019). The contribution of CD8+T autoreactive cells has been recently better characterized (Salou et al., 2015). Although MS/EAE are classically viewed as T cell-mediated pathologies,observations from anti-CD20-treated patients suggest a much more sophisticated role of B lymphocytes. In addition to their classical participation as antibody-producing cells,they also likely act as APCs, antigen carriers to secondary lymphoid organs, proinflammatory secretory cells, and as regulatory cells (Sospedra, 2018).
T cell licensing in the periphery
A relatively new concept in MS is that T cells must undergo peripheral licensing, which is understood as alterations that prepare these cells to reach and act at the CNS. As claimed by a few authors that investigated this process, it mostly occurs in the lungs (Odoardi et al., 2012) and spleen (Tan et al.,2017). According to these two research groups, the licensing stage occurs by reprogramming of their gene expression profile, which is characterized by the upregulation of genes that control CD4 T cell motility and their subsequent migratory potential.
Central nervous system entrance: a crucial step
Consistent experimental findings indicate that a meningeal inflammatory event precedes cellular entry into the CNS. This idea is in accordance with the current concept that meninges are strategic immunosurveillance sites for detecting infectious or other threats to the CNS. By using the EAE model, it has been demonstrated that meningeal leukocyte infiltration occurs and includes T lymphocytes,monocytes, and polymorphonuclear cells preceding clinical manifestations (Walker-Caulfield et al., 2015). Moreover, a clonal expansion of T cells reactivated by the recognition of a cognate antigen presented by local APCs has also been previously described (Kivisakk et al., 2009). To reach the CNS, these myeloid and lymphoid cells must cross the BBB,which is a molecular and cellular structure that restricts most of the traffic of soluble mediators and leukocytes from the periphery to the CNS in homeostatic conditions. The disruption of this barrier by a variety of mechanisms is a major hallmark in MS/EAE immunopathogenesis since it will allow leukocyte entrance and ensuing deleterious events to occur in the CNS. It is believed that most of the leukocyte trafficking to the CNS in MS will occur through parenchymal capillaries and post-capillary venules at the level of the neurovascular units. These units are composed of specialized endothelial cells referred to as BBB-endothelial cells (BBBECs), myocytes, neurons, astrocytes, pericytes, and extracellular matrix components (Muoio et al., 2014). BBB-ECs lack fenestrations and express a plethora of different molecules as tight junctions (claudins, occludin, and junctional adhesion molecules), zona occludens, cingulin, and Ca2+-dependent serine protein kinase. This specialized BBB endothelium is lined by embedded pericytes and by two vascular basement membranes, with the second one being covered by astrocytic endfeet (Muoio et al., 2014). The space between the vascular and parenchymal basement membrane is known as the perivascular space and is the region where lymphocytes are reactivated after crossing the BBB-ECs (Lopes-Pinheiro et al., 2016). In the context of MS/EAE, the architecture of this barrier can be altered at distinct disease time points by mediators from the periphery, by mediators and cells coming from the meningeal inflamed niches, by compounds released from the neurovascular unit-components, or from the inflammatory process that occurs at the CNS. An array of different molecules such as cytokines (IFN-γ, TNF-α, IL-1β, IL-6, IL-17, IL-21, IL-22, and oncostatin), neutrophins,reactive oxygen species, and matrix metalloproteinases have been described as able to trigger BBB disruption (Larochelle et al., 2011).
Central nervous system inflammation and neurodegeneration: cellular and molecular players
Clear evidence implicates innate and specific immune response as well as CNS resident cells in both the inflammation and neurodegeneration that typically characterize this disease. It is widely acknowledged that CNS attacks are initiated by a few specific Th1, Th17, and CD8+T lymphocytes that cross the BBB. Once at the target organ, these cells are again activated and expanded before they begin the immune-mediated attack. The contribution of different cell types, as well as the mechanistic details of neuroinflammation and ensuing degeneration, have been extensively investigated by recognized experts in the area (Filippi et al., 2018). The primary cells and mediators involved in immunopathogenesis are presented in Table 1. While we emphasize the pathogenetic outcomes in this review, it remains important to consider that most of these cells may, under certain conditions, perform immunoregulatory activities. Although highly relevant,the immunoregulatory potential of many cell types, including the classical regulatory CD4+Foxp3+T cells, will not be addressed.
