Adsorption characteristics of chitooligosaccharides onto activated charcoal in aqueous solutions*
YU Yu , LI Kecheng
1 Marine Science and Engineering College, Qingdao Agricultural University, Qingdao 266109, China
2 Key Laboratory of Experimental Marine Biology, Center for Ocean Mega-Science, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China
3 Laboratory for Marine Drugs and Bioproducts of Qingdao National Laboratory for Marine Science and Technology, Qingdao 266237, China
Abstract To investigate the adsorption characteristics of chitooligosaccharides in solution onto activated charcoal, we studied the optimal adsorption conditions and the adsorption mechanisms of the chitooligosaccharides onto activated charcoal, which will greatly promote the application of activated charcoal in the chitooligosaccharides separation and purif ication. We studied the eff ects of particle size of activated charcoal, pH of solution, contact time, temperature, and initial concentration of chitooligosaccharides on the adsorption behavior in batch mode experiments. Activated charcoal in f ine particle size showed a high uptake of chitooligosaccharides. Weak alkaline solution (pH 8-9) was the most favorable to the adsorption.The adsorption equilibrium after 60 min was established, which followed a pseudo-second-order kinetic model. The adsorption capacity ( Q max) reached 0.195 g/g (chitooligosaccharides/activated charcoal) at 298 K. The adsorption was temperature-insensitive, and the adsorption isotherms could be best described by the Langmuir equation. Chitooligosaccharides adsorbed on activated charcoal could be desorbed in 50% ethanol solution in combination with an acidic condition (pH 2), reaching desorption effi ciency of 96.0%. These f indings are of great signif icance for the production and purif ication of amino oligosaccharides including chitooligosaccharides using activated charcoal.
Keyword: chitooligosaccharide; adsorption; activated charcoal; kinetics; isotherm
1 INTRODUCTION
Chitin is a natural polysaccharide, which is composed of β(1→4)-linked N-acetyl-D-glucosamine(GlcNAc) residues. Chitin is considered as the second most abundant polysaccharide in nature following plant cellulose, which exists generally as a structural component in the exoskeletons of insects, nematodes and arthropods (e.g., crabs, crawf ish, lobsters and shrimps). Chitosan is a heteropolymer of GlcNAc and D-glucosamine (GlcN) residues, which can be produced via deacetylation of chitin. Chitosan with less acetyl group has a greater solubility in dilute acid solutions but it is still not soluble in neutral conditions.The low solubility restricts the use of chitosan,particularly in medicine and food industry (Domard,2011; Laroche et al., 2018).
Chitooligosaccharides (COS) is the hydrolyzed product of chitin and chitosan, with higher solubility in neutral aqueous solutions. It exhibits numerous biological functions such as antifungal (Tikhonov et al., 2006), antibacterial (Mengíbar et al., 2011;Benhabiles et al., 2012), antitumor (Xu et al., 2012),antioxidant (Kim and Thomas, 2007; Ngo et al., 2008),anti-inf lammation (Zhao et al., 2018), immunityenhancing activity (Yang et al., 2019), and so on. In combination with its non-toxicity, biocompatibility,and biodegradability, chitooligosaccharides exhibits huge potential for applications in agriculture, food,and medicine (Muzzarelli, 2010; Liaqat and Eltem,2018). In particular, chitooligosaccharides have been authorized as a new food material by China Food &Drug Administration in 2014, which greatly promotes the application of chitooligosaccharides in food industry. At present, large amounts of chitooligosaccharides can be produced in many technological ways, including acid hydrolysis,oxidative degradation, and enzymatic methods (Einbu and Varum, 2008; Tishchenko et al., 2011). Moreover,some physical methods can be used to improve the degradation of chitosan, including microwave (Li et al., 2012c), ultrasonication (Tsaih et al., 2004), and γ-irradiation (Duy et al., 2011). All the reported techniques generally produce a complicated chitooligosaccharides mixture, which can be classif ied in terms of the degree of N-acetylation (DA), the degree of polymerization (DP), the molecular weight(MW), and the molecular weight distribution (PD, for polydispersity). Recently, separation and purif ication of chitooligosaccharides have attracted an increasing interest. Diff erences in biological activity of separated chitooligosaccharides