Could non-invasive brain-stimulation prevent neuronal degeneration upon ion channel re-distribution and ion accumulation after demyelination?
Friederike Pfeiffer , Alia Benali
1 Werner Reichardt Centre for Integrative Neuroscience (CIN), University of Tübingen, Tübingen, Germany
2 Section for Computational Sensomotorics, Department of Cognitive Neurology, Hertie-Institute for Clinical Brain Research and Centre for Integrative Neuroscience, University of Tübingen, Tübingen, Germany
Abstract Fast and efficient transmission of electrical signals in the nervous system is mediated through myelinated nerve fibers. In neuronal diseases such as multiple sclerosis, the conduction properties of axons are disturbed by the removal of the myelin sheath, leaving nerve cells at a higher risk of degenerating. In some cases, the protective myelin sheath of axons can be rebuilt by remyelination through oligodendroglial cells. In any case, however, changes in the ion channel organization occur and may help to restore impulse conduction after demyelination. On the other hand, changes in ion channel distribution may increase the energy demand of axons, thereby increasing the probability of axonal degeneration. Many attempts have been made or discussed in recent years to increase remyelination of affected axons in demyelinating diseases such as multiple sclerosis. These approaches range from pharmacological treatments that reduce inflammatory processes or block ion channels to the modulation of neuronal activity through electrical cortical stimulation. However, these treatments either affect the entire organism (pharmacological) or exert a very local effect (electrodes). Current results show that neuronal activity is a strong regulator of oligodendroglial development. To bridge the gap between global and very local treatments, non-invasive transcranial magnetic stimulation could be considered. Transcranial magnetic stimulation is externally applied to brain areas and experiments with repetitive transcranial magnetic stimulation show that the neuronal activity can be modulated depending on the stimulation parameters in both humans and animals. In this review, we discuss the possibilities of influencing ion channel distribution and increasing neuronal activity by transcranial magnetic stimulation as well as the effect of this modulation on oligodendroglial cells and their capacity to remyelinate previously demyelinated axons. Although the physiological mechanisms underlying the effects of transcranial magnetic stimulation clearly need further investigations, repetitive transcranial magnetic stimulation may be a promising approach for non-invasive neuronal modulation aiming at enhancing remyelination and thus reducing neurodegeneration.
Key Words: ion channel; multiple sclerosis; neuronal activity; oligodendrocyte; (re-)myelination; repetitive transcranial magnetic stimulation; transcranial magnetic stimulation
Introduction
Multiple sclerosis (MS) is a chronic disease that affects the central nervous system. In the course of the disease, inflammatory processes occur in the brain, spinal cord and optic nerves, leading to demyelinated plaques in the white and gray matter (Lassmann, 2018). Myelin is removed from the internodes of axons in the affected region, interfering with their conduction properties. As a response, altered expression and distribution of sodium channels along demyelinated axons has been observed and this may lead to restoration of conduction of the affected axons. Increased expression of sodium channels can provoke axonal degeneration via energy failure (Waxman, 2006), thus specific blocking of sodium channels could protect axons from degeneration (Waxman,2006). While these attempts are still under investigation,another direction of research for new therapies addresses the enhancement of remyelination to prevent neurodegeneration, and some promising treatments are currently being tested (Cole et al., 2017).
Myelination of axons by oligodendrocytes occurs in parallel with clustering of sodium channels at the Node of Ranvier, permitting saltatory conduction. In addition, clustering of sodium channels was detected on unmyelinated fibers prior to myelination (Freeman et al., 2015). The distribution and clustering of voltage-gated sodium channels regulates axonal impulse conduction, adding an additional mode of regulation of impulse conduction (Figure 1). Demyelination therefore contributes to altering conduction of axons in several diseases. Although the mechanisms of nodal assembly during remyelination are not understood yet, it is possible that clustering of channels precedes remyelination in order to restore conduction (Freeman et al., 2016).
