Tumor necrosis factor-alpha (TNF-α) in spotted halibut Verasper variegatus at the embryonic and metamorphic stages*
LI Zan , LIU Xiumei , DU Xinxin ZHANG Kai CHEN Yan WANG Xubo WANG Zhigang YU Haiyang ZHANG Quanqi
1 School of Agriculture, Ludong University, Yantai 264025, China
2 College of Life Sciences, Yantai University, Yantai 264005, China
3 Key Laboratory of Marine Genetics and Breeding, Ministry of Education, College of Marine Life Science, Ocean University of China, Qingdao 266003, China
Abstract As an aquatic f ish, the spotted halibut Verasper variegatus is highly susceptible to bacterial and virus infections. Tumor necrosis factor-alpha (TNF-α) as a cytokine could control the inf lammatory responses. The functions of TNF-α in many species have been widely studied, particularly in mammals.However, little is known about the TNF-α functions in V. variegatus. We f irst cloned and sequenced the TNF-α gene in V. variegatus (VvTNF-α). The two conserved cysteine residues, transmembrane sequence,Thr-Leu motif, and TNF family signature, as well as the TA-rich motifs of its proteins related to inf lammatory responses had high similarity to those of the other teleost and mammalian TNF-α. The phylogenetic analysis showed that VvTNF-α was consistent with TNF-α genes of other vertebrates. The VvTNF-α transcripts were extensively distributed in the peripheral blood leukocytes (PBLs), spleen, and gill, indicating that the VvTNF-α had a role in immune function. Furthermore, treatment with pathogen-associated molecular patterns (PAMPs) could induce a rapid and signif icant increase of VvTNF-α in the PBLs, which reveals that VvTNF-α does participate in the host immune responses against bacterial and viral pathogens. We found that VvTNF-α had an interesting expression pattern during metamorphosis, showing that the f latf ish TNF-α may have some novel functions during specif ic developmental stages. In addition, the 3D structure prediction of VvTNF-α provided an indication of how it is likely to interact with other proteins. Therefore, VvTNF-α has multiple functions, and provides valuable information to explore novel functions of TNF-α.
Keyw ord: tumor necrosis factor-alpha (TNF-α); Verasper variegatus; metamorphosis; peripheral blood leukocytes (PBLs); 3D modeling; pathogen-associated molecular patterns (PAMPs)
1 INTRODUCTION
Tumor necrosis factor-alpha (TNF-α) belongs to the “TNF superfamily”, a large family of structurally related proteins. TNF, a pleiotropic cytokine, is critical to the control of an extensive array of immunological responses of monocytes, macrophages,T- and B-lymphocytes, and NK cells (Sherry and Cerami, 1988; Camussi et al., 1991; Nascimento et al., 2007; Li and Zhang, 2016). It can increase cell survival, induce cellular diff erentiation, apoptosis,and necrosis, and contribute to both physiological and pathological processes (Li and Zhang, 2016).Furthermore, TNF-α can serve as a signaling molecule and interact with its receptors TNFR-1 and TNFR-2 to transduce the exterior signals into the cells(Loetscher et al., 1991; Idriss and Naismith, 2000; Li and Zhang, 2016; Qi et al., 2016).
The functions of TNF-α are manifold (Beg and Baltimore, 1996; Blobel, 1997; Uysal et al., 1997;Idriss and Naismith, 2000; Ellis, 2001; Locksley et al., 2001). First, this protein can confer resistance to infections. TNF-α receptor knockout leads to less resistance against pathogens and weaken the inf lammatory response (Acton et al., 1996). Second,TNF-α has also been reported to be related to the embryonic development process (Wride and Sanders,1995). Third, TNF-α is involved in physiological sleep regulation in animal models (Idriss and Naismith, 2000). Fourth, TNF-α can regulate apoptosis either during normal embryonic development or during organelle destruction (Wride and Sanders, 1995; Idriss and Naismith, 2000). These studies show that TNF-α is a crucial signaling protein during an immune reaction, and it is also involved in developmental and other biological processes.