in diff erent DPs or DAs have been reported, which greatly facilitated in-depth the understanding of the relationship between the structure and the function of chitooligosaccharides and its action mode (Le Dévédec et al., 2008; Li et al.,2016). Activated charcoal, as the most widely and eff ectively used adsorbent, is known of its porous structure with large surface area and low cost. It can bind with diverse organic molecules through a variety of physicochemical mechanisms and forces, such as Van der Waals forces, H-bond, dipole-dipole interactions, ion exchange, covalent bonding, cation bridging, and water bridging (Aksu and Yener, 2001).Activated charcoal has been widely used in the separation and purif ication of chitooligosaccharides.For example, activated charcoal chromatography has been used to separate single chitoligosaccharides on a lagre scale (Semeñuk et al., 2001; Kim and Rajapakse,2005), and has been applied for the isolation of chitooligosaccharides from an acidic solution (Xiong et al., 2009). In addition, activated charcoal could also be used to extract chitooligosaccharides fractions in aqueous solution for its desalination after ionexchange chromatographic separation (Li et al.,2012a & b, 2013b). It is worth noting that the chitooligosaccharides sample loss is always severe in these applications due to lack of theoretical guidance.Actually, all these applications are based mainly on the adsorption interaction between activated charcoal and chitooligosaccharides. However, to the best of our knowledge, no data were available on the optimal adsorption conditions and the adsorption mechanisms of chitooligosaccharides onto activated charcoal,which greatly restricted the application of activated charcoal in the chitooligosaccharides processing.
In this study, the adsorption characteristics of chitooligosaccharides in aqueous solutions onto activated charcoal were studied systematically as a function of activated charcoal particle size, solution pH, shaking time, temperature, and initial chitooligosaccharides concentration. The adsorption data could be f itted to both kinetics and isotherms model in order to better understand the adsorption mechanisms. Meanwhile, the adsorption of some monosaccharides in aqueous solutions onto activated charcoal was also determined, and the desorption process was investigated.
2 MATERIAL AND METHOD
2.1 Material
The chitooligosaccharides sample (molecular weight 1 420 Da, degree of deacetylation 93.7%) was obtained from Shandong Weikang Bio-Tech Co.,Ltd., China. N-acetyl glucosamine (≥98%) and glucosamine hydrochloride (≥99%) were purchased from Aladdin Industrial Co. (Shanghai, China). Darco G-60 activated charcoal (20-40 and 100 mesh) was purchased from Sigma Chemicals Co. (USA). Sulfuric acid and phenol reagent used for the phenol-sulfuric acid method were guaranteed reagent. Glucose and all other chemicals and reagents were of analytical grade.
2.2 Determination of point of zero charge (pH pzc)for activated charcoal
Point of zero charge of activated charcoal(100 mesh) was determined according to the method of Chingombe et al. (2005). An amount of 25-mL 0.1 mol/L NaCl standard solution was added into a 150-mL conical f lask, and its pH value was adjusted to 2-11 respectively using 0.1 mol/L HCl or 0.1 mol/L NaOH. An amount of 0.25 g activated charcoal(100 mesh) was added into the solution and then conical f lask was shaken at 150 r/min at 298 K for 48 h.The resultant pH was recorded using a Leici PHS-3C pH meter (Shanghai, China). A f inal pH against the original pH was plotted to obtain the pH titration curves. The point of zero charge was the solution pH without any change after adding activated charcoal.
2.3 Adsorption experiments
Batch mode adsorption experiments of chitooligosaccharides onto activated charcoal were performed to investigate the eff ects of some important factors on the adsorption process, including particle size of activated charcoal, solution pH, contact time,temperature, and the initial concentration of chitooligosaccharides. The batch experiment steps were as following: 15 mL of chitooligosaccharides solution of diff erent concentrations and 250 mg of activated charcoal were mixed in a 150-mL sample f lask, and then mounted on a water bath shaker at 150 r/min to keep the adsorbent in suspension. The adsorption time for other studies except for the contact time experiment was set to 12 h, which was supposed to be enough to reach adsorption equilibrium.
