Neuroprotection mediated by natural products and their chemical derivatives
Fei Fei , Ning Su , Xia Li , Zhou Fei
1 Department of Ophthalmology, Xijing Hospital, the Fourth Military Medical University, Xi’an, Shaanxi Province, China
2 Department of Radiation Oncology, Xijing Hospital, the Fourth Military Medical University, Xi’an, Shaanxi Province, China
3 Department of Neurosurgery, Xijing Hospital, the Fourth Military Medical University, Xi’an, Shaanxi Province, China
Abstract Neuronal injuries can lead to various diseases such as neurodegenerative diseases, stroke, trauma, ischemia and, more specifically, glaucoma and optic neuritis. The cellular mechanisms that regulate neuronal death include calcium influx and calcium overload, excitatory amino acid release, oxidative stress, inflammation and microglial activation. Much attention has been paid to the effective prevention and treatment of neuroprotective drugs by natural products. This review summarizes the neuroprotective aspects of natural products, extracted from Panax ginseng, Camellia sinensis, soy and some other plants, and some of their chemical derivatives. Their antioxidative and anti-inflammatory action and their inhibition of apoptosis and microglial activation are assessed. This will provide new directions for the development of novel drugs and strategies to treat neurodegenerative diseases.
Key Words: antioxidant effect; brain injuries; Camellia sinensis; chemical derivatives; curcumin; genistein;natural products; neurodegenerative diseases; neuroprotection; Panax
Introduction
Neuronal damage and death in the central nervous system is a common pathological process in neurological diseases,such as neurodegenerative diseases, stroke, brain injury, glaucoma and optic neuritis (Fei et al., 2015). In the past few decades, the incidence of neurodegenerative diseases, including Parkinson’s disease (PD), Huntington’s disease, amyotrophic lateral sclerosis and Alzheimer’s disease (AD), has increased dramatically worldwide (Wang et al., 2019). These diseases not only seriously threaten the health of older adults, but also greatly increase the social burden. The cellular mechanism that regulates neuronal cell death includes calcium influx and calcium overload, excitatory amino acid release, oxidative stress, inflammation and glial dysfunction (Cheng et al., 2018). However, the underlying mechanism of neuronal death is still not fully understood and there are no effective therapies for these neurological diseases. Therefore, more research and trials of neuroprotective drugs are in progress(Ganguly et al., 2018).
Neuroprotection is not just a treatment to slow disease progression and prevent neuronal death, but also to defer age related neuronal death, a natural phenomenon and extremely complex process (Cai et al., 2017). The neuroprotective effects of natural products and their chemical derivatives have attracted much attention (Araujo-Filho et al., 2016) and are discussed and summarized in this review.
Retrieval Strategy
The PubMed online database was searched for studies from 1942 to 2019, using the following queries in all fields: (Brain injuries (MeSH Terms) OR Neurodegenerative diseases(MeSH Terms)) AND (Natural Products (MeSH Terms)).Non-SCI experiments and reviews were excluded.
We examined the potential usefulness ofPanax ginseng,green tea/EGCG/Camellia sinensis,genisteinand curcumin in the prevention and treatment of brain injuries or neurodegenerative diseases in human clinical studiesin vivoor cellsin vitro. We reviewed the molecular mechanisms involved in effective prevention and treatment of neuroprotective drugs from natural products.