TNF-α was f irst cloned in humans by Pennica et al.(1984). It has been identif ied and characterized in teleosts, such asCynoglossussemilaevis(Li and Zhang, 2016),Paralichthysolivaceus(Hirono et al.,2000),Oncorhynchusmykiss(Zou et al., 2002; Hong et al., 2013),Scophthalmusmaximus(Ordás et al.,2007),Sparusaurata(García-Castillo et al., 2002;Saeij et al., 2003),Ctenopharyngodonidella(Zhang et al., 2012),Cyprinuscarpio(Forlenza et al., 2009),Oplegnathusfasciatus(Hwang et al., 2014),Dicentrarchuslabrax(Nascimento et al., 2007),Sinipercachuatsi(Xiao et al., 2007),Carassius auratus(Grayfer et al., 2008), andIctaluruspunctatus(Zou et al., 2003). TNF-α inC.idellawas found involved in the regulation of the NF-κB pathway(Zhang et al., 2012). InO.mykiss, two types of TNF-α were found, of which TNF-α3 could induce the expression of macrophage growth factor, antimicrobial peptides, and proinf lammatory cytokines (Hong et al.,2013). The expression ofI.punctatusTNF-α was found up-regulated in the peripheral blood leukocytes(PBLs) by phorbol-12-myristate-13-acetate (PMA)/calcium ionophore treatment (Zou et al., 2003). Given that TNF-α is generally regarded as an immunity gene, many researchers have studied the immunological function of TNF-α in f ishes. However,the role of TNF-α in cellular diff erentiation and apoptosis in f ishes has been rarely studied.
AsV.variegatushas a high market value in Asia, it is widely considered a promising candidate for aquaculture and f ishery enhancement. However, there are several problems in the current culture process ofV.variegatus. In the process of artif icial breeding, we have not found an eff ective way to make its broodf ish mature and ovulate spontaneously (Xu et al., 2012).In addition,V.variegatusis susceptible to virus and bacterial infections, which makes it endangered.Thus, the function studies of immunity genes could help us to f ind eff ective methods to improve the survival rate in its aquaculture. TNF-α has not been identif ied, and its function remains unclear inV.variegatus. To better understand the function of TNF-α gene inV.variegatus(VvTNF-α), we f irst cloned its full-length cDNA and predicted the secondary and 3D structure of VvTNF-α protein.Then we studied its expression pattern in diff erent adult tissues and during embryonic development,including metamorphosis. Additionally, we chose the PBLs ofV.variegatusto detect the immune challenge response stimulated by pathogen-associated molecular patterns (PAMPs). The above data are essential for us to understand the functions of VvTNF-α in immune response and its novel functions during the metamorphic stages.
2 MATERIAL AND METHOD
2.1 Ethics statement
Veraspervariegatussamples were obtained from a commercial hatchery. This research was conducted in accordance with the protocols of the Institutional Animal Care and Use Committee of the Ocean University of China (protocol number 11-06) and the China Government Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research,and Training (State Science and Technology Commission of the People’s Republic of China for No. 2, October 31, 1988. http://www.gov.cn/gongbao/content/2011/content_1860757.htm).
2.2 Sample collection
TheV.variegatusused in this study were raised in a commercial hatchery in Weihai, Shandong Province,China. The f ish were anesthetized (MS-222 at 30 μg/mL) and then killed by severing spinal cord. To research the expression pattern in diff erent tissues,heart, liver, spleen, kidney, brain, gill, muscle,intestine and PBLs were collected from three 1-yearold samples. Each of these samples was collected in triplicate. Samples were snap frozen in liquid nitrogen and then stored at -80°C until further use. Fertilized eggs were released by artif icial fertilization. The eggs and larvae were incubated and reared in plastic tanks(length: 94.6 cm; width: 65.1 cm; height: 49.7 cm;volume: 0.306 m³; f ish density: 2.3 kg/m³) withaerated seawater (dissolved oxygen: 4.6 mg/L;salinity: 30; pH value: 7.9; light intensity: 800 lx;temperature: 11±1°C) for several days before random sampling. The diff erent embryonic stages were observed under a stereomicroscope. Three pools of samples at 10 embryonic stages and 6 metamorphic stages were separately collected from mixed families with a nylon net (100 mesh). The embryonic stages includes unfertilized egg, 1-cell, 4-cell, morula,blastula, gastrula, neurula, somite, tail bud, and hatching stages. And the metamorphic stages includes 3 days post hatching, 10 days post hatching, premetamorphosis, early-metamorphosis, midmetamorphosis, late-metamorphosis. The above samples were immersed in 1.5 mL of RNAwait liquid(Solarbio, Shanghai, China) overnight at 4°C and then stored at -80°C until further use.

