A new look on the electric spark sensitivity of nitramines
Svatopluk Zeman ,Ning Liu
a Institute of Energetic Materials,Faculty of Chemical Technology,University of Pardubice,CZ-432 10 Pardubice,Czech Republic
b Xi'an Modern Chemistry Research Institute,Xi'an,Shaanxi,710065,China
Keywords:Electric spark Free volume Initiation Nitramines Sensitivity
ABSTRACT Electric spark energy,E ES,for a 50%probability of initiation of the 14 nitramines was determined using a measuring instrument in which the electrodes are in direct contact with the sample.Indirectly proportional relationships were established between the logarithm of the E ES values and the length of the longest N-N bond in the nitramine molecule.This finding is compatible with the mechanism of the first step in the electro-reduction of the nitramine grouping.Directly proportional relationships were found to exist between the E ES values and the crystal lattice free volumes,ΔV(i.e.an increase in the ΔV values increases the nitramine's resistance to electric sparks)but there were several nitramines with the opposite course of this relationship.Also a semilogarithic relationships between the E ES values and a ratio of intrinsic volumes of molecule,V int,to the ΔV values were described as well as ambiguous linear dependence between these energies and a sum of the positive and negative extremes of the molecular surface electrostatic potentials,V S,Σ.Several nitramines studied(always the same ones)display roughly the same distribution in the coordinate systems of relationships with lengths of the longest N-N bonds,the V int/ΔV ratio and the sum V S,Σas the independent variables.It was found that,typically,such relationships start from a single identical point,in effect a point corresponding to data for a structural unit from which the studied nitramines can be hypothetically generated,and/or are converging on another point,often the one corresponding to the data for HNIW.All the findings point to a fundamental influence of the intermolecular forces on reactivity of nitramines exposed to electric sparks.©2020 China Ordnance Society.Production and hosting by Elsevier B.V.on behalf of KeAi Communications Co.This is an open access article under the CCBY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
1.Introduction
There are several different approaches and types of apparatus reported in the literature for measuring and solving electric spark sensitivity,EES(see Ref.[1]and references therein).The EESvalues corresponding to these various options are to be found in these papers[2-5].However,it seems that,in some European countries,the Czech Defense Standard(a part of STANAG[6])is gaining ground[1].This fact relates with the results of work undertaken at the University of Pardubice in the years 1995-2003,and subsequently with the later activity carried out by OZM Research[7].
At the University,two kinds of instrument were built for studying electric spark sensitivity[1-5].They differed in their electrode configuration and their circuit structure[3,4]but,more importantly,in the construction of their spark gaps.In the first machine,the discharge from the spark traversed an air gap(model RDAD[1,3,4])while in the more recent model of the second instrument(ESZ KTTV[3]in its latest version,X Spark 10[7])the electrode is in direct contact with the sample.In the first example(i.e.in the former system,RDAD)there is a considerable electrical energy loss in the air gap between the upper electrode and the sample surface and a large part of the discharge is converted into the thermal component expressed here[1,3,4].How ever,the values obtained with the RDAD instrument were comparable with those of the Los Alamos Natl.Lab.[8],and they also correlate relatively well with the molecular structure of polynitro compounds[2-4,9,10].How ever,these values are about an order of magnitude higher than the results from ESZ,and thus they cannot be taken as values representing a“pure”(real)electric spark sensitivity.It is therefore inappropriate to use them for the quantum-chemical reasoning concerning reactivity caused by the transfer of the electron into the nitro groupings(for example see Refs.[9,10]).These relationships and/or differences between the two types of instrument are well documented in these papers[3-5].
Thus,the outputs from the later instrument,i.e.ESZ KTTV(currently know n as X Spark 10)[1-5,7],become a focus of interest from the point of view of the electron entering the nitro grouping in polynitro compounds.Their molecular-structural dependences have been relatively widely described in papers[1-4]including the problem of the hot spot formation during electric spark initiation[11].Since the publication of these facts,new knowledge has emerged in the literature about the initiating reactivity of energetic materials(EMs),which could usefully be incorporated into the study of EM sensitivity to electric sparks.This concerns mainly the relationship of the crystal lattice free volume(CLFV)of nitramines and their initiation by impact[12],friction[13]and heat[14].But it also describes the use of the sum of the negative and positive extremes of molecular surface electrostatic potentials,VS,Σ,of these nitro compounds in the study of their thermal decomposition[15]and detonation[16].In the current study,these characteristics have been introduced into an analysis of the electric spark effect,together with new information concerning electric spark initiation of nitramines.The choice of the 14 nitramines for this purpose was largely dictated by their molecular structures being relatively simple and the mechanism of the primary homolysis of their N-NO2bonds being well understood[17-20].
2.Data sources
2.1.Nitramines used in this study
The chemical names and code designations of the nitramines studied,their electric spark sensitivity(energy of the electric spark,EES,in m J),the lengths of their longest N-N bonds and the sum of the positive(VS,max)and negative(VS,min)extremes of the molecular surface electrostatic potentials,VS,Σ,are summarized in Table 1.The justification for the derivation of this VS,Σ,sum,including its values for the nitramines under study,is presented in the studies[16,17].For a clearer illustration,the structural formulae of the nitramines used are presented in Scheme 1.