Mechanisms of Neuronal Damage and Neuroprotection
The mechanism of neuronal damage and death, from the organ level to the molecular level, has been explored over many years. In 1889, the concept of “brain damage” was first reported; however, the neuronal changes in the brain were not studied. In 1942, Sandler first proposed “neuronal injury” and found that hypoglycemia complicated with poliovirus infection caused neuronal injury. Later on, the study of neuronal damage went down to the molecular level. For example, in 1988, O’Shaughnessy and Lodge found that the activation of excitatory amino acid receptors could lead to neuronal damage by enhanced Ca2+mobilization and these could be blocked by selective antagonists. In 1995, Ziv et al.also verified the importance of the effects of changes in intracellular calcium concentration on neuronal damage. The theory of the molecular mechanisms of neuronal damage is focused on calcium influx and calcium overload, excitatory amino acid release and free radical formation (Abushouk et al., 2017). Calcium is of particular importance in neuronal damage. Calcium overload can trigger either necrotic or apoptotic cell death of neurons. Normally, nitric oxide plays a physiological role as a neurotransmitter, but in the pathological concentrations it can cause damage to neuronal cells(Gokay et al., 2016). Excitatory amino acids are important neurotransmitters in the central nervous system with their release and uptake controlled under normal circumstances. However, their accumulation can lead to brain damage.Brain tissue is rich in iron and unsaturated fatty acids but lacks the antioxidant systems of catalase, glutathione and vitamin E (Sakr et al., 2015). As a result, neurons are more vulnerable to damage by oxygen free radicals. The expression of reactive oxygen species (ROS, such as peroxides and hydroxyl groups) and reactive nitrogen clusters increase significantly in cerebral ischemia. Extensive production of ROS leads to lipid peroxidation and causes post-ischemic nerve injury (Lei et al., 2015). Glutamate-induced activation of N-methyl-D-aspartic acid receptors can lead to calcium overload, protein kinase activation, nitric oxide synthase and phospholipase, which are associated with the damaged mitochondria and increased free radicals, resulting in neuronal damage (Simoes et al., 2018; Figure 1). Various epidemiological studies have indicated that natural products such as ginsenoside and soy isoflavone glycitein may reduce the risk of neurodegenerative disorders and neuroinflammatory diseases. It has been reported that ginkgo biloba extract 761 and resveratrol can protect neuronal damage by inhibiting the generation or aggregation of β amyloid peptide (Aβ; Yang et al., 2018). Other research into the effects of ginsenosides, including Re, Rb1 and Rg1, showed they have neuroprotective effects and can promote neurite outgrowth in cultured neurons (Li et al., 2017). These and other studies indicate that natural products may contribute towards neuroprotection after neuronal damage.
Neuroprotective Effects of Some Natural Products and Their Chemical Derivatives Ginsenoside from Panax ginseng (WEG)
Ginseng is a perennial plant, belonging to the Panax genus under the family Araliaceae. The herbal medicine of ginseng is extracted from the dried root of the perennial plant.
Among the family of Araliaceae, WEG is a precious herbal medicine used in many countries, especially in Korea, China and Japan, for more than five thousand years. People named the plant WEG because they believed that WEG had properties to resist the adverse influence and restore homeostasis from the damage of multiple physical and chemical factors to the patients. Russian botanist C. A. Meyer named the genus“Panax” which derived from ancient Greek words “pan” and“axos”, meaning “all” and “cure”. Chinese named the plants“ginseng” from ancient literary Chinese “renshen”, which means “human”. Nowadays ginseng has become a popular,worldwide drug with a wide variety of medicinal uses. A recent scientific study demonstrated that ginseng has positive effects on preventing skin aging and increasing vigor (Park et al., 2016b).