Table 1 Primers used in this study
2.3 RNA extraction and cDNA synthesis
As per the manufacturer’s instructions, we used TRIzol Reagent (Invitrogen, Carlsbad, CA, USA) to extract total RNA. The quality and quantity of the total RNA were evaluated by 1.5% agarose gel electrophoresis and spectrophotometry with the NanoPhotometer Pearl (Thermo Scientif ic, Carlsbad,CA, USA). We used RNase-free DNase I (TaKaRa,Dalian, China) to remove the DNA contamination of the extracted total RNA and then frozen at -80°C.
As per the manufacturer’s protocol of M-MLV kit(TaKaRa, Dalian, China), reverse transcription and cDNA synthesis were performed with 1 μg of the above RNA and using random hexamer primers.
2.4 Molecular cloning and sequence analysis of VvTNF-α
The genome information ofV.variegatushas not yet been available. To obtain the conserved region of VvTNF-α, we designed a pair of degenerate primers(TNF-Fw/Rv, Table 1) in accordance with the conserved sequences of TNF-α in other teleosts. The species and GenBank accession numbers utilized as reference to the construction of the degenerated primers were as follows:P.olivaceusTNF-α(BAA94969.1),P.maximaTNF-α (ACN41911.1),P.majorTNF-α (AAP76392.1) andD.labraxTNF-α(AAZ20770.1). The PCR reaction system in the above experiment used to obtain the open reading frame (ORF) sequence was as follows: 2.5 μL 10×buff er (15 mmol/L), 0.5 μL dNTP (10 mmol/L),0.5 μL Primer-Fw (10 mmol/L), 0.5 μL Primer-Rv(10 mmol/L), 1 μL cDNA template (20 ng/μL),0.25 μL Taq DNA polymerase (5 U/μL), and 19.75 μL sterile water. PCR amplif ication program was as follows: 95°C for 5 min; 30 cycles of 95°C for 30 s,55°C for 30 s, and 72°C for 1 min; and 72°C for 15 min. To obtain the TNF-α full-length cDNA from the spleen sample ofV.variegatus, we performed the 5′- and 3′-rapid amplif ication of cDNA ends (RACE)by using the SMART RACE cDNA Amplif ication Kit(Clontech, CA, USA) based on the manufacturer’s instruction. The gene-specif ic primers (GSPs) for the nested PCR assay were TNF-5′Rv1/Rv2 for the 5′-RACE and TNF-3′Fw1/Fw2 for the 3′-RACE(Table 1). The PCR was conducted in accordance with the SMART RACE amplif ication protocol. We separated the PCR products by using 1.5% agarose gel electrophoresis. The separated PCR products were purif ied by using the Zymoclean Gel DNA Recovery Kit (Zymo Research, CA, USA) and cloned into the pMD-18T vector (TaKaRa) according to the manufacturer's instruction, and then the above products were sequenced.
ORF of VvTNF-α was predicted by using the ORF Finder (http://www.ncbi.nlm.nih.gov/gorf/gorf.html).
2.5 Quantitative real-time PCR (qRT-PCR)
TNF-α-RT-FW/RV (Table 1, amplif ication effi ciency=0.99), the specif ic primer pair used in this experiment, was designed according to the characterized of TNF-α. Pre-experiments with qRTPCR amplif ication using specif ic primers and spleen cDNA template were conducted to conf irm whether the melt curve was unimodal. The unimodal melt curve corresponded to a single cDNA qRT-PCR products that could be used for subsequent experiments. As18SrRNAwas the most stable reference gene in diff erent tissues and at diff erent developmental stages, it was used as the reference gene in the relative expression analysis of VvTNF-α.
Three biological replicates of each sample were analyzed, with each sample ran in triplicate. The qRTPCR reaction system was as follows: 1 μL cDNA template (10 ng/μL), 0.4 μL Primer-Fw (10 mmol/L),0.4 μL Primer-Rv (10 mmol/L), 8.2 μL sterile water,and 10 μL SYBR Premix Ex Taq II (TaKaRa). qRTPCR was performed by using LightCycler 480 at 95°C for 5 min pre-incubation, followed by 45 cycles of 95°C for 15 s and 60°C for 45 s. We detected single amplif ication by analyzing the melting curve. Under the control of LightCycler 480 Software 1.5,f luorescent signal accumulation was recorded at the 60°C 45 s phase during each cycle. We used the 2-ΔΔCtcomparative Ct method to calculate the relative quantities of VvTNF-α expressed as fold variation over reference gene.