The interplay of these different cell types is characterized by a tremendous complexity that has slowly been unveiled.Three of the most relevant mechanisms of this multifarious immunopathogenesis will be briefly detailed below. Solid data supports the notion that oxidative stress considerably contributes to the immunopathogenesis of both MS and its animal models. Macrophages and microglia are considered the dominant producers of excessive amounts of reactive oxygen species that will ultimately trigger axonal damage and demyelination (Ohl et al., 2016). Oxidative stress can cause severe damage to cellular macromolecules as membrane injury due to lipid peroxidation, changes in protein structure and function due to protein oxidation, and DNA strand breaks by OH radical attack.
Notably, reactive oxygen species have been described as the major inflammasome activating signal. Inflammasomes are multiprotein complexes that can sense molecular patterns displayed by pathogens or associated with damage. NLRP3,which is the most studied member of these complexes, has been clearly connected with many kinds of CNS disorders, including MS. Activation of the NLRP3 inflammasome triggers inflammation via the production of pro-IL-1β and pro-IL-18 and their further conversion into active cytokines. IL-1β contributes to CNS autoimmunity by inducing the differentiation and infiltration of Th17 toward the CNS, which elicits the GM-CSF production considered a prerequisite for T cell encephalogenicity (Ronchi et al., 2016). More recently, it was also demonstrated in EAE that IL-1β allows pioneer monocytes CCR2+to cross the BBB, regulates GM-CSF production by CNS endothelial cells, and activates myelin-reactive TCD4+cells to release neurotoxic factors (Pare et al., 2018).During MS/EAE development, the inflammasome is mainly activated in microglia and infiltrating macrophages; however,other cells such as astrocytes and neurons also express these inflammatory platforms and are being investigated regarding their contribution to disease pathogenesis (Albornoz et al.,2018). Recent research advances also highlighted the contribution of the glutamatergic pathway to neurodegeneration.Glutamate is the major excitatory neurotransmitter in the nervous system, serving a variety of essential roles. Nonetheless, in excessive amounts, as detected in MS and EAE, it has been implicated in immunopathogenesis. Some of the most evident deleterious consequences of this glutamatergic dysfunction include the increased migration and proliferation of autoreactive T lymphocytes, apoptosis of regulatory T cells,excitotoxic cell death of oligodendrocytes, myelin damage,and BBB breakdown (Macrez et al., 2016).
Microbiome and intestinal barrier alterations
There is growing evidence that gut microbiome alterationsfound in MS patients are somehow linked to immunopathogenesis and that the restoration of a healthy microbiota could contribute to MS therapy. Gut dysbiosis has been associated with increased intestinal permeability and changes in bile acid metabolism (Camara-Lemarroy et al., 2018). Particularly, this elevated permeability would allow the translocation of intestinal bacteria and their toxic products, which could affect the peripheral autoimmune response or even reach the CNS. Underlying mechanisms by which microbiota impacts MS evolution have mostly been learned from preclinical studies with EAE. These investigations indicate that the microbiome can regulate BBB permeability, activate microglia, control myelin gene expression, and reduce astrocyte pathogenicity (Chu et al., 2018). A breakthrough in this area was the revelation that the gut microbial community regulates Th17 differentiation in both MS and EAE (Esplugues et al., 2011).
The Possible Contribution of Mast Cells to Multiple Sclerosis/Experimental Autoimmune Encephalomyelitis Immunopathogenesis
MCs could affect disease course by acting during different phases of disease development as outlined in Figure 2B.
Mast cells play a role in the peripheral immune response
MCs could act, for example, during the assembly of peripheral autoimmune response. One potential site of action is through either their direct or indirect influence on the generation of primary T cell response. MCs reside near DCs in many tissues and are present at sites of initial T cell activation, including in the secondary lymphoid organs (as described previously in this review). They express several molecules known to influence DC maturation and T cell activation (Carroll-Portillo et al., 2015). Gregory et al. (2005)provided the firstin vivoevidence that MCs can amplify the magnitude of the initial autoreactive T cell response, which will promote disease progression after initial priming. By using a MC-deficient murine model (W/Wv mice) and the selective repletion of the MC compartment, they demonstrated that specific activation of CD4+and CD8+T cells, IFN-γ production and the expression of CD44 and CD11a levels were all downmodulated in these animals following MOG35-55priming in comparison to wild type animals. Normally present at low frequencies within secondary lymphoid organs,the number of MCs increase in lymph nodes in response to immune challenge.