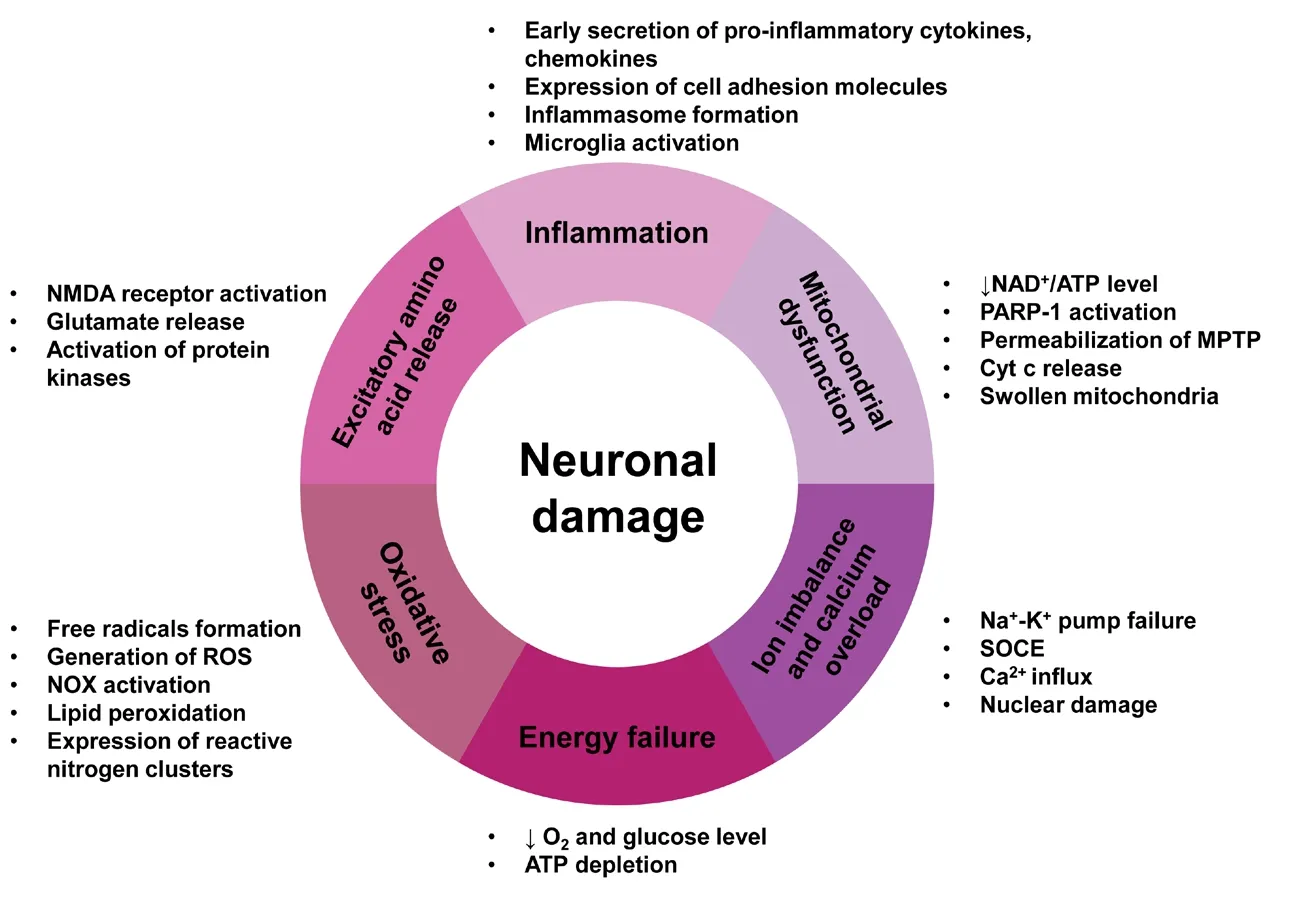
Figure 1 The main molecular mechanisms of neuronal damage.
A number of compounds have been identified in the root of Ginseng, including ginsenosides, the elements calcium,magnesium and manganese, various vitamins and amino acids (Chen et al., 2019b). However, the two major active ingredients named ginsenosides or ginseng saponins are found exclusively in the Panax species (Chen et al., 2019b).More than 100 different ginsenosides have been named and extracted from whole ginseng of which 40 ginsenosides have been found in WEG (Fernandez-Moriano et al., 2017). All of the identified ginsenosides have a four-ring hydrophobic, steroid-like chemical structure in common. However,depending on the different number of sugar moieties and binding positions, there are two main groups of ginsenosides: 20(S)-protopanaxadiol and 20(S)-protopanaxatriol saponins (Jin et al., 2019). The different numbers of sugar moieties might be responsible for the unique activity of each ginsenoside. The different chemical structures result in each ginsenoside having its own specific tissue-dependent pharmacological effects. Many studies have shown that ginseng has numerous therapeutic effects, including cardiovascular and hepatic benefits (Shi et al., 2019), immunomodulatory effects (Chen et al., 2019a), cancer suppression (Wang et al.,2018), hypoglycemic activity (Jang et al., 2017) and beneficial effects on the central nervous system, such as improved cognitive performance and neurotransmitter modulation(Reay et al., 2019). Many reports have shown that ginsenosides have neuroprotective effects on the central nervous system through their anti-apoptosis, oxidation resistance,and anti-inflammation properties (Zhou et al., 2018). Ginsenosides have been used to treat some neurodegenerative diseases including PD and AD (Razgonova et al., 2019).Several reports demonstrate that ginseng and its active components ginsenosides exhibit neuroprotective effects on dopaminergic neurons bothin vitroandin vivo(Cheng et al.,2019; Razgonova et al., 2019). PD, characterized by both the progressive degeneration of the nigrostriatal dopaminergic pathway and the accumulation and aggregation of misfolded α-synuclein, is a progressive neurodegenerative disease affecting 2% of the population aged over 60 years worldwide.Nowadays, most PD treatments provide only symptomatic therapies, and no medicine has been proven to prevent the progressive degeneration. However, ginsenosides Rb1 and Rg1 can attenuate Ca2+over-influx into mitochondria and increase energy production, leading to partial neuroprotectionin vivo. It is reported that in 1-methyl-4-phenylpyridinium (MPP+) treated SH-SY5Y cells, WEG exerted an inhibitory effect on cell death, alleviated the overproduction of ROS, increased Bax/Bcl-2 ratio, downregulated the expression of cytochrome c and prevented the activation of caspase-3 (Bell et al., 2017). These experiments suggested that ginseng has potential therapeutic value in the war against PD.