2.6 PAMPs-induced VvTNF-α expression in PBLs
We collected the blood from the caudal veins of theV.variegatussamples, and preared the PBLs using Percoll as previously reported (Zhou et al., 2014). The trypan blue exclusion method was used to determine the viability of PBLs and the average percent viability of PBLs is 93.6%. After full mixing,V.variegatus PBLs were cultured in a 24-well plate (Thermal Scientif ic, 9×106cells/well) overnight at 24°C. And then theV.variegatus PBLs were separately stimulated with lipopolysaccharide (LPS) (50 μg/mL),polyinosinic: polycytidylic acid (poly(I:C), Sigma)(50 μg/mL), and PBS (control). After 0, 0.5, 1, 2, 4, 6,12, and 24 h of PAMPs treatment, the samples were separately obtained. In order to study the response of VvTNF-α to LPS, poly(I:C), and PBS challenge, the qRT-PCR was constructed using18SrRNAas reference gene. The above experiment was repeated three times, so three biological replicates of each sample were analyzed, with each sample ran in triplicate.
2.7 Bioinformatics analysis and phylogenetic tree reconstruction
The homologous nucleotide and protein sequences of VvTNF-α were conf irmed by a BLAST search against the databases of NCBI and Ensembl. Multiple sequence alignments were performed with ClustalX 2.1 and DNAMAN 7.0. The phylogenetic tree was constructed through MrBayes 3.2.3.
The motifs of vertebrate TNF-α sequences were searched using the Pfam and CCD databases. Use Phyre2 (http://www.sbg.bio.ic.ac.uk/phyre2/html/page.cgi?id=index) and PDBsum Generate (http://www.ebi.ac.uk/thornton-srv/databases/pdbsum/Generate.html) to predict the secondary and 3D structure of protein VvTNF-α. The docking simulation of protein was performed with the ZDOCK server (http://zdock.umassmed.edu/).
2.8 Statistical analysis
Use a one-way ANOVA to analyze qRT-PCR data statistically followed by LSD test by SPSS 20.0 (IBM,New York, USA).P<0.05 is considered statistically signif icant. The data are showed as mean±SD (n=3).
3 RESULT AND DISCUSSION
3.1 VvTNF-α is highly conserved among vertebrates
TheV.variegatusgenome information has not yet been available. Thus, we designed a pair of degenerate primers (TNF-Fw/Rv, Table 1) in accordance with the conserved sequences of other vertebrates TNF-α and gained the conserved region of VvTNF-α. Then, we used the RACE method to obtain the unknown 5′- and 3′-regions of VvTNF-α cDNA. To obtain the VvTNF-α cDNA sequence, we assembled all the cloned sequences using SeqMan. The full-length cDNA of VvTNF-α is 1 273-bp long (GenBank:KY038170) and includes a 100-bp 5′-untranslated region (UTR), a 417-bp 3′-UTR with a poly(A) tail,and a 756-bp ORF. VvTNF-α has a complete polyadenylation signal in the 3′-UTR in comparison withP.olivaceusTNF-α. In addition, TA-rich motifs(TTATTTAT) in the 3′-UTR of mammalian TNF-α are also present in the VvTNF-α cDNA. These motifs inf luence the half-life (Caput et al., 1986) and translational effi ciency (Han et al., 1990) of TNF-α mRNA. The 3′-UTRs of human and mouse TNF, as well as those of human lymphotoxin, human CSF,human and rat f ibronectin, human and mouse IL-1,and a majority of sequenced human and mouse IFNs,have the consensus sequence TTATTTAT (Caput et al., 1986). Except for lymphotoxin mRNA, all of these mRNAs have no homology to the coding region of TNF mRNAs (Caput et al., 1986). One of the characteristic feature of inf lammatory mediator genes is the TTATTTAT instability motif (Sachs, 1993),whose presence shows the VvTNF-α transient expression. TA-rich motifs are common in sequences encoding inf lammatory response-related proteins(Caput et al., 1986).
The ORF of VvTNF-α encoded a polypeptide comprising 251 amino acid residues, whose molecular weight is 27.94 kDa and theoretical isoelectric point is 5.57. Like other vertebrates, there was a transmembrane (TM) domain (residues numbers 37-54) at the N-terminus of VvTNF-α predicted by Singer’s classif ication for membrane topology(Singer, 1990). The predicted VvTNF-α polypeptide also contains the TNF domain in the C-terminus of the protein. The sequence identities of VvTNF-α with the TNF-α ofS.maximus,O.fasciatus,P.olivaceus,andHomosapiensare 83%, 82%, 81%, and 54%,respectively. These results imply that the TNF-α protein may be highly conserved among vertebrates.