A recently emerging concept is that myelination of axons is influenced by their electrical activity during development but as well during learning, giving an additional level of plasticity to the brain. Neurotransmitter release from vesicles along the axon could be responsible for transmitting information to myelinating oligodendroglial cells, promoting proliferation and differentiation of these cells (Fields, 2015).Thus, there seems to be a correlation between neuronal activity and the development of oligodendroglial cells and the extent of myelination.
Search Strategy and Selection Criteria
The articles used in this review were retrieved using an electronic search of the MEDLINE database for literature describing Transcranial magnetic stimulation, neuronal activity and Multiple sclerosis. The search was performed for all years until December 2019 using the following conditions: Transcranial magnetic stimulation (MeSH Terms) or TMS AND rTMS (MeSH Terms); Multiple sclerosis (MeSH Terms) or MS (MeSH Terms); ion channel (MeSH Terms), oligodendrocyte precursor cell (MeSH Terms) or myelin (MeSH Terms).The abstracts of the results were further checked for their relevance for the review. Only articles that are related to MS or give deep insights in the TMS-physiology were inserted.A lot of histological work from TMS-research could not be inserted because it would go beyond the scope of this review.
Core
In a recent study, an increase in the occurrence of synaptic vesicles was shown through ultrastructural analysis along axons exposed to toxin-induced removal of mature oligodendrocytes in the Cuprizone mouse model (Pfeiffer et al., 2019).Moreover, after remyelination and functional recovery, an increase in the number of nodes of Ranvier was detected, indicating structural alterations within the axonal organization as well as in the length of internodes. These observations indicate an intrinsic mechanism of axons to enhance signaling to the glial compartment, in particular the oligodendrocyte precursor cells (OPC) after removal of myelin.
The mechanism of signaling to oligodendroglial cells through synaptic vesicles has already been proposed in Zebrafish during myelin sheath initiation. It could be demonstrated that decreasing neuronal activity reduced the number of myelin sheaths, while stimulating neuronal activity increased this number, pointing towards activity-dependent myelination (Mensch et al., 2015). More recently, stimulating neuronal activity could be linked to increased myelin generation after demyelination in a mouse model. Moderate and repeated photo-stimulation of axons promoted the maturation of OPCs into mature oligodendrocytes. This increase in mature oligodendrocytes translated into remyelination and restoration of conduction (Ortiz et al., 2019).
The major challenge in protecting axons from degeneration is in finding the balance between blocking neuronal activity - to avoid accumulation of toxic levels of calcium - on the one hand, but not blocking neuronal activity too much- as this will interfere with restoration of conduction- and possibly also with communication to myelinating glial cells.To this end, it might even be beneficial to increase neuronal activity. While evidence is accumulating in animal models that enhancing neuronal activity during or after the induction of demyelination is beneficial for the process of remyelination and regeneration, it is of course more challenging to enhance neuronal activity in humans suffering from MS.Based on lesion location, different cognitive and motor tasks have been proposed to improve remyelination in patients depending on the location of lesions (Jensen and Yong, 2016).
To support remyelination in patients one could think about a pharmacological modulation of neuronal activity by blocking or enhancing ion channels by drugs, but this will influence all organs and systems related to neuronal activity and have multiple adverse events. The application (oral or injections) will be not limited to the affected lesions and therefore the benefits of the patient must outweigh the side effects.
After the removal of myelin sheaths in demyelinating diseases like MS, sodium and potassium channels are redistributed along the previous internodal region of the axon and/or their expression is increased in order to restore conduction (Judge and Bever, 2006; Faissner et al., 2019). Studies from demyelinating models reveal that the myelin sheath is required for maintenance of the normal localization of the potassium channels at the juxtaparanode/node, whereas the sodium channels are mainly localized at the node (Rasband et al., 1998; Baba et al., 1999). Therefore, one could imagine that future MS-therapies could aim to induce a redistribution of potassium channels in regions where they do not affect conduction. Calcium ion concentration has also been shown to rise in axons due to ion exchangers e.g., the sodium/calcium exchanger that are upregulated to decrease the sodium ion concentration (Waxman, 2006). In order to prevent axonal degeneration as a consequence of ion accumulation therapeutic agent blocking sodium channels have been tested, but thus far the outcome is still controversial (Faissner et al., 2019). A therapy blocking potassium channels is currently approved for treatment of MS patients and has been shown to improve walking abilities (Goodman and Stone,2013; Baird et al., 2018), but there is still the need to improve the efficacy, decrease the side effects of these treatments and investigate the mechanistic properties.