AD usually affects older adults, destroys memory and thinking skills with gradual neuronal death. Aβ accumulation is widely believed to be the key leading to AD pathogenesis. According to current research, Aβ deposition is caused by the abnormal metabolism of amyloid precursor protein(Wang et al., 2016a). Nowadays therapies in AD include decreasing the generation or aggregation of Aβ, increasing the disaggregation of Aβ in neurons, and using stronger antioxidant and anti-inflammatory drugs. There is still no definitive evidence to document any novel drug effective in preventing the progressive loss of neurons in AD patients. However,studies have suggested that ginsenosides could be protective and trophic against AD.
Korean red ginseng can be used as an adjuvant treatment adding to conventional therapies in AD patients (Heo et al.,2008). The results showed that 15 patients taking 12 weeks of high-dose Korean red ginseng obtained significant improvement in the Alzheimer’s disease Assessment Scale and Clinical Dementia Rating compared with the control group(n= 31) but not using the Korean version of MMSE (Heo et al., 2008). Ginseng may exert its neuroprotective effect by its anti-inflammatory effects. Ginseng extracts can significantly reduce the activation of nuclear factor-κB (NF-κB) and MAP kinase. Lipopolysaccharide (LPS)-induced inducible nitric oxide synthase (iNOS) and LPS-induced activation of STAT signaling pathway can be significantly inhibited by ginsenosides Rh2, Rh3 and compound K (Yu et al., 2017). Therefore, ginseng extracts can be beneficial in treating neuroinflammatory diseases. Another study found that American ginseng selectively inhibits the expression of inflammatory intermediates in the STAT cascades in keratinocytes and the expression of LPS-induced tumor necrosis factor-α and other cytokines in macrophages (Ahn et al., 2016). It has been reported that ginsenoside Rg2 inhibits Na+influx through the channels and consequently reduce both Ca2+influx and catecholamine secretionin vivo. Na+channel blockers have neuroprotective effects on neuronal damage caused by cerebral ischemia, hypoxia, and brain trauma (Leipold, 1995). In conclusion, this natural herb, ginseng, and its components,ginsenosides, provide important natural sources of protection for neuronal damage.