Mast cells can be abundant in T cell licensing sites
To the best of our knowledge, a possible contribution of MCs to peripheral self-reactive T cell licensing, which is believed to occur before EAE and MS development, has not yet been investigated. However, data from the literature indicate a surprisingly high prevalence of asthma in MS patients(Hill et al., 2019). Regardless of the tremendous complexity of this subject, lung accumulated MCs or the high frequency of MC progenitors in the blood circulation of asthmatic patients could theoretically support disease initiation or aggravation (Mendez-Enriquez and Hallgren, 2019). Evidence on the association of food allergies and MS have also been recently reported. For example, Fakih et al. (2019) described that MS patients with food allergies presented more relapses and a higher probability of gadolinium-enhancing lesions compared to non-allergic patients. Interestingly, Toyoshima et al. (2017) point the spleen, which is one of the possible lymphoid organs involved in licensing, as a unique site associated to the development of pathogenic MCs.
Mast cells disrupt the blood-brain barrier and recruit inflammatory cells to the central nervous system
The distribution of MCs in the CNS is a pivotal aspect of the ability of these cells to affect BBB permeability. As previously mentioned, MCs are found scattered at the dura and pia mater, in very close proximity to BBB vasculature.According to Sayed et al. (2010), the first inflammatory leukocyte influx into the CNS is mast cell-dependent. In line with their findings, peripheral T cells would be activated and clonally expanded at the leptomeninges. Locally,these autoreactive cells would activate MCs, triggering an encephalitogenic scenario characterized by the release of three types of molecules that seem critical to increasing BBB’s permeability: matrix metalloproteinases, TNF-α, and IL-1β. Matrix metalloproteinases would disrupt the BBB by degrading the extracellular matrix, whereas TNF-α would recruit polymorphonuclear cells that would contribute to increasing BBB permeability by affecting endothelial cell integrity. Seminal investigations concerning the role of IL-1β in this initial CNS invasion by leukocytes were performed by the Melissa Brown research team. According to them, IL-1β released by MCs is essential for Th1 and Th17 encephalogenicity, that is, their ability to produce GM-CSF (Russi et al., 2016), which subsequently facilitates the further recruitment of peripheral monocytes to the CNS.
Mast cells and their products contribute to central nervous system inflammation and degeneration
MCs’ contribution to MS/EAE immunopathogenesis seems to take place throughout the entire disease process. Most CNS MCs reside on the abluminal side of the blood vessels and are thus able to interact with neurons, glial cells, astrocytes, endothelial cells of the extracellular matrix, and with the peripheral leukocytes that invade the CNS. Most of the crosstalk between MCs and these other residing or infiltrating cells is believed to occur through the release of different mediators. According to Christy and Brown (2007), some of the most detrimental consequences of MC presence in the CNS during MS/EAE development include alteration in BBB permeability and a subsequent increase in the number of cells that reach the CNS, which is mediated by histamine,chemokines, and leukotrienes. MCs could also contribute to epitope spreading through tissue damage provoked by proteases, Th1 and Th17 differentiation by releasing polarizing cytokines (IL-12 for Th1 and IL-6 plus TGF-β for Th17), and a reduction in the number of Tr1 regulatory cells by a direct interaction involving the OX40L/OX40 pair.
Mast cells contribute to intestinal Th17 differentiation
The largest MC population in the human body is found in the gut, where they are involved in key physiological activities such as the regulation of epithelial function and integrity, the modulation of innate and specific immunity, and the preservation of neuro-immune intercommunications(Albert-Bayo et al., 2019). Moreover, MCs are equipped with a broad assortment of receptors that allow them to interact with the intestinal microbiota. These interactions can either induce or reduce the activation status of these cells. In the case of activation, MCs can secrete proinflammatory mediators, release extracellular traps, and act as local APCs (De Zuani et al., 2018). In this context, it has been described that the small intestine environment plays an essential role in the immunopathogenesis of EAE and MS by promoting Th17 expansion (Cosorich et al., 2017). Considering this and other evidence supporting the occurrence of gut microbiome dysbiosis in MS (Gandy et al., 2019) and the participation of MCs during Th17 expansion in mucosal barriers (Suurmond et al., 2016), it is tempting to speculate that MCs also contribute to these neurodegenerative diseases by acting duringTh17 differentiation in the small intestine.