2.2.Results of calculations for crystal lattice free volume of nitramine explosives
All the compounds were optimized at computational level of B3LYP/6-311+g(d,p)by using the Gaussian 09 software[31].All optimized structures were characterized as true local energy minima on the potential energy surfaces without imaginary frequencies.The crystal volume[V(0.003)]was calculated by using the Multiwfn 3.3.9 software[32].The effective volume per molecule(Veff)was calculated as:Veff=M/ρ,where M is molecular mass,ρ is crystal density.The intrinsic gas phase molecular volume(Vint)was calculated by the 0.003 au surface according to Ref.[33],Vint=V(0.003).Therefore,the free space per molecule(ΔV)is given by:

Results of these calculations are summarized in Table 2,which also includes the minimum ionization potentials of several nitramines,taken from paper[34].
The way of using of the ΔV values themselves is in this paper principle essentially identical with the approach in work[33,39].
3.Results and discussion
As already mentioned earlier in this paper,measuring the electric spark sensitivity gives very different results depending on the instrument used-between the one where the discharge goes through an air gap(model RDAD[1,3,4,8]),and the one where the electrodes are in direct contact with the EM sample(model ESZ KTTV[1,3-5]).The results differ by an order of magnitude and,in addition,the correlations with the molecular structure of the EMs measured are totally different(this is well described in papers[3-5]).Taking into account the findings in paper[36]about the influence of an external electric field on the potential trigger bond in EM molecules,it can be stated that,during measurement with the RDAD instrument,the N-N bonds in the nitramines concerned are activated by such an electric field with their subsequent thermolysis by heat from the electric spark passing through air gap;that means that any transfer of electrons into nitramine groupings cannot be under consideration here.
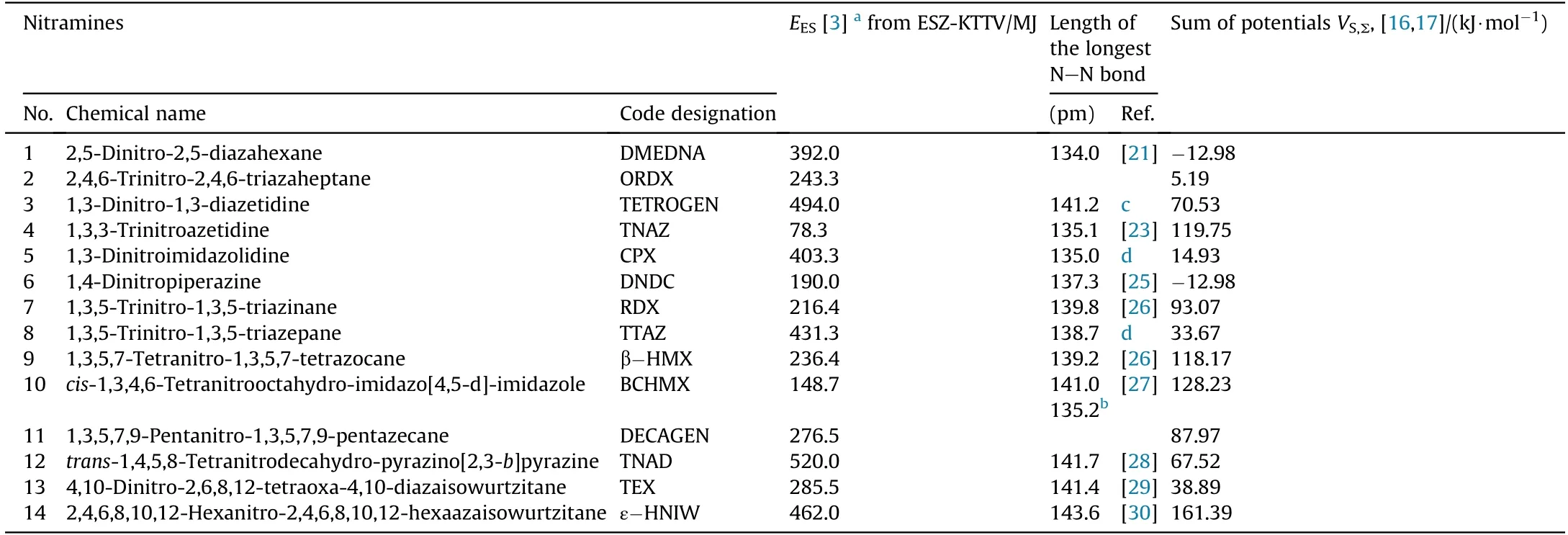
Table 1 Details of the nitramines studied show ing their electric spark sensitivity(energy of the electric spark,E ES,in MJ),lengths of their longest N-N bonds and the sum of positive(V S,m ax)and negative(V S,m in)extremes of the molecular surface electrostatic potentials,V S,Σ,[16,17].