Green tea epigallocatechin-3-gallate
Green tea (Camellia sinensis) is a traditional beverage consumed worldwide, especially in Asian countries. This traditional drink is linked with a low incidence of human cancer,obesity and slowing of the aging process. Thus, the effect of green tea in the prevention and mitigation of neurodegenerative diseases has recently received particular attention (Tyagi et al., 2016). Green tea (Camellia sinensis) is generally composed of four derivatives based on their different structures:epicatechin, epigallocatechin, epicatechin gallate and green tea epigallocatechin-3-gallate (EGCG). However, the most abundant and anti-oxidative component in green tea is (-)-EGCG (Shirani et al., 2016). The potential of EGCG for antioxidant, anti-inflammatory (Ohishi et al., 2016) and fibrogenic instability is responsible for the neuroprotective effects of green tea (Xing et al., 2019). An epidemiological survey has shown that American people drinking two or more cups of tea every day have a low risk of PD (Barranco Quintana et al.,2009). EGCG may prevent ROS by scavenging free radicals,metal chelation and adjusting the oxidation and anti-oxidant enzyme system. EGCG can inhibit Cd2+-induced apoptosis by acting as a ROS scavenger rather than a metal chelating agent (An et al., 2014). EGCG can promote the survival of PC12 cells treated with neurotoxic compounds 6-hydroxydopamine, MPP+, paraquat, levodopa, and hydrogen peroxide(He et al., 2017). It was suggested that green tea polyphenol EGCG not only upregulates the expression of antioxidant enzymes such as superoxide dismutase, catalase and GPX but also effects a variety of apoptotic factors including caspase modulation, Fas ligand (FasL), growth arrest and DNA damage proteins and members of the B cell lymphoma 2 (Bcl-2)family (Wang et al., 2015b). It is believed that these effects lead to EGCG’s antioxidative roles. In addition, EGCG is more effective at scavenging free radicals than other common factors, vitamin C and E (Zahr et al., 2018). Chen et al. (2019a,b) confirmed that orally administered EGCG could modulate other pathways including protein kinase C, extracellular signal-regulated kinase, and Akt/glycogen synthase kinase-3 axis. The neuroprotective effect of EGCG could be exerted through another mechanism that involves scavenging ROS,iron chelation and stabilization of the mitochondrial source of activated caspase. EGCG directly reduced neuronal damage caused by ROS by inhibiting NF-κB (Zhang et al., 2016).Wei et al. (2011) reported that EGCG directly inhibited the expression of nNOS through inhibition of the secretion of nitric oxide and tumor necrosis factor-α by LPS activated microglia, thereby reducing the role of oxidative stress.
The neuroprotective effect of green tea has been confirmed in many clinical trials (Prado Lima et al., 2018; Kim et al.,2019). Green tea, especially EGCG, can be an adjunctive method in the treatment of neurodegenerative diseases and neurological diseases.
Soy isoflavone glycitein
Soybeans and soy products are highly consumed in traditional Asian diets. A previous study demonstrated that the soybeans and soy products can reduce the risk of cancer,heart diseases and diabetes (Namazi et al., 2018). Soy isoflavone glycitein, the most recognized biologically active compound in soybeans and soy products, is considered to have therapeutic potential for different types of neurodegenerative diseases. Soybeans are rich in isoflavones including genistein(4′,5,7-trihydroxyisoflavone), daidzein (4′,7-dihydroxyisoflavone), glycitein (6-methoxydaidzein) and their glycosides.Genistein can defend human cortical neurons against free radical generating toxins such as tertiary-butylhydroperoxide(t-BuOOH) by regulating bcl-2 (Sonee et al., 2004). Glycitein could decrease the expression of β amyloid-induced paralysis in the transgenicCaenorhabditis elegans(Gutierrez-Zepeda et al., 2005). Therefore, soy isoflavones may have therapeutic potential for Aβ-related neurodegenerative disease through combinations of antioxidant activity and inhibition of Aβ deposition. Genistein, which has structural similarities to the sex hormone estrogen, exhibits estrogen agonist and antagonist characteristics and exerts protective effects on hippocampal neurons and dopaminergic neuronsin vitroandin vivo(Park et al., 2016a). Genistein can promote the cascade reaction to inhibit production of ROS and expression of BAD mRNA, regulate nuclear factor-erythroid 2 p45-related factor 2/heme oxygenase-1, Nrf 2/HO-1 signal transduction pathway, upregulate the expression of apoptosis suppressing gene and reduce the deposition of Aβ (Bai and Wang, 2019).
All these findings show that soybeans and soy products are potential new therapies for neurodegenerative disorders.