Mast Cell-Modulating Drugs
As previously described, MCs are involved in the pathogenesis of various human disorders such as mastocytosis,urticaria, allergic rhinitis, asthma, tumors, and autoimmune diseases. Considering the increased prevalence of these pathological conditions in recent years, MC-modulating drugs and their pathway signaling targets have been largely investigated (Siebenhaar et al., 2018). A variety of these MC-modulating drugs are already approved by the Food and Drug Administration Agency (FDA), especially for the treatment of hypersensitivity diseases (Table 2). A few examples of these drugs, based on their previous utilization to control inflammatory pathologies (including EAE and MS) will be briefly described below. To improve clarity, their mechanisms of action will be grouped into four categories, including a reduction in cell number, interference in cell activation,inhibition of signal transduction, and blockage of the release or activity of their mediators. However, it is important to emphasize that some drugs can act through more than one mechanism.
Reduction in cell number
Reduction in MC number is an important strategy since this avoids the recruitment of these cells to the sites of disease manifestation. This is achieved through the induction of apoptosis or by blocking the molecules responsible for their differentiation, survival, or migration. For example,cladribine is a synthetic adenosine analog already approved in therapies for aggressive forms of mastocytosis, which induces cell death through the disruption of DNA synthesis and repair (Barete et al., 2015). Moreover, tyrosine kinase inhibitors (TKI) such as imatinib also reduce MCs number in mastocytosis patients (Alvarez-Twose et al., 2017) and were recently approved by the FDA for use in patients with gastrointestinal stromal tumors (Zitvogel et al., 2016). Protein tyrosine kinases (PTK) such as c-kit, Syk (spleen tyrosine kinase), and Src family protein tyrosine kinases play crucialroles in MC activation. The signaling triggered by the binding of IgE to FcεRI anchored on MC’s surfaces is enhanced by the interaction of SCF with c-kit, while the Src family protein tyrosine kinases and Syk are also required to control the signaling events dependent on FcεRI activation (Gilfillan and Rivera, 2009).
Sphingosine-1-phosphate (S1P) inhibitors are another example of drugs that work by decreasing MC numbers by altering their differentiation, proliferation, survival, and migration. S1P is a lipid metabolite essential for angiogenesis, neurogenesis, and lymphocyte trafficking. Its activity on the innate and adaptive immune response is mediated by binding to S1P receptors that are expressed in T and B lymphocytes, MCs, eosinophils, DCs, and endothelial cells. S1P signaling via S1P receptors regulates crucial cellular functions such as proliferation, survival, migration,and adhesion (Aoki et al., 2016). The therapeutic potential of S1P inhibitors has been mainly investigated in allergic asthma experimental models (Price et al., 2013; Roviezzo et al., 2016). In this context, an isoenzyme-specific SphK1 inhibitor attenuated ovalbumin-induced allergic inflammation and airway hyperresponsiveness in C57BL/6 mice. This effect was associated with reduced eosinophil recruitment to the lungs and lower levels of proinflammatory cytokines and chemokines in bronchoalveolar lavage fluid (Price et al.,2013). Other authors described that disodium cromoglycate,a chromone used to treat asthma and allergies, inhibits asthma-like features induced by S1P. This beneficial effect was attributed to the lower recruitment of MCs and B lymphocytes to the lungs and the reduced expression of CD23, which is a regulatory receptor that amplifies IgE-associated immune response on pulmonary T and B cells (Roviezzo et al., 2016).
Interference in mast cell activation
The second therapeutic strategy is to interfere with the process of MC activation. Several drugs act on activating or inhibitory receptors expressed on MC surfaces, such as ketotifen, omalizumab, and imatinib. Ketotifen is a histamine H1 receptor antagonist that is also endowed with MC-stabilizing properties that have been correlated with exocytosis inhibition (Baba et al., 2016). Omalizumab, an anti-IgE recombinant humanized monoclonal antibody, prevents the binding of IgE to FcεRI, thereby blocking the interaction between IgE and MCs/basophils (Busse et al., 2001). A recent clinical trial confirmed the efficacy of imatinib in the control of severe refractory asthma. This drug is an inhibitor of the SCF receptor (c-kit), which affects PTK activity and is essential for MC development and survival (Cahill et al., 2017). All of these MC-stabilizing drugs are largely employed to treat allergic rhinitis, conjunctivitis, asthma, and psoriasis. A diverse range of MC stabilizers has been identified over the last decade, including substances from natural, biological,and synthetic sources. Interestingly, clinical indications of these compounds are extrapolating the allergic conditions toward cancer therapies, antihypertensives, antidepressants,antimalarials, and immunomodulators (Zhang et al., 2016).