3.2 Phylogenetic and protein domains of VvTNF-α are consistent with those of other teleosts TNF-α
The alignment of VvTNF-α with the other vertebrates TNF-α shows that the VvTNF-α contains all the basic elements of the TNF gene family characteristic in the f ish and higher vertebrate. These elements include the TNF family signature [VL]-x-[LIVM]-x3-G-[LIVMF]-Y-[LIVMFY]2-x2-[QEKHL](with the exception of I instead of V or L at position 1), a TM domain, and two conserved cysteine residues, which are critical for the correct folding of mature TNF-α (Rink and Kirchner, 1996) (Fig.1a). As shown in Fig.1a, like other f ish TNF-α, the VvTNF-α also contains the Thr-Leu motif which could be recognized by the TACE metalloproteinase. In soluble mouse TNF-α, the Thr-Leu motif was conf irmed as the cleavage site for the release of mature protein(McGeehan et al., 1994). Moreover, most sequence identities are located at the C-terminus domain, which is consistent with the other teleosts TNF protein(Praveen et al., 2006) (Fig.1a).
A search for all the proteins against the Pfam database (http://pfam.xfam.org/) revealed that each of these proteins contains one TNF-α domain located at the C-terminus of the protein (Fig.1b). As shown in Fig.1b, the vertebrate TNF-α protein phylogenetic tree constructed with MrBayes shows that TNF-α is clustered into one group but separated from the outgroupH.sapiensTNF-β. This indicates that the production of the TNF-α and TNF-β is due to TNF ancestor gene duplication, which occurred before the species formation. The f ishes TNF-α are clustered,and the TNF-αofV.variegatus,P.maxima, andP.olivaceusare clustered into one group.
3.3 VvTNF-α is highly expressed in the PBLs, gill,spleen and at the hatching stage
The expression of TNF-α can be regulated(Hawiger, 2001); thus, we explored the expression patterns of VvTNF-α in diff erent tissues and at diff erent embryonic developmental stages with qRTPCR. The VvTNF-α expression level was normalized to the housekeeping genes,18SrRNA. As shown in Fig.2, VvTNF-α is widely expressed, which is consistent with the tissue distribution results forC.carpio,I.punctatus,S.aurata, andD.labrax(Zou et al., 2003; García-Castillo et al., 2004; Savan and Sakai, 2004; Nascimento et al., 2007). The presence of the VvTNF-α transcript in the brain is of interest,given that TNF-α is believed to be involved in the physiological sleep regulation in mammals (García-Castillo et al., 2002). However, our result shows that,VvTNF-α had the least amount of expression in the brain. The VvTNF-α transcripts are most expressed in the PBLs, followed by the spleen and gill. The other tissues, such as liver, heart, kidney, muscle, and intestine show moderate level of VvTNF-α transcripts.This result is similar to the phenomenon inC.auratus,S.aurata,I.punctatus, andO.mykiss (García-Castillo et al., 2002; Zou et al., 2003; Grayfer et al., 2008;Hong et al., 2013).