Curcumin
Curcumin, extracted from the root of ZingiberaceaeCurcuma longa, is used as a food preservative in ancient Asia (Arablou and Kolahdouz-Mohammadi, 2018). Curcumin, derived mainly from turmeric, has been associated with the beneficial effects of several human ailments for centuries, as recorded in the Ayurveda. Epidemiological studies have shown that the usage of turmeric may be behind the low incidence rate of AD in India compared with that in Caucasians (Tiwari et al.,2016). Turmeric is effective in protecting neurons in the PD mouse. It is believed that turmeric contains a variety of active ingredients, however, the most important component of turmeric is curcumin, which has strong antioxidant properties,radical scavenging and metal chelating activities.
Curcumin can effectively attenuate the production of pro-inflammatory mediators, including cytokines, activator protein 1, NF-κB, iNOS, cyclooxygenase 2 and c-Jun N-terminal kinase (Yang et al., 2018). Curcumin has been shown to directly detoxify reactive nitrogen species such as peroxynitritein vitro(Liu et al., 2011). Curcumin can protect neuronal mitochondria against peroxynitrite-induced protein nitration. In addition, curcumin suppresses the inflammation in microglia through inhibiting the ganglioside-,LPS-, or interferon-γ-stimulated induction of cyclooxygenase 2 and iNOS, which are key steps in inflammatory processes (Pan et al., 2012). The enhancement of suppressor of cytokine signaling 1 expression and the inhibition of Janus kinase-signal transducer and activator of transcription signaling lead to the anti-inflammatory effect of curcumin on activated microglia (Kim et al., 2003). Furthermore, curcumin can not only reduce oxidative and inflammatory damage,but also decrease the accumulation of Aβ in mutant amyloid precursor protein transgenic plaque-forming mouse model(Du et al., 2019). The molecular structure of curcumin is similar to that of the β-amyloid receptor and studies have shown that low-dose curcumin not only effectively disaggregates Aβ, but also prevents fibril and oligomer formation(Ouberai et al., 2009). This indicates how curcumin may contribute to the prevention or treatment of AD.
It is concluded that curcumin has significant neuroprotective effects, such as anti-inflammatory, antioxidant, and anti-protein-aggregate effects, and has a great potential in the treatment of different neurodegenerative disorders and neuroinflammatory diseases.
Other products and their chemical derivatives
Resveratrol, a natural polyphenol with multiple biological functions (Jeong et al., 2016), is mainly found in grapes, giant knotweed, peanuts and mulberries. Resveratrol can attenuate early neurological dysfunction in transgenicC. elegans(Pandey et al., 2015) and neuropathic pain in mice by activating Sir2 (silent information factor 2) (Shao et al., 2014). In rat models, resveratrol can lessen neuronal damage induced by the dopamine neurotoxin, MPP+(Chen et al., 2016). In the ischemic model of Mongolian gerbils, the resveratrol delayed neuronal death was accompanied by microglial activation(Bhattacharjee et al., 2016; Liu et al., 2016). Early data have demonstrated that resveratrol potently inhibited the production of NO and iNOS, increased the activity of superoxide dismutase and decreased malondialdehyde concentration (Li et al., 2015b). It showed that resveratrol had neuroprotective effects on LPS-induced and N-methyl-D-aspartic acid-induced neuronal death. This neuroprotective effect may be associated with antioxidant and anti-inflammatory activity.In addition, resveratrol has been shown to activate PI3K/Aktand extracellular signal-regulated kinase 1/2 pathway, resulting in the inactivation of glycogen synthase kinase-3, which may contribute to its neuroprotective effects (Wang et al.,2015a).