Another possibility to improve remyelination is by increasing the activity in the lesion areas that could be considered are induced electric fields along the neuronal membrane by transcranial magnetic stimulation (TMS) (Figure 1). Computational models of the electric field predict an activation of sodium channels at the node of Ranvier, where the diameter of the axons changes (Roth, 1994) or at axon terminals(Aberra et al., 2018). The thresholds for activation of these structures are within the range of the conventional magnetic stimulators.
In animal experiments, it has been shown that repetitive TMS (rTMS) can be applied in a regular or in a patterned rhythm. High-frequency bursts interleaved with brief periods of no stimulation applied continuously tend to lower cortical excitability as low-frequency stimulation (< 1 Hz)does. By contrast, if a burst of high-frequency stimulation is applied intermittently it tends to increase cortical activity, as regular high-frequency stimulation (> 5 Hz ) does (Huang et al., 2009). Depending on the stimulation parameter (frequency, intensity and pattern) rTMS can increase or decrease the neuronal firing rate (Funke and Benali, 2011; Tang et al., 2015) and these modulations outlast the stimulation period (Benali et al., 2011). Regarding ion channels, it has been shown in cultured neurons and acute rat brain slices that rTMS transiently opens voltage gated sodium channels,increases with a delay the intracellular calcium-concentrations (Banerjee et al., 2017), affects the potassium channels(Tan et al., 2013), and promotes the release of growth factors(Tang et al., 2015; Lenz et al., 2016). All these parameters are key regulators of oligodendrogenesis (Figure 2) and adaptive myelination (Cullen et al., 2019). Beyond these studies in animal models as well as inin vitrosystems, TMS is being discussed as a potential biomarker in the clinical assessment(Snow et al., 2019) and as a therapeutic method of MS (Gaede et al., 2018).
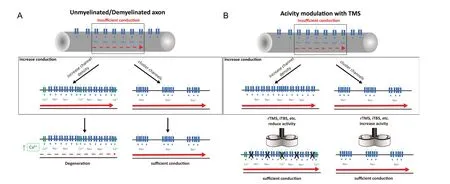
Figure 1 Neuromodulation of brain activity by TMS.
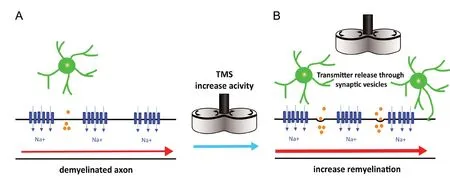
Figure 2 Induction of myelination by increasing the neuronal activity by TMS.
Although the mechanisms of these effects clearly need further investigation, it would be one possibility to alter neuronal activity and hopefully positively enhance regenerative processes in the affected regions. Indeed, the enhancement of neuronal activity through rTMS was shown to increase the number of newborn oligodendrocytes in adult mouse cortex by enhancing their survival (Cullen et al., 2019).Whether myelination can be increased through the release of neurotransmitter evoked by neuronal activity (Figure 2),as it has already been shown in a model of demyelination(Gautier et al., 2015) and is currently assumed to be one of the mechanisms regulating oligodendrocyte development(Butt et al., 2019) remains to be elucidated. Moreover, could TMS also be used to locally decrease neuronal activity in areas affected with demyelination in order to slow down or even prevent axonal degeneration? While further research is required to elucidate the mechanisms of this effect, these attempts may represent a possibility to non-invasively manipulate neuronal activity in order to either enhance remyelination and regeneration or decrease neurodegeneration.