Inhibition of signal transduction
Another subtype of MC-modulating drug acts on signal transduction by blocking the effects of individual mediators.For example, inhibitors of Syk, phosphoinositide 3-kinase(PI3K), and Bruton’s tyrosine kinase (BTK), which block allergen IgE-mediated signaling, are currently being trialed for allergic rhinitis, rheumatoid arthritis, and chronic urticaria(Harvima et al., 2014). Fostamatinib, which is an oral Syk inhibitor, was recently approved by the FDA to treat adult persistent and chronic immune thrombocytopenia (Bussel et al., 2018). Moreover, Idelalisib, a PI3K inhibitor, was the first drug of its class of inhibitors to be approved to treat patients with hematologic malignancies (Raedler, 2015). Some BTK inhibitors, such as imidazolepyrazines, aminocinnolines,carbazoles, and pyridinones, have demonstrated high efficacy in controlling experimental rheumatoid arthritis. For this reason, acalabrutinib, a highly selective irreversible BTK inhibitor, was tested in a phase II clinical trial to treat rheumatoid arthritis patients (Lv et al., 2018).
Blockage of the release or activity of mediators
The last group of drugs is exemplified by calcineurin inhibitors such as cyclosporine A and tacrolimus (FK506),which interfere in calcium (Ca2+) mobilization and are very effective in controlling the most aggressive forms of mastocytosis (Harrison et al., 2007). Calcineurin is an enzyme that triggers the expression of a variety of cytokines by dephosphorylating the cytosolic nuclear factor of activated T cells in the presence of intracellular Ca2+. Harrison et al. (2007)demonstrated that cyclosporine A and FK506 inhibit the release of histamine from MCs and basophils by blocking the calcineurin pathway. Notably, this group contains many drugs that neutralize the effects of histamine (ketotifen)and leukotrienes (montelukast) or inhibit TNF-α release(etanercept) (Harvima et al., 2014). MCs are also rich in proteases such as tryptases, chymases, and carboxypeptidase A. Although a variety of protease inhibitors has already been therapeutically tested in inflammatory conditions, none have yet been approved for clinical use (Caughey, 2016).
The Effect of Mast Cell-Modulating Drugs on Experimental Autoimmune Encephalomyelitis and Multiple Sclerosis
As previously described, emerging evidence suggests that MCs play a critical role in both the initiation and progression of MS and EAE. MCs accumulate in the demyelinating plaques found in the CNS of MS patients (Kruger, 2001) and also contribute to EAE immunopathogenesis (Brown and Weinberg, 2018). Therefore, drugs with an inhibitory effect on MC activity could be therapeutically explored to control the inflammatory process that targets the CNS in MS. Some drugs that have already proven to be effective in preclinical and clinical trials will be briefly mentioned below.
Hydroxyzine, a first-generation antihistamine, efficiently controlled the initiation and progression of EAE in Lewis rat,which is an acute monophasic paralytic disease with spontaneous recovery (Dimitriadou et al., 2000). Although this investigation indicated that MC inhibition correlated positively with clinical improvement, the protective mechanism was not entirely described. More recently, the TKI imatinib was demonstrated to reduce CNS inflammation, demyelination,and T cell recruitment in a DA rat model of recurrent MS by enhancing BBB integrity and establishing an anti-inflammatory phenotype in the periphery (Adzemovic et al., 2013).Interestingly, imatinib efficiently controlled neuroinflammation even when treatment began after EAE clinical manifestations. This is certainly a relevant drug feature since most patients are treated after the onset of clinical manifestations.Therapy with the TKI tyrphostin AG126 was also effective in controlling EAE symptoms in C57BL/6 mice, which is a chronic disease model. Treated animals displayed less Th17 differentiation and CNS inflammatory infiltration, decreased microglial activation, and limited myelin damage (Menzfeld et al., 2015). The authors also demonstrated that AG126 directly inhibits BTK, which is associated with B cell receptor and TLR signaling. According to Menzfeld et al. (2015), this drug has the potential to serve in the therapeutic management of disturbed innate and adaptive immune functions in MS patients. A selective PI3K inhibitor ameliorated EAE symptoms in C57BL/6 mice by reducing leukocyte infiltration and enhancing remyelination and the number of axons in the CNS (Li et al., 2013). Since remyelination can restore neuronal function and prevent further neuronal loss and clinical disability in MS patients, this PI3K inhibitor seems to be a promising drug for MS therapy.