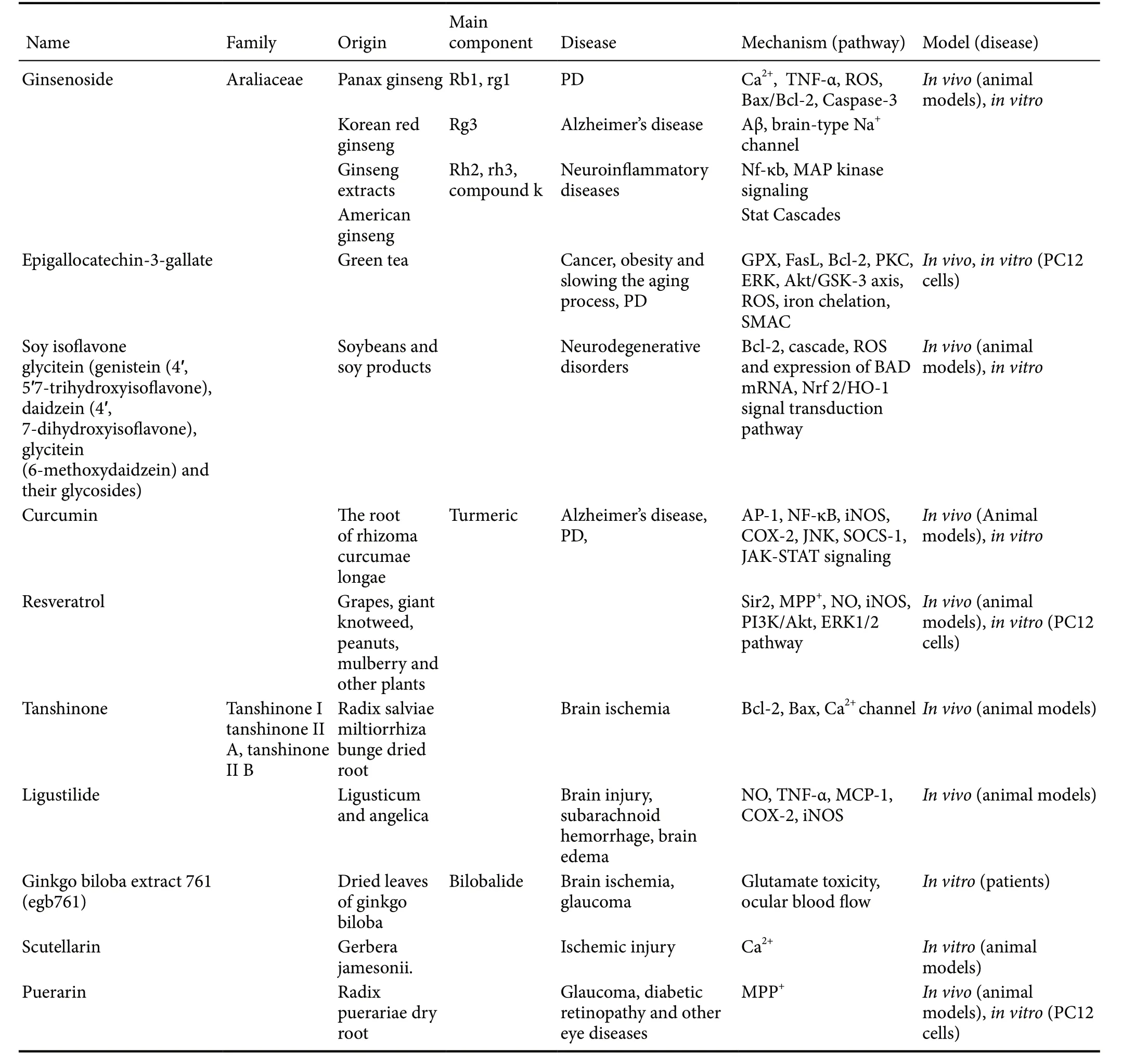
Table 1 Summary of neuroprotective effects of some natural products and their chemical derivatives
Tanshinones are lipid soluble abietane diterpenes extracted from the dried root, RadixSalviae MiltiorrhizaBurge.The main constituents are tanshinone I, tanshinone II A, and tanshinone II B but tanshinone II A is the most abundant component. Studies have shown that tanshinone II A may have neuroprotective effects in ischemic animal models (Lee et al., 2013). Tanshinone II A pretreatment can promote the activation of astrocytes and reduce brain damage in ischemic models. The neuroprotective effects of tanshinone II A may be associated with the increased activity of superoxide dismutase, decreased concentration of malondialdehyde, higher expression of Bcl-2, and lower expression of Bax, nitric oxide and iNOS (Li et al., 2015a). In addition, tanshinone II A can block calcium channels to reduce intracellular Ca2+accumulation after brain damage (Xu et al., 2016).