Discussion
How can we locally modulate neuronal activity in affected brain areas and thus reduce neuronal loss? How can we promote remyelination by enhancing the input onto OPCs? Is there a transition between neuronal activity and the induction of myelination/remyelination? Will we interfere with the communication towards OPCs and the onset of remyelination by blocking the activity of sodium channels? Is the intrinsic neuronal activity enough to induce remyelination or will it be beneficial to stimulate neurons to further enhance it? In addition, while there is more and more evidence accumulating that neuronal activity can alter proliferation and differentiation of oligodendroglial cells, the mechanisms of this interaction are still under intensive investigation.
Clarifying these questions will hopefully lead to the development of new therapies that finally promote remyelination and protect axons from degeneration in neurodegenerative diseases like MS.
Modulation of neuronal activity in the brain, and its neural networks as well as in the glial compartment should be further explored in order to prevent neurodegenerative processes in neurological diseases. Patients with MS show adaptive mechanisms in order to compensate neural disorganization or damage (Nasios et al., 2018). A tool to modulate non-invasively neuronal activity and induce neuronal plasticity is rTMS. Recent attempts used rTMS as an engine to treat cognitive impairment in MS patients seem to be promising, but the underlying knowledge about the cellular mechanisms are weak.
Therefore, the development of new or the combination of different methods that allow us to gain a deeper understanding of cellular mechanisms of TMS by translating human stimulation protocols to an animal model and study its mechanisms of actionin vivois essential. Using extracellular electrophysiology, Li et al. (2017) translated a human stimulation protocol that has the potential as a biomarker in the MS diagnostics. They developed anin vivotool to investigate cortical neuronal dynamics under TMS with a minimal time-loss (1 ms) due to the TMS artifact. The reported data unveil the TMS dynamics and show a pattern of neuronal activity that is largely congruent with human TMS-data (Li et al., 2017).
We believe that the study of neuronal dynamics under TMS undoubtedly improves our understanding of remyelination, because TMS activates axonal fibers and neurons and promotes the number of newborn oligodendrocytes (Cullen et al., 2019). By gaining a deeper understanding of human TMS-protocols on the cellular level, the path is smoothed for the development of new stimulation protocols combined with pharmacological therapies to increase or stabilize the treatment. The effects of TMS directly on oligodendrocyte lineage cells are largely unknown and should be subject to further investigations. As there are currently no therapies available that would promote remyelination and thus regeneration, stimulation of axons to enhance interactions with oligodendroglial cells in combination with a pharmacological therapy is a potentially promising direction of future research.
The major challenge is now to develop methods that will enhance physiological processes already taking place between neurons and glial cells in order to improve regeneration of damaged fibers and thus preventing their degeneration.
Acknowledgments:The authors thank Dr. Julia Fitzgerald for helpful comments on the manuscript.
Author contributions:Both authors contributed to the preparation of the manuscript and approved the final manuscript.
Conflicts of interest:The authors declare no conflicts of interest.
Financial support:This work was supported by the DFG BE 6048/2-1 (to AB) and DFG PF574/5-1 (to FP).
Copyright license agreement:The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Tetsuya Akaishi, Tohoku University, Japan.
Additional file:Open peer review report 1.
- 中国神经再生研究(英文版)的其它文章
- The role of the TrkB-T1 receptor in the neurotrophin-4/5 antagonism of brain-derived neurotrophic factor on corticostriatal synaptic transmission
- The role of exercise in brain DNA damage
- Combined effect of repetitive transcranial magnetic stimulation and physical exercise on cortical plasticity
- Should mast cells be considered therapeutic targets in multiple sclerosis?
- Neuroprotection mediated by natural products and their chemical derivatives
- Reliable cell purification and determination of cell purity: crucial aspects of olfactory ensheathing cell transplantation for spinal cord repair