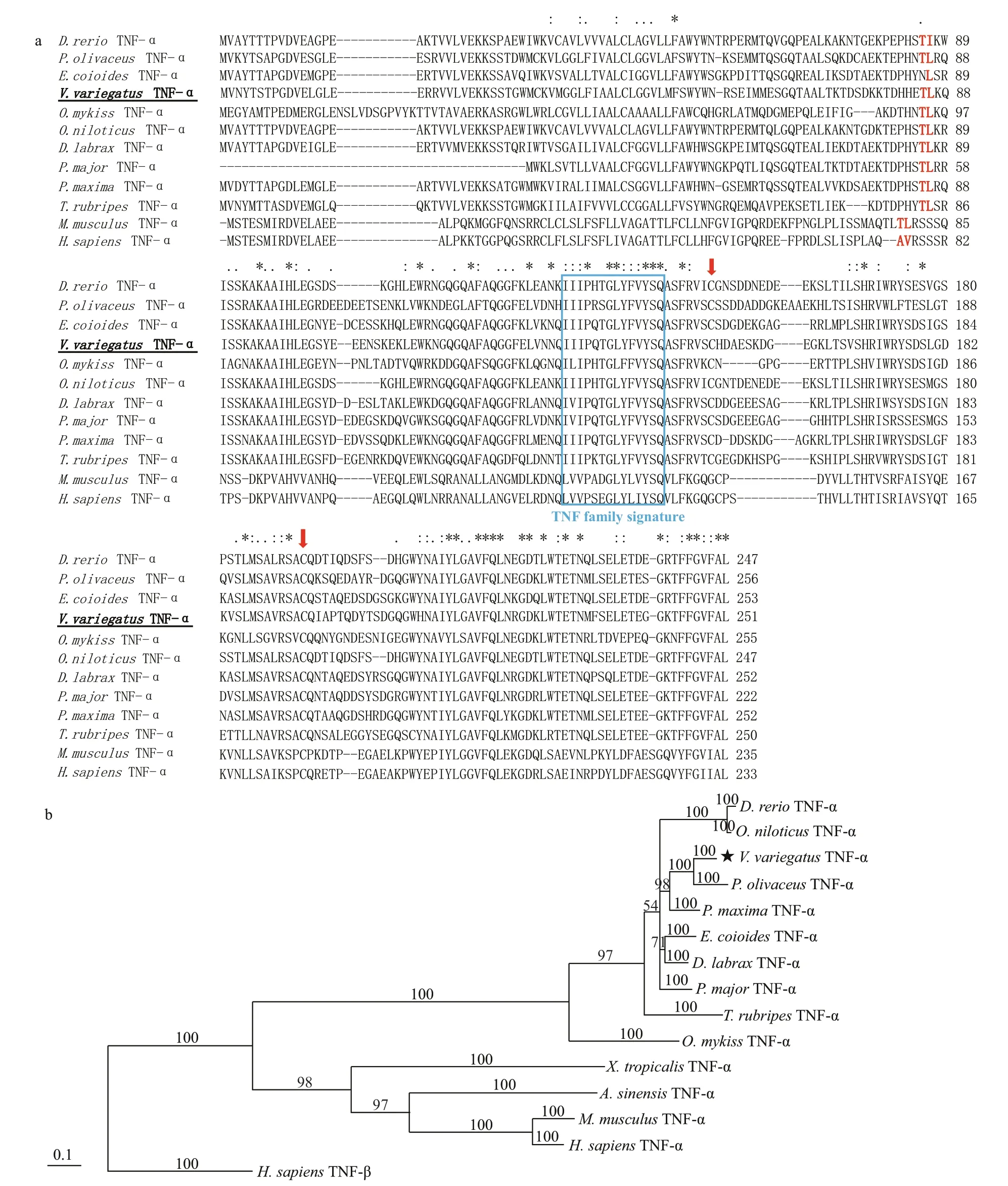
Fig.1 The amino acid sequence alignment and phylogenetic tree analysis of vertebrates TNF

Fig.2 Relative expression of VvTNF-α in diff erent adult tissues
In the early stages of embryonic development, the egg envelope protects the f ish embryo from the attacks by pathogens in the water environment. After hatching,the f ish embryos exposed to the environment are vulnerable to pathogen. At present, we know very little about the immune defense mechanism of f ish embryo in their hostile environment. Thus, we explored the temporal expression pattern of VvTNF-α during the embryonic stages. The result showed that VvTNF-α mRNA was detected until the somitestage (Fig.3). This phenomenon indicates that VvTNF-α is not a maternally expressed gene. During embryonic development,TNF-α may have major roles in the programmed cell death, in the remodeling of the extracellular matrix and in the cellular growth and diff erentiation (Wride and Sanders, 1995). Furthermore, the embryonic cells diff erentiate begin at the somite stage. The TNF-α function during the embryonic development may be supported by our results. Before the hatching stage, the VvTNF-α remained relatively low expression.However, VvTNF-α had a high expression level at the hatching stage (Fig.3). The exposed aquatic environment of most f ish embryos and hatchings is full of a large number of diff erent microorganisms,including potential pathogens; and during this period,the immune systems of those f ishes have not yet fully developed (Wang et al., 2016). Furthermore, during the hatching stages, embryos are more susceptible to attack by pathogens due to loss of egg envelope. Therefore,high expression of VvTNF-α in the hatching stage indicates that it may plays a role in resisting the pathogens around the environment. However, TNF-α has not yet been identif ied in the other f ish eggs. Thus,the role of TNF-α in early developmental stage of f ish remains largely unclear. In this study, we found that VvTNF-α is not a maternal gene, but its transcripts gradually increases and peaks at the hatching stage.The VvTNF-α expression pattern are similar to those of f ish-egg lectin (FEL), which plays a critical role in the immunity of zebra f ish and rock bream (Kim et al.,2011; Wang et al., 2016).