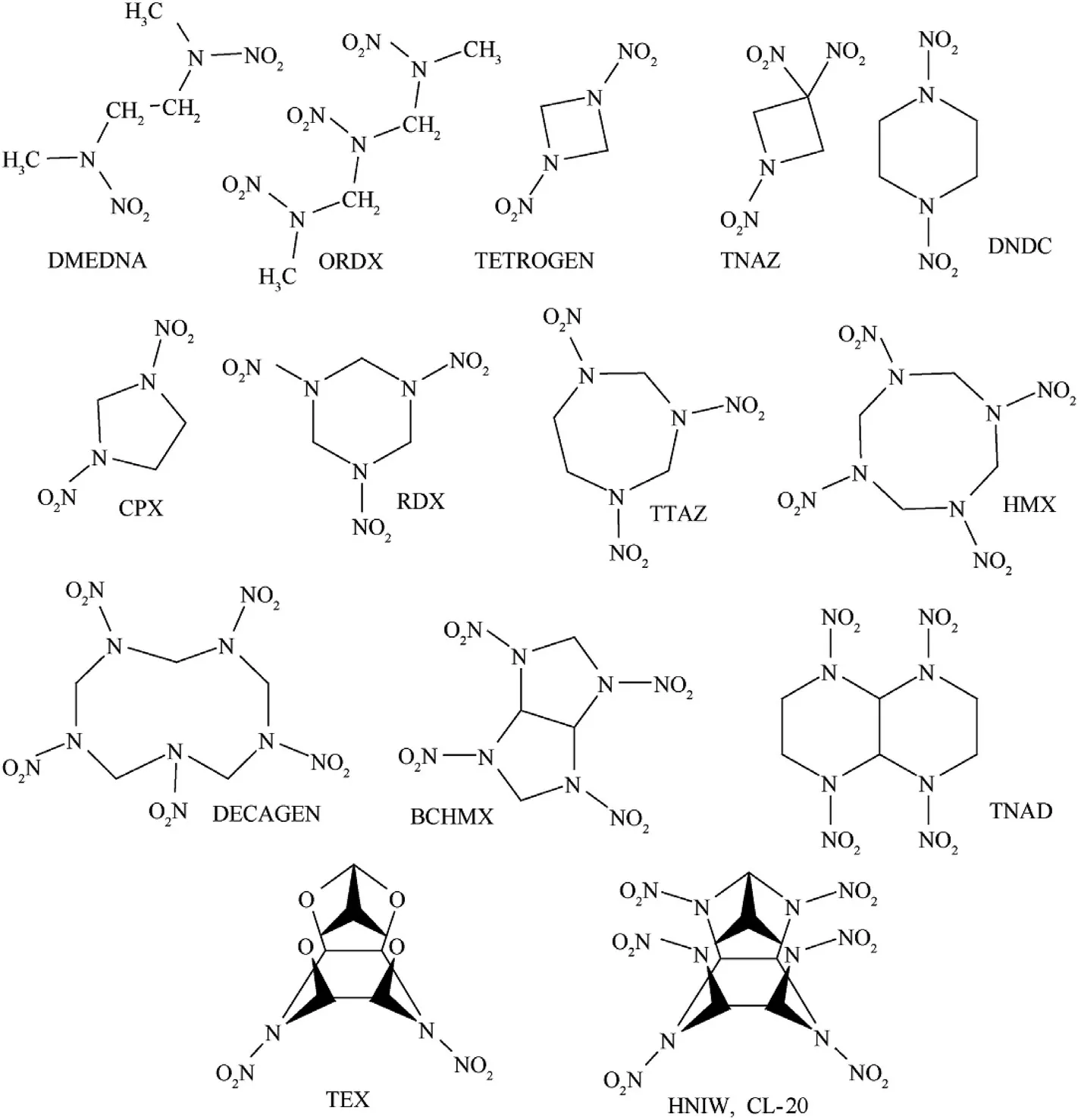
Scheme 1.Structural formulae of the nitramines studied.