At the end of the adsorption, the solution was f iltrated and the concentration of chitooligosaccharides was determined by the phenol-sulfuric acid method at 490 nm (Dubois et al., 1956). Each sample was analyzed in three repetitions and each experiment was performed in duplicate. The amount of adsorbed chitooligosaccharides (Qt, g/g) was calculated as following:

whereC0andCtare the initial and f inal concentrations of chitooligosaccharides (g/L), respectively,Vis the volume of the solution (L), andmis the amount of the activated charcoal used (g).
Two activated charcoals with diff erent particle sizes (20-40 mesh and 100 mesh) were used for the adsorption experiments to reveal the eff ect of activated charcoal particle size on the chitooligosaccharides adsorption. A chitooligosaccharides solution of 4 g/L was used and the pH values of the solution was adjusted to 7 using dilute NaOH solutions.
The pH eff ect on chitooligosaccharides adsorption onto activated charcoal was investigated over the pH 2-14 range with an increment of pH 1. The pH of each chitooligosaccharides solution was adjusted to the required pH value by adding dilute HCl or NaOH.
The eff ect of diff erent contact times on the adsorption of chitooligosaccharides was determined at diff erent time points (5, 20, 30, 40, 60, 90, 120,180, 240, and 300 min, respectively).
The eff ect of various initial concentrations of chitooligosaccharides on its adsorption was studied in parallelled experiments at 298, 308, and 318 K,respectively. The initial chitooligosaccharides concentration was 0.5, 1.0, 2.0, 3.0, and 4.0 g/L,respectively. The equilibrium isotherm data were collected at the same time.
The adsorption capacities of monosaccharides on activated charcoal were investigated, including N-acetyl glucosamine (GlcNAc), glucose (Glc), and glucosamine (GlcN). Similarly, 15 mL of 4 g/L GlcNAc, Glc, and GlcN were added into 150-mL conical f lask. The pH values of the solutions were adjusted to 7 using dilute HCl or NaOH solutions.250 mg of activated charcoal (100 mesh) were added and then the conical f lask was shaken at a 150-r/min at 298 K for 12 h to reach adsorption equilibrium.Then the solution was f iltered and the concentrations of these monosaccharides were determined using the phenol-sulfuric acid method. The amount of monosaccharide adsorbed on activated charcoal was also calculated using Eq.1.
2.4 Desorption experiments
Filter residue of activated charcoal was collected after equilibrium adsorption experiments. Then the activated charcoal was mixed with 15 mL of ethanol solutions of varied concentrations, and shaken at 150 r/min at 298 K for 12 h. pH values of the ethanol solutions were adjusted by dilute HCl or NaOH solutions. Desorption effi ciency of chitooligosaccharides was calculated as following:
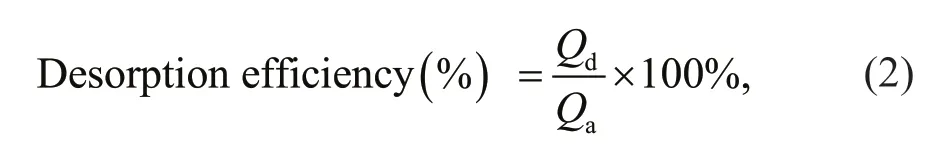
whereQaandQdare the amounts of adsorbed chitooligosaccharides by the activated charcoal and desorbed chitooligosaccharides after ethanol elution(g/g), respectively.
2.5 Statistical analyses
Statistical evaluation was carried out using SPSS 22.0 package. Data are presented as mean±SD and statistical comparisons between groups were performed usingt-test.P<0.05 was considered to be statistically signif icant.
3 RESULT AND DISCUSSION
3.1 Eff ect of particle size of activated charcoal on chitooligosaccharides adsorption
As is shown in Fig.1, two activated charcoals with diff erent particle sizes (20-40 mesh and 100 mesh)were used for the adsorption experiments of chitooligosaccharides. The activated charcoal with smaller particle size showed a higher chitooligosaccharides uptake, with an increase inQtby 20.1%. Thus, the f ine activated charcoal (100 mesh)was used for the following adsorption experiments.