The presented results show that the expression of inf lammatory factor VvTNF-α is tissue and stage specif ic.

Fig.3 Relative expression of VvTNF-α at diff erent embryonic developmental stages
3.4 VvTNF-α shows the highest expression at the mid-metamorphosis stage
Metamorphosis of f ish in which larva transited to juvenile is a crucial development stage.Pleuronectiformes could change from a symmetrical larva to an asymmetrical juvenile during this stage,which is a dramatic morphological reorganization.Thus, Pleuronectiformes can be used as a representative of teleost to study metamorphosis.Also as f latf ish,V.variegatusandP.olivaceusare evolutionary relatives (Li et al., 2011, 2012; Xu et al.,2012), and both undergo the metamorphic stage.Metamorphosis arises from a series of processes including apoptosis, biochemical changes and so on(Power et al., 2001).T3 (Thyroid hormones 3), T4(Thyroid hormones 4), and TR (thyroid hormone receptor) have been reported to drive f latf ish metamorphosis (Inui and Miwa, 1985; De Jesus et al.,1991; Yamano et al., 1994; Campinho et al., 2007),which are related to apoptosis and cellular diff erentiation (Yamano and Miwa, 1998; Power et al., 2001; Liu and Chan, 2002; Marchand et al., 2004;Klaren et al., 2008).

Fig.4 Relative expression of VvTNF-α during metamorphosis

Fig.5 Expression analysis of VvTNF-α in PBLs treated with 50 μg/mL LPS
TNF-α gene plays an important role in cellular diff erentiation, proliferation, and apoptosis (Wride and Sanders, 1995; Li and Zhang, 2016) and interact with T3, T4, and TR (Aust et al., 1996; Kalashnikova et al., 2009; Kiss-Toth et al., 2013). For instance,thyroid modulation is realized as more intense induction of TNF-α apoptosis in the cells(Kalashnikova et al., 2009) and TNF-α in the thyroid can inf luence its functions (Aust et al., 1996; Kiss-Toth et al., 2013). InV.variegatusdevelopment,VvTNF-α expression began to decrease at 3 days post hatching (dph) and gradually increased again during metamorphic stage followed by another decline at the end of metamorphosis (Fig.4). In this period,VvTNF-α expression was predominantly detected in mid-metamorphosis, which is the most violent period of metamorphosis (Fig.4). The results show that VvTNF-α has a consistent expression pattern in comparison to T3, T4, and TR at the metamorphic stage ofS.maximusandP.olivaceus(Yamano and Miwa, 1998; Marchand et al., 2004). The relative expression level of TR gene and concentration of T3 and T4 inP.olivaceuswas gradually growing during pre-metamorphosis, early-metamorphosis, and midmetamorphosis. They reached a peak in midmetamorphosis and then began to decline (Yamano and Miwa, 1998). In summary, our results strongly imply that VvTNF-α may be involved in a series of regulated processes during f latf ish metamorphosis.These results contribute to the further understanding of the molecular mechanism of f latf ish metamorphosis.

Fig.6 Expression analysis of VvTNF-α in PBLs treated with 50 μg/mL poly(I:C)
3.5 Poly(I:C) and LPS induce signif icant and rapid increase of VvTNF-α
As an immunity gene, TNF-α participates in the immune defense against pathogens. Mammalian TNF-α plays a crucial role in the antibacterial immune response induced byMycobacteriumtuberculosis,ListeriamonocytogenesandMycobacteriumbovis(Pasparakis et al., 1996; Olleros et al., 2002; Saunders et al., 2005). Because TNF-α is released from macrophages, monocytes, neutrophils, NK cells, and T-cells (Covello et al., 2009), we investigated the VvTNF-α expression patterns in the PBLs exposed to PAMPs. In our study, theV.variegatusPBLs were co-incubated with LPS, poly(I:C) and PBS as a control, respectively. In the qRT-PCR results, we normalized the VvTNF-α expression level to the control. As shown in Figs.5 and 6, the VvTNF-α fold change levels were signif icantly and rapidly upregulated at 0.5 h and 1 h after LPS and poly(I:C)treatments, respectively. This result indicates that TNF-α expression may be more sensitive to the PAMPs of LPS than to those of poly(I:C).