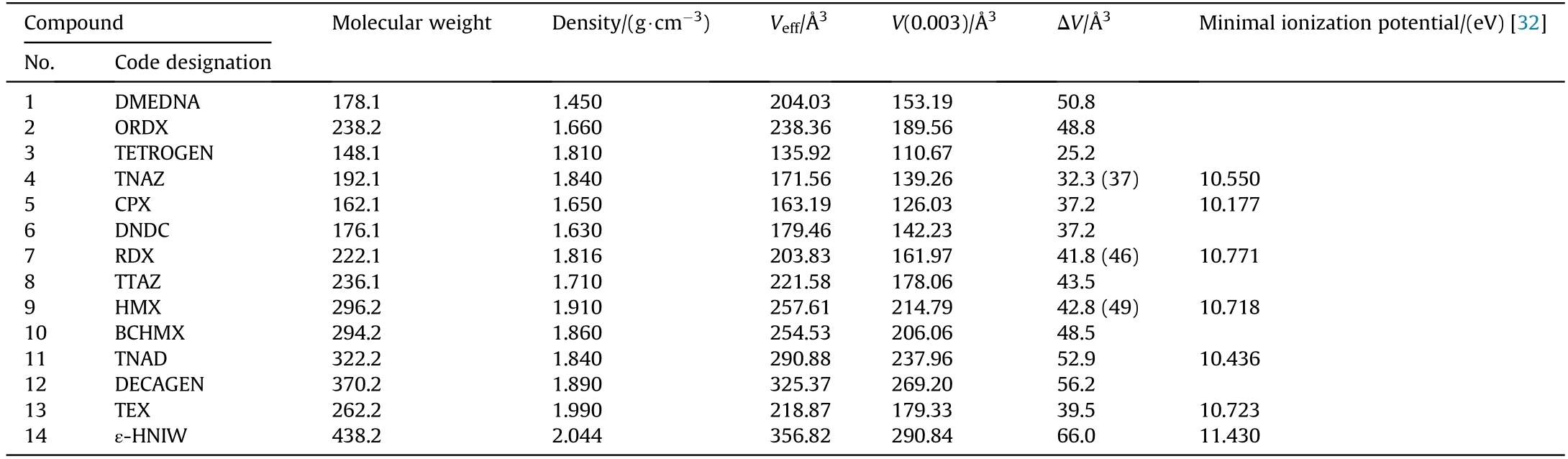
Table 2 Details,for each nitramine,of the molecular weight,M,effective volume,V eff,crystal volume,V(0.003),free space per molecule,ΔV,and minimum ionization potential(taken from Ref.[34]).
3.1.The electric spark initiation mechanism
It has been adequately shown that,in nitramine-containing molecules,the nitramine grouping is a trigger for initiation by electric sparks[3,36].Cleavage of the σ-bonds by electro-reduction begins by electron transfer into the nitramine group[37,38],in general forming an aza-radical and a nitrite anion.This transfer and the subsequent fission may be formulated as in Scheme 2:
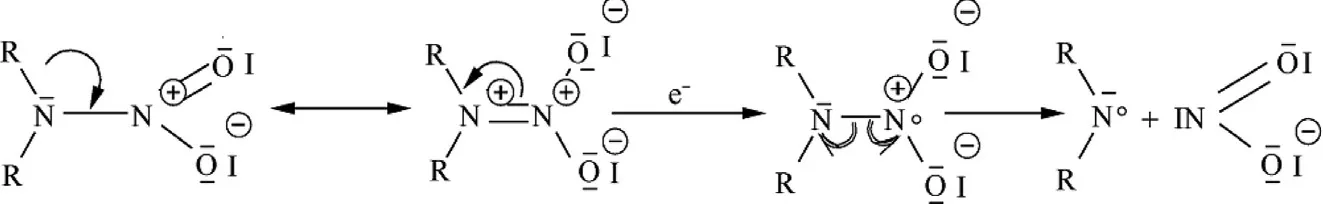
Scheme 2.Electron attack on the nitramine grouping producing an aza-radical and a nitrite anion(the first step in the nitramine electro-reduction).
In the“normal”electro-reduction in an aprotic milieu an additional electron is transferred,transforming the aza-radical into an amine.Under the conditions of the electric spark sensitivity testing(here using the ESZ KTTV model)the nitramine's primary fission products should be a reason for further decomposition,or even possibly sample explosion.
3.2.The role of the N-N bond lengths
It could be assumed that the length of the trigger bond would be proportional to the initiating reactivity of any given EM;for the group of nitramines here it was demonstrated that this is not the case[22].Therefore,it is better to speak about a trigger or the weakest bonds.Nevertheless,accepting this assumption and using data from Table 1,the chart in Fig.1 can be constructed.
With the exception of TTAZ,where the most reactive position is position 1 while its longest N-N bond is in position 3(this nitramine is as if dimerized and so that stabilized in its molecular crystal through mutual interaction nitramino groupings in position 3)[22],the positions of the longest N-N bonds in the nitramine molecules featured in Fig.1 correspond to the most reactive nitramine groupings,as specified by means of the15N NMR chemical shifts[1,3]or DFT calculations[3].Fig.1 shows that resistivity of nitramines to spark initiation increases with an increase in the lengths of their longest N-N bonds.This fact corresponds to Scheme 2,according to which deeper conjugation of the amine electron pair with the nitro group should increase the affinity of the nitro nitrogen atom to an electron and at the same time compress the length of the corresponding N-N bond,which supports an increase in this sensitivity.But problem is here in the case of BCHMX in whose molecule a biggest difference exists between the longest and shortest N-Nbond among of all the studied nitramines(see in Table 1and also[27]).After inserting data of its shortest bond into coordinates of Fig.1 a point was obtained,which relatively well correlates with straight line of“DNDC-β-HMX-ε-HNIW”;it might be analogy of the TTAZ molecule what needs further investigation.From what has been presented so far and also published in the literature[1-5],it follows that the reaction centers in electric spark initiation should be the same as in the case of initiation by mechanical and thermal stimuli[1-5],even if certain additional aspects might be in play,mainly in crowded molecules such as BCHMX.It is,how ever,necessary to point out that,when using this measurement method the resulting sensitivity to the electrical spark is inversely proportional to the dissociation energies of the respective N-N bonds[3],which is in agreement with the mechanism in Scheme 2.For results obtained by means of the RDAD instrument,the situation is completely the reverse,confirming the idea of thermal decomposition of the sample during measurements.