Encouraging results from the use of MC-targeted drugs in MS patients have also been observed. For example, it has been described that people exposed to antihistamine sedatives presented a reduced risk of developing MS (Yong et al., 2018). According to these authors, this beneficial effect could be related to the ability of these drugs to cross the BBB and to their effects within the CNS. Moreover, a TKI named masitinib triggered a therapeutic benefit in patients with progressive MS, which was characterized by an improvement in MS functional composite score (Vermersch et al., 2012). In this multicenter, randomized, placebo-controlled trial, masitinib was orally administered for at least 12 months. This therapy was relatively well tolerated, while the adverse events included asthenia, rash, nausea, edema, and diarrhea, all of which were considered manageable by symptomatic treatment. Cladribine, which is an apoptotic drug that reduces the amounts of MCs, T and B lymphocytes,was also tested. In a phase III, 96-week, placebo-controlled,multicenter study, the administration of cladribine oral tablets resulted in encouraging effects in both clinical and neuroimaging outcomes in relapsing-remitting MS (Giovannoni et al., 2010). Treated patients presented significantly lower annualized relapse rates, higher relapse-free rates, and a significant reduction in the number of brain lesions determined by magnetic resonance imaging (MRI). Lower risk of 3-month sustained progression of disability observed in patients treated with cladribine supports the importance of this drug for controlling the main manifestations of this pathology, acute relapses, and progressive disability. However,this efficacy was accompanied by lymphocytopenia and herpes zoster infection.
Recently, a double-blind, randomized, phase II trial performed over 24 weeks showed that evobrutinib, a selective oral BTK inhibitor, triggered beneficial effects in relapsing MS. In patients treated daily with 75 mg, there was a significant reduction in the total number of enhancing MRI lesions. Notably, a change in measures of disease activity on MRI is commonly used as a marker of treatment response.Interestingly, only elevations in liver aminotransferase levels were detected in these patients (Montalban et al., 2019).These results suggest that evobrutinib represents another promising drug for MS therapy. However, longer and larger trials are still required to corroborate its beneficial effect.
Our experience
In recent years, we have dedicated our scientific efforts to test alternative therapies for MS. For this, we adopted EAE,which is widely accepted as a model for therapeutic and immunomodulatory drug screening. Our main strategy has been to test pharmaceuticals that are already prescribed to humans without causing unacceptable deleterious effects.Active vitamin D3 is one of these substances, which demonstrated prophylactic and therapeutic potential (Chiuso-Minicucci et al., 2015; Mimura et al., 2016). Vitamin D and its analog paricalcitol also worked well as tolerogenic adjuvants(Mimura et al., 2016; Zorzella-Pezavento et al., 2017).
More recently, based on outstanding discoveries concerning the contribution of MCs to EAE development (Secor et al., 2000; Christy et al., 2013; Brown and Weinberg, 2018)and the availability of numerous drugs that control their activity, we tested the hypothesis that ketotifen fumarate (Ket)could control EAE development. This drug is a second-generation antihistamine substance that is widely used to treat allergic disorders such as asthma, chronic rhinitis, urticaria,and allergic conjunctivitis. It acts mainly by antagonizing histamine H1 receptors and inhibiting MCs exocytosis (Baba et al., 2016). In our investigation, C57BL/6 mice were submitted to EAE induction and treated daily with Ket from the 7thday onward following disease induction. This early intervention with Ket was very effective in controlling both disease prevalence and severity (Pinke et al., 2019). Markers of disease severity, such as inflammasome activation status, oxidative stress, and T cell infiltration, were all significantly reduced following therapy. Additionally, a clear restoration of BBB permeability levels, which was disrupted during EAE development, was observed following Ket administration. The primary findings of this investigation are illustrated in Figure 3.