TNF-α could be expressed in the human lymphocytesp, monocytes/macrophages and eripheral blood mononuclear cells stimulated by PAMPs (Jang et al., 2006). In addition, reports showed that bacteria,LPS, virus, poly(I:C) and parasites could induce the high expression of TNF-α in a variety of f ish (Chang et al., 2006; Roca et al., 2008; Covello et al., 2009;Teles et al., 2011; Hwang et al., 2014). In our study,the VvTNF-α was expressed in various tissues under normal physiological conditions; the VvTNF-α transcripts signif icantly and rapidly increased after LPS and poly(I:C) challenges. These results indicate VvTNF-α is involved in host immune responses against bacterial and viral pathogens. As shown in Figs.5 and 6, the VvTNF-α expression level decreased at 24 h after co-incubation with the PAMPs. TNF-α can induce cell necrosis, apoptosis and survival (Chu,2013). Therefore, we speculate that the reduction of TNF-α mRNA is to protect PBLs from damage.
3.6 Protein 3D modeling analysis and protein docking simulation of VvTNF-α predict its potential function
The secondary structure of TNF-α was predicted with Phyre2 tools (Fig.7a). Initially, the 3D structure of VvTNF-α was predicted by SWISS-MODEL Workspace. However, the quality index, such as QMEANscore4 was -0.69, indicating that this tool was unsuitable for the 3D structure prediction in this study. Thus, we used Phyre2 to construct the 3D model (Fig.7b) and selected d2tnfa as the template.The coverage of the protein could reach 59%, which is larger than that in SWISS-MODEL, and the conf idence level could reach 100.0%. The PDBsum Generate software was used to illustrate the Ramachandran f igure and predict the stability of the model (Fig.7c). There were only a few amino acid residues in the forbidden zone. Thus, the predicted 3D structure of the VvTNF-α protein has good quality.
The interaction of TNF-α and its receptor (TNFR)is the basis of the function of TNF-α (Locksley et al.,2001; Praveen et al., 2006; Xiao et al., 2007; Li and Zhang, 2016). Thus, the interaction between the predicted VvTNF-α and a potential receptor of the amyloid precursor protein (APP) (Fig.7d), which belongs to the human TNFR super family, was tested.As shown in Fig.7e, VvTNF-α can interact with APP through the predicted receptor binding sites.
The above results show that the 3D structure of VvTNF-α is similar to those of the other vertebrate TNF-α and imply that VvTNF-α may also perform its function by interacting with its conserved receptor binding domains.
4 CONCLUSION
We identif ied the mRNA sequence of VvTNF-α and characterized its signature. The two conserved cysteine residues, TM sequence, Thr-Leu motif, and TNF family signature of this gene, as well as the TArich motifs of its proteins related to inf lammatory responses were similar to the TNF-α of mammals and other reported f ish. The VvTNF-α expression patterns strongly imply that it has some universal immune functions and may perform certain novel actions during the metamorphic stages. The VvTNF-α could signif icantly and rapidly respond to PAMPs (LPS or poly(I:C)), which indicates that this gene actively participates in host immune responses against bacterial and viral pathogens. In addition, we used the 3D structure prediction and protein docking analysis to f ind that the VvTNF-α function relies on the interaction with its receptors.
5 DATA AVAILABILITY STATEMENT
The sequence of TNF-α gene in spotted halibut is available from GenBank under the accession number KY038170. Additional supporting data can acquire from the corresponding author upon reasonable request.
6 CONFLICT OF INTEREST STATEMENT
We declare no conf lict of interest.

Fig.7 The secondary structure, 3D structure, and protein docking simulation of the VvTNF-α
 Journal of Oceanology and Limnology2020年2期
Journal of Oceanology and Limnology2020年2期
- Journal of Oceanology and Limnology的其它文章
- Contribution of surface wave-induced vertical mixing to heat content in global upper ocean*
- Upper ocean response to typhoon Kujira (2015) in the South China Sea by multiple means of observation*
- Inf luence of simulating deep-sea environmental factors on cathodic performance of seawater battery*
- Adsorption characteristics of chitooligosaccharides onto activated charcoal in aqueous solutions*
- Eff ects of hypoxia on survival, behavior, and metabolism of Zhikong scallop Chlamys farreri Jones et Preston 1904*
- Distinct inf luence of trimethylamine N-oxide and high hydrostatic pressure on community structure and culturable deep-sea bacteria*