3.3.The role of minimum ionization energy
The authors of paper[34]have made a detailed analysis of the electric spark sensitivity of 12 nitramines,but using sensitivity data corresponding to measurement using the RDAD instrument(i.e.to thermal decomposition in an external electric field).They also used the calculated minimum ionization energies which we have inserted in Fig.2(the lower the ionization potential,the easier the ionization[34]).
Fig.2 shows a difference between nitramine molecules depending on whether they are cyclic or globular,but with TEX showing characteristics of both types,and DNCD tending to associate with globular molecules(its molecular skeleton forms a part of those in TEX and HNIW).This chart also shows that,for compounds with globular molecules,a greater resistance to the electron detachment from the nitramine molecule(to the higher Iminvalue)corresponds to a lesser sensitivity to electric spark,while for the cyclic nitramines(TNAD,TETROGEN,HMX and RDX)the reverse is true.
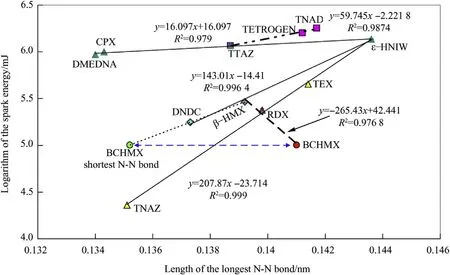
Fig.1.Semi-logarithmic relationship between electric spark sensitivity,expressed as spark energy,E ES,and lengths of the longest N-N bond in molecules of the nitramines studied;for BCHMX also its shortest N-N bond is used.

Fig.2.Approximate relationship of electric spark sensitivity,expressed as the energy of electric spark,and the minimum ionization energy of several of the nitramines studied.
3.4.Relationship with the crystal lattice free volume
Some authors have assumed that increasing the crystal lattice free volume(CLFV)corresponds to an increase in impact sensitivity[39].We have found that this is not quite so clear[12],and in the case of friction sensitivity it tends towards the reverse[13]and in the sensitivity to heat it is completely the reverse[14].Fig.3 shows how this relationship appears from the point of view of sensitivity to electric spark(in the sense of the electron transfer into the nitramine grouping).
Fig.3 presents relatively tight structural-molecular relationships,mostly showing increasing resistance to electric sparks with an increase in the ΔV values.A group consisting of“TETROGENTEX-RDX-(HMX)-BCHMX”shows the opposite trend.There is an interesting comparison here with the relationship between the activation energies of thermal decomposition and energies of electric spark for the nitramines studied,which is mostly directly proportional,with the exception of the group mentioned above,for which there is an opposite trend[40].
How ever,when the free volume is replaced by the ratio of the intrinsic molecular volume,Vint,to the ΔV value,along with the logarithm of the EESvalues,another view of the relationship in Fig.3 is created-shown in Fig.4.When looking at dependency on ΔV values,it is frequently the case that they start from a single point,i.e.from a point corresponding to data for the structural unit from which the compound can be hypothetically generated,and/or converge on another point,often the one corresponding to data for HNIW[14,15](see also Fig.5).In contrast to similar dependencies for thermal decomposition[14]and detonation[15],Fig.3 shows this starting point as a multiple one,which is represented by the data for nitramines DMEDNA,CPX,DNDC and TTAZ.This complexity may be related to the possible influence of an external electric field on the trigger N-N bond,especially on its length and electron density(see paper[36]);in this connection it instructive to compare the positions of data for DMEDNA,CPX,TTAZ,HNIW,TETROGEN and TNAD in Fig.1 with those in Fig.4,which would seem to support this presumption.