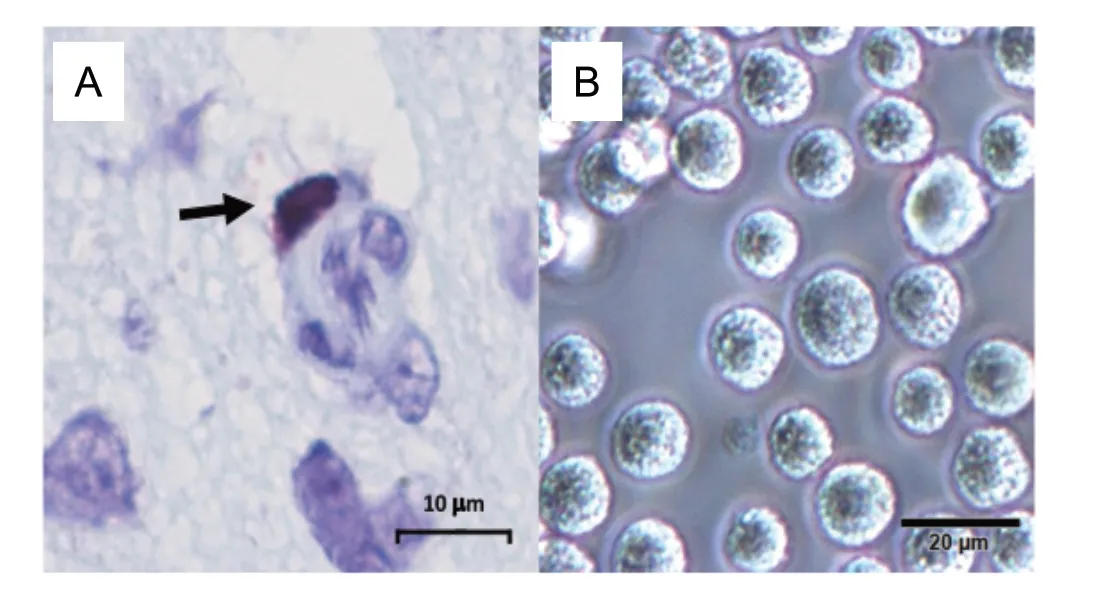
Figure 1 Morphological aspect of mast cells found in tissues (A) and in cell culture after in vitro differentiation (B).

Figure 2 MC contribution to MS/EAE development.
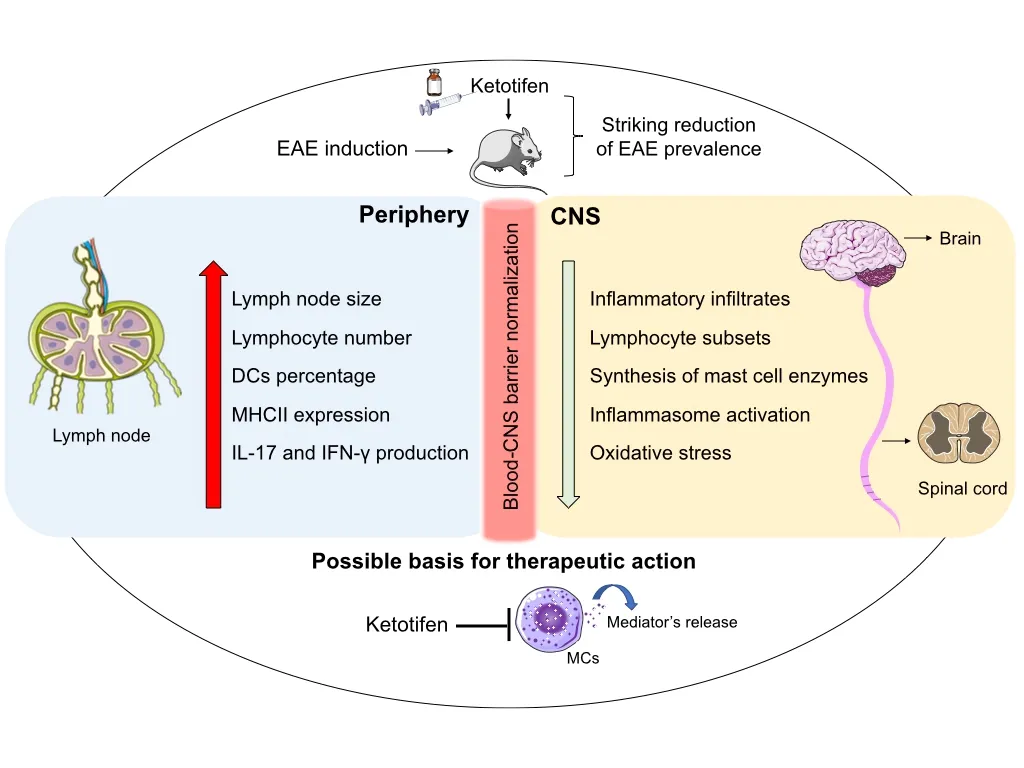
Figure 3 Overview of the ketotifen effects on EAE immunopathogenesis.