Ligustilide is a main active ingredient inLigusticumand Angelica. Numerous studies have reported that ligustilide can reduce NO production, tumor necrosis factor-α, monocyte chemotactic protein-1, cyclooxygenase-2 and iNOS expression to protect neurons (Su et al., 2011; Ma and Bai,2012). Moreover, ligustilide can reduce the fatality rate, improve the neurobehavioral defects, attenuate brain edema and alleviate cerebral vasospasm through the blood brain barrier after subarachnoid hemorrhage in mice (Xiao et al.,2015).
Ginkgo biloba extract 761 (EGb761), from the dried leaves of Ginkgo biloba, has been used to treat numerous diseases in traditional Chinese medicine for thousands of years. EGb761 is also used as a natural neuroprotective medication for brain ischemia (Wan et al., 2015). In a neuronal toxicity modelin vitro, 12 M bilobalide, a purified terpene lactone component of the EGb761, reduced neuronal death induced by glutamate toxicity, demonstrating that bilobalide may have neuroprotective potential (Chandrasekaran et al.,2003). The retina, as part of the central nervous system, was also protected by EGb761. The vision in normal-tension glaucoma patients was improved after Ginkgo biloba extract treatment compared with the controls. It was suggested that the effect of Ginkgo biloba extract was related to the increased ocular blood flow (Cho et al., 2019).
Scutellarin is a flavonoid compound, extracted fromGerbera jamesonii. Scutellarin may exert its neuroprotective effects by inhibiting Ca2+overload, resistance to lipid peroxidation, maintenance of the mitochondrial membrane structure and improvement of cerebral energy metabolism after ischemic injury (Tang et al., 2014).
Puerarin, an isoflavone compound, is extracted and isolated from the dry root ofRadix Puerariae. In recent years,puerarin has been used to treat glaucoma, diabetic retinopathy and other eye diseases. Some researchers have reported an anti-apoptosis role for puerarin in ischemic models (Wang et al., 2016b). The results showed that pretreatment of puerarin in PC12 cells can reduce MPP+-induced neurotoxicity and apoptosis. The neuroprotective mechanism of puerarin may be linked to inhibition of mitochondrial dysfunction and the activation of caspase-3 (Wang et al., 2016b; Table 1).
Conclusion
The mortality and morbidity in patients with disease or injury of the central nervous system are still high. Neuronal damage is central to the high figures but its pathophysiology is complex. Although tens of thousands of studies have investigated various neuroprotective mechanisms, including anti-oxidation, inhibition of apoptosis, anti-inflammation and inhibition of microglial activation, the outcomes of neurologically diseased patients or injured people are still poor.As we indicated in our review, evidence has accumulated that natural products and their chemical derivatives can reduce neuronal damage. They and their derivatives provide a new opportunity for the development of novel drugs and strategies for the treatment of these diseases.
Author contributions:Conceptualization, writing—original draft preparation: FF and NS; writing—review and editing: ZF and XL. All authors approved the final version of the manuscript.
Conflicts of interest:The authors have no actual or potential conflicts of interest.
Financial support:This work was supported by the National Natural Science Foundation of China, Nos. 81600738 (to FF), 81771239 (to ZF),81430043 (to ZF), 81801300 (to NS). The funding sources had no role in paper writing or deciding to submit this paper for publication.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Muscovite nanoparticles mitigate neuropathic pain by modulating the inflammatory response and neuroglial activation in the spinal cord
- Knocking down TRPM2 expression reduces cell injury and NLRP3 inflammasome activation in PC12 cells subjected to oxygen-glucose deprivation
- Neuroprotective mechanisms of ε-viniferin in a rotenone-induced cell model of Parkinson’s disease:significance of SIRT3-mediated FOXO3 deacetylation
- Amyloid-beta peptide neurotoxicity in human neuronal cells is associated with modulation of insulin-like growth factor transport, lysosomal machinery and extracellular matrix receptor interactions
- MicroRNA regulatory pattern in spinal cord ischemiareperfusion injury
- Sequencing analysis of matrix metalloproteinase 7-induced genetic changes in Schwann cells