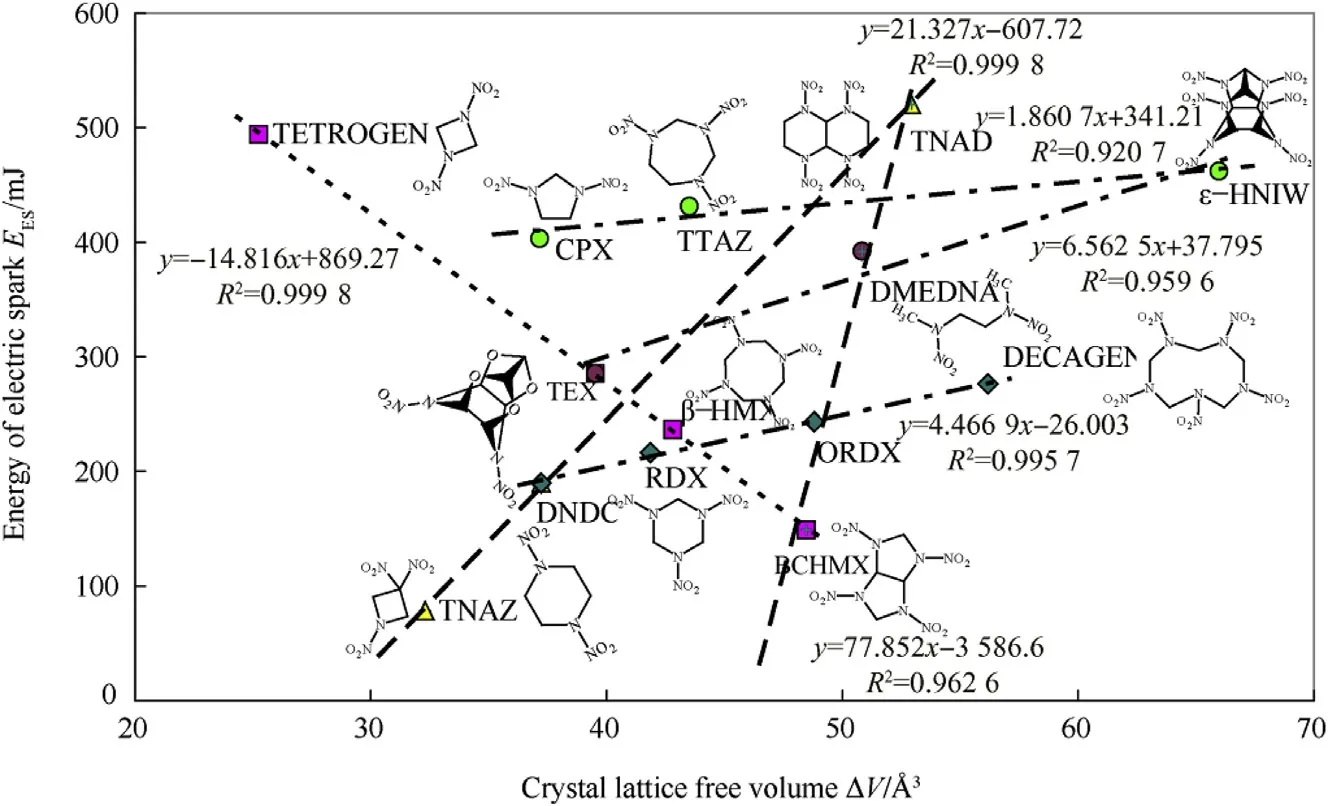
Fig.3.Relationship between electric spark sensitivity,expressed as energy of electric spark E ES,and crystal lattice free volume,ΔV.
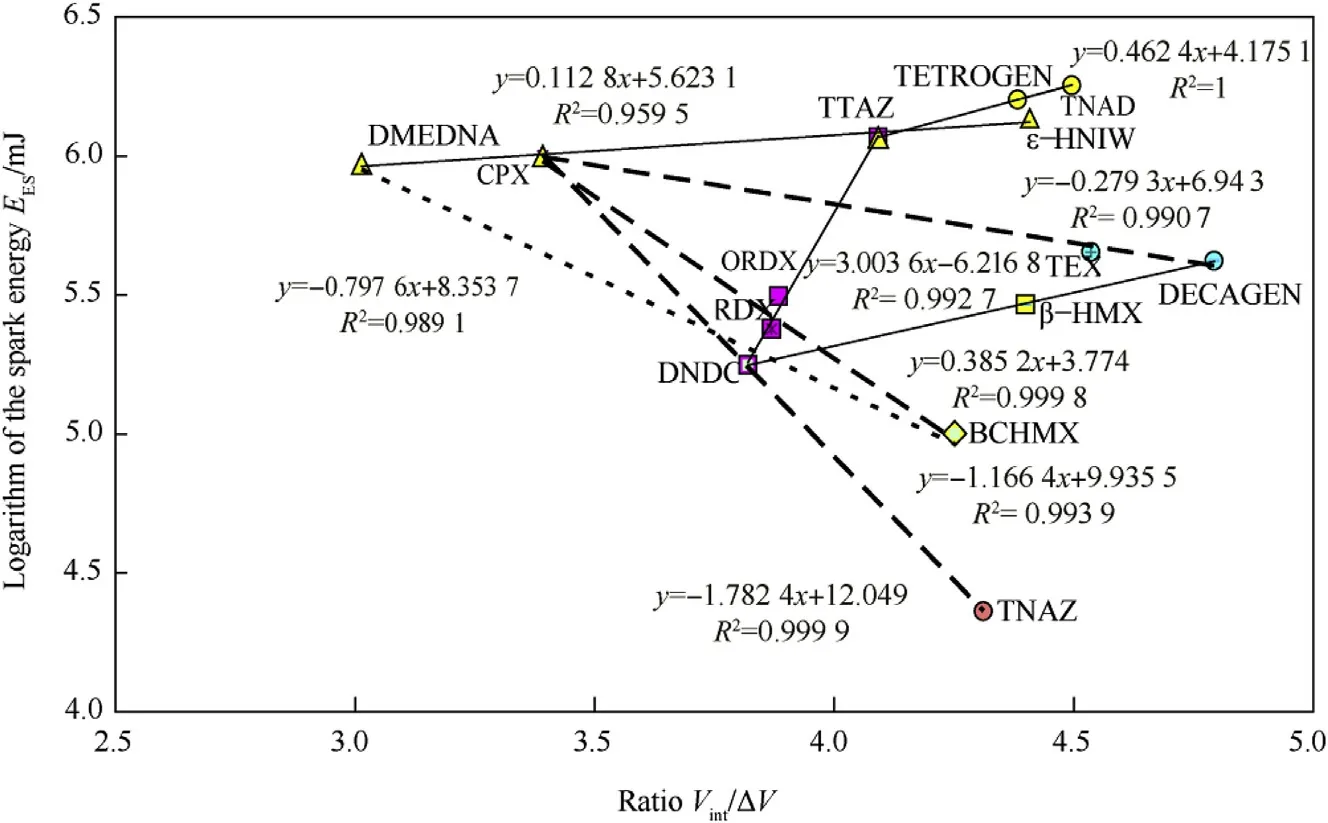
Fig.4.Semi-logarithmic relationship between the electric spark sensitivity and the ratio of the intrinsic molecular volume,V int,to the corresponding crystal lattice free volume,ΔV,of the nitramines studied.