Considering the abundant supporting literature, we interpreted these results as a preliminary yet significant proofof-concept that this and other MC activity-inhibiting drugs could be useful in MS therapy. In this regard, we envision a series of aspects that deserve to be theoretically considered and experimentally investigated. To illustrate these points,we believe that a wide-open comparison between MCs from patients (preferentially presenting distinct disease forms)and healthy individuals based on omics data analysis could be particularly enlightening. The EAE model would certainly be useful to scrutinize the various aforementioned drug groups and to suggest the stages of disease progression wherein the drugs would be more effective. Additionally,this model could be used to test a variety of possible drug associations, not only among these MCs targeted drugs, but also between them and the array of immunomodulatory substances that are currently available for MS treatment.
Conclusion
The past several years have witnessed significant advancements in knowledge regarding MS and EAE immunopathogenesis and therapeutic approaches, with investigations revealing the pivotal role of MCs in disease evolution being especially enlightening. The possible role of MCs in MS has been predominantly investigated using the EAE model.Strong evidence supports the substantial contribution of MCs to BBB disruption, recruitment of inflammatory cells to the CNS, and local degeneration. While most of these effects have been attributed to their soluble products, their contribution to the other stages of EAE/MS immunopathogenesis remains a matter to be clarified. However, this possibility is reasonably supported by literature data. In this context,the possibility to employ MC-targeted drugs to control this disease can be viewed as a promising therapeutic avenue for exploration. Many of these drugs are already approved by the FDA and considered safe enough to be tested in clinical trials either alone or associated with currently adopted therapies. Additionally, preclinical studies taking advantage of recent advances in genome editing tools and classical methodologies in the immunology field could contribute to an improved understanding of how MCs contribute to each of the distinct pathological steps of EAE development in order to define the most responsive disease stages.
Acknowledgments:The authors thank the Brazilian site fCiências -Ciência e Tecnologia for providing a free image bank that was used in the assembly of figures 2 and 3.
Author contributions:All authors contributed to the content of the review and approved the final manuscript.
Conflicts of interest:The authors declare no conflicts of interest.
Financial support:This work was supported by São Paulo Research Foundation (FAPESP, grant Nos. 2015/03965-2 and 2014/00239-6), the National Council for Scientific and Technological Development (CNPq,grant Nos. 307603/2018-0 and 307269/2017-5), and Coordination for the Improvement of Higher Education Personnel (CAPES, Finance Code 001).
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Hong Fan, Fourth Military Medical University,China.
- 中国神经再生研究(英文版)的其它文章
- Muscovite nanoparticles mitigate neuropathic pain by modulating the inflammatory response and neuroglial activation in the spinal cord
- Knocking down TRPM2 expression reduces cell injury and NLRP3 inflammasome activation in PC12 cells subjected to oxygen-glucose deprivation
- Neuroprotective mechanisms of ε-viniferin in a rotenone-induced cell model of Parkinson’s disease:significance of SIRT3-mediated FOXO3 deacetylation
- Amyloid-beta peptide neurotoxicity in human neuronal cells is associated with modulation of insulin-like growth factor transport, lysosomal machinery and extracellular matrix receptor interactions
- MicroRNA regulatory pattern in spinal cord ischemiareperfusion injury
- Sequencing analysis of matrix metalloproteinase 7-induced genetic changes in Schwann cells