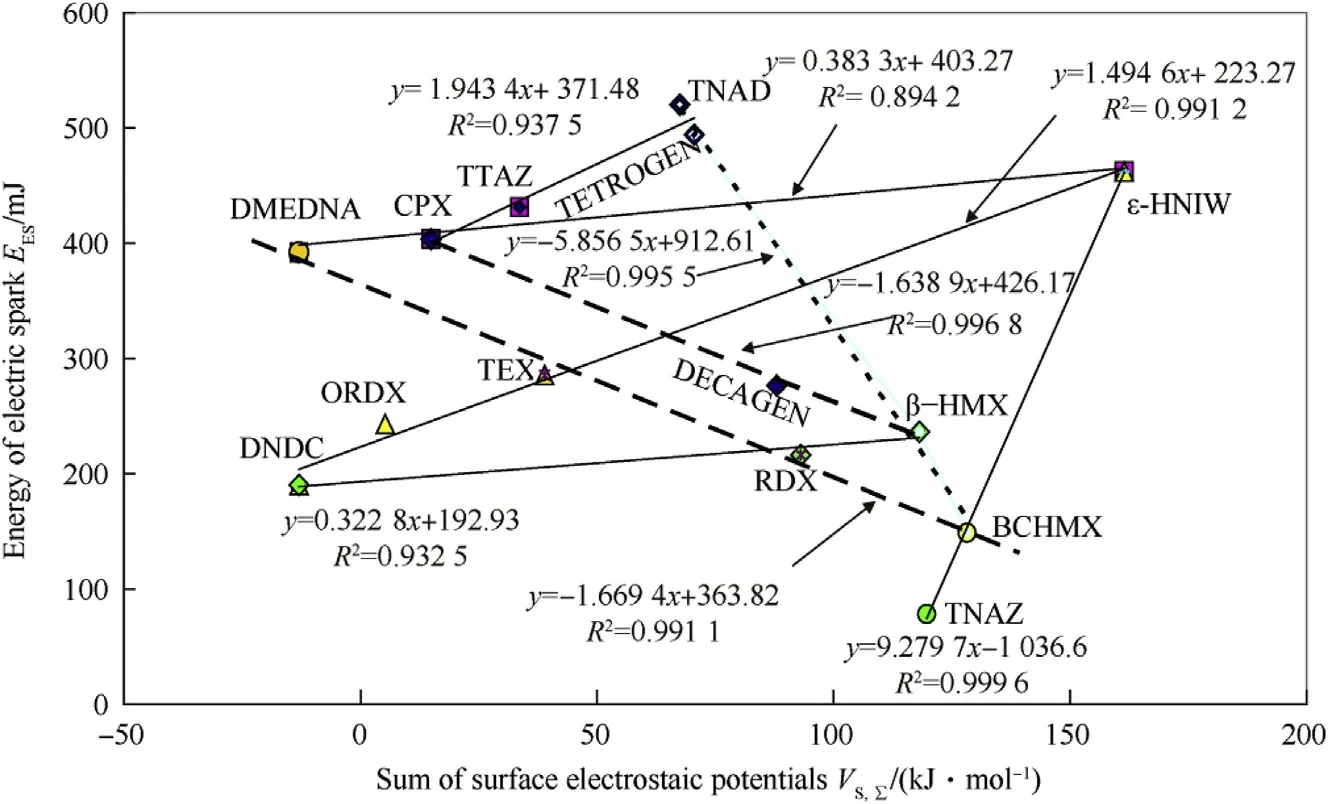
Fig.5.Relationship between electric spark sensitivity,expressed as energy of electric spark E ES,and the sum of the positive(V S,max)and negative(V S,min)extremes of the molecular surface electrostatic potentials,V S,Σ,.
3.5.Relationship with the sum of the surface electrostatic potentials,VS,Σ
Papers[16,17]discuss thermal decomposition[16]and detonation[15,17]of nitramines and provide the reasoning and calculations for the sum of the positive(VS,max)and negative(VS,min)extremes of the molecular surface electrostatic potentials.Comparison of electric spark sensitivity with this sum for the nitramines under study is presented in Fig.5.
In terms of the positioning in the coordinate system of data for DMEDNA,CPX,TTAZ,HNIW,TETROGEN and TNAD,this Figure is similar to Fig.4.In other words,it is also possible here to consider the influence of the external electric field[36]on the electron transfer into the nitramine grouping,as being a reason for the complication in the particular molecular-structural relationships.
4.Comments
The relationships presented here are formed from a relatively small amount of data.How ever,in the overwhelming majority of cases,they form narrow molecular structural row s,having in common a single structural characteristic or even a complete structural unit.This is best seen in the CPX-TTAZ-HNIW series in Fig.3,in which the first two molecular skeletons enter the globular skeleton of the latter.Here the change in conformation(intermolecular interactions)of molecules and the lengths of the longest bonds,which fundamentally affects their general reactivity,can be clearly seen.In Figs.1 and 4,this group of three nitramines is extended by the addition of DMEDNA because its molecular skeleton is also a part of the molecular skeletons of the others.In this way it is possible to analyse all the nitramine groups formed from the same base.
The straight lines with the positive slopes in Figs.4 and 5 could well correspond with the prevailing influence of intermolecular forces in the crystal lattice on the electron transfer into the nitramine grouping,while those with a negative slope would correspond with the prevailing influence of the close surroundings of the reaction center(around the most reactive nitramine group in the molecule,predominantly with a pyramidal configuration[41]).This is probably what causes the differentiation of the nitramines in Fig.2 into two groups which is similar to the differentiation based on the relationship of the EESvalues to the sum of surface electrostatic potentials in Fig.5.
In comparison with recent studies in the area of the relationships discussed here[12-15],the analogous relationships in the area of electric spark sensitivity seem to be somewhat more complicated,with the exception of those presented in Fig.1.As has already been mentioned,this might be explained by the influence of an external electric field on the nitramine grouping(see in Ref.[34]).How ever,it is possible to see here an analogy with the relationships with impact sensitivity,where limitations in the relationships based on molecular structure similarity have been found.This would imply a greater importance attributed to the inter-molecular interactions in the crystal lattice in the initiation of crystalline EMs-as also found when an electric spark is used.
Taking into account the findings in paper[34]about the influence of an external electric field on the potential trigger bond in the EMs molecules,it can be stated that,during measurement with the electrical discharge passing across the air gap over the nitramine under study,the N-N bonds in the molecule concerned are activated by such an electric field with their subsequent thermolysis by the heat of the discharge.This implies that any transfer of electrons into nitramine groupings cannot be involved here.
5.Conclusion
As previously published results[3]show,the initiation of nitramines by electron transfer into the nitramine grouping is characterized by an indirectly proportional relationship between the respective spark energy,EES,and the dissociation energy of the N-N bond.This fundamental finding corresponds very well with the indirectly proportional relationship between the logarithm of the energy of the electric spark and the length of the longest N-N bond in the nitramine molecules.Both facts also agree well with the supposed mechanism of the first step in electro-reduction of the nitramine grouping but only in the case,when the electrodes during measurement are in a direct contact with the measured sample.
As far as minimum ionization energy is concerned,it seems that an increase in this energy leads to an increase in resistance against electric spark for nitramines with globular molecules,whereas for the cyclic ones it should be the reverse.Several partial directly proportional relationships exist between the EESvalues and the crystal lattice free volumes,ΔV(i.e.an increase in the ΔV values causes an increased resistance of nitramines to an electric spark)but there exist several nitramines in which the reverse of this relationship is observed.
It is possible to explain this by the semi-logarithmic relationship between the EES,values and the ratio of intrinsic volumes of molecules,Vint,to the ΔV values,and also by the linear relationship between these EESvalues and the sum of the positive and negative extremes of the molecular surface electrostatic potentials,VS,Σ.It is often seen to be typical that these relationships start from a single point,i.e.from data for a structural unit from which the nitramines studied can be hypothetically generated,and/or converge on another point,often the one corresponding to the HNIW data.Several always the same nitramines studied display roughly the same distribution in the coordinate systems of relationships with lengths of the longest N-N bonds,the Vint/ΔV ratio and the sum VS,Σas the independent variables.From the facts outlined in this paper,it can be seen that beside the sensitivity to mechanical impulses and heat,the intermolecular forces have a fundamental influence also on the reactivity of nitramines,exposed to electric spark initiation.
Acknowledgement
The work described in this paper was funded partially by the Faculty of Chemical Technology at the University of Pardubice,and partially by the financial support for a six-month traineeship in 2016 for Dr.LIU Ning in the Institute of Energetic Materials at University of Pardubice from the State Administration of Foreign Experts Affairs,Peoples Republic of China.
- Defence Technology的其它文章
- An approach for predicting digital material consumption in electronic warfare
- Initial alignment of compass based on genetic algorithm-particle swarm optimization
- The non-isothermal gravimetric method for study the thermal decomposition kinetic of HNBB and HNS explosives
- A non-myopic scheduling method of radar sensors for maneuvering target tracking and radiation control
- Research on construction of operation architecture based on complex network
- Estimating the metal acceleration ability of high explosives