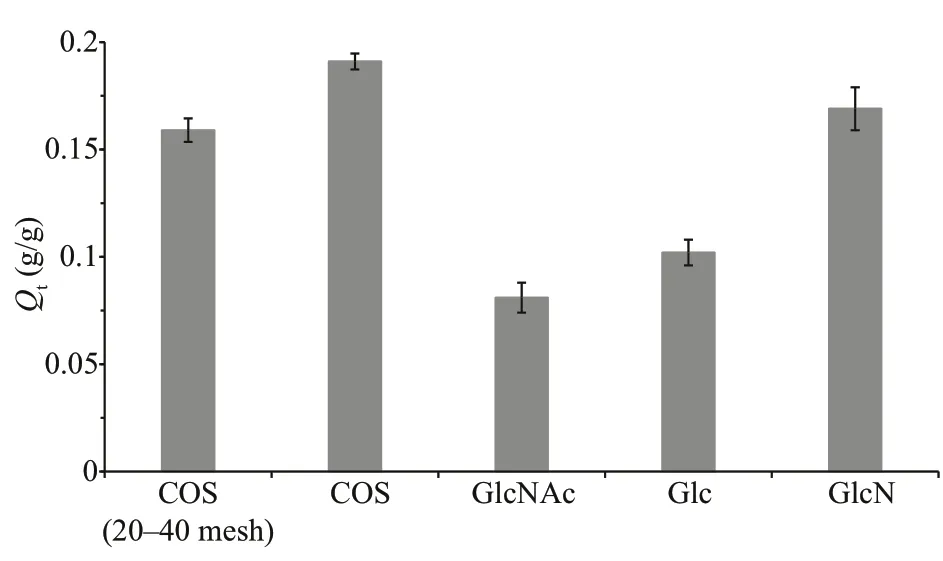
Fig.1 Adsorption of chitooligosaccharides and three monosaccharides by activated charcoal with diff erent particle sizes
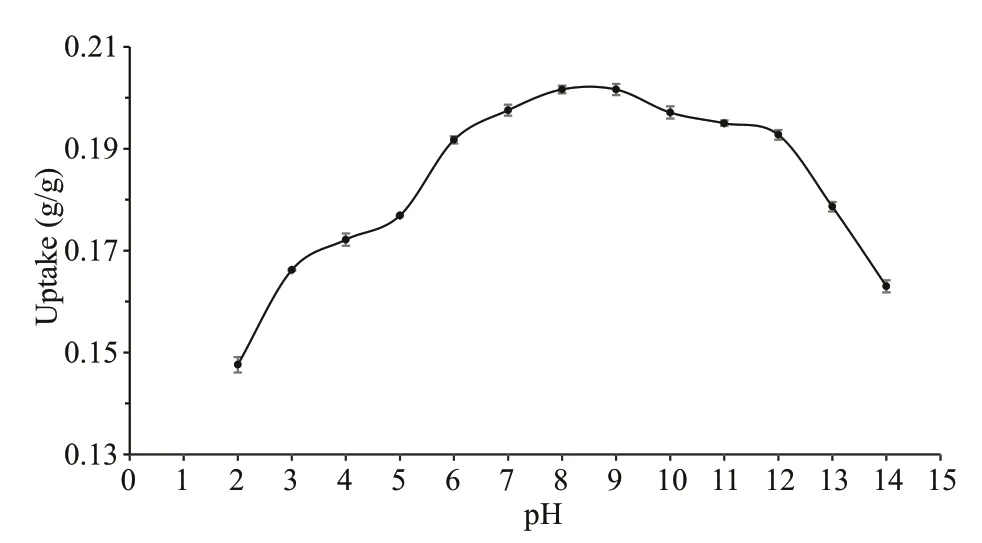
Fig.2 Eff ect of the initial pH on the uptake of chitooligosaccharides by activated charcoal
3.2 Adsorption of monosaccharides onto activated charcoal
To understand the adsorption behavior of chitooligosaccharides, several monosaccharides,including GlcNAc, Glc, and GlcN, were used in the adsorption experiments for comparison (Fig.1). All the three monosaccharides could be adsorbed by activated charcoal. However, the amounts of adsorbed monosaccharides were diff erent and all were less than that of chitooligosaccharides adsorbed by activated charcoal as shown in the order of chitooligosaccharides> GlcN > Glc > GlcNAc. The result indicates that long-chain oligosaccharides can be more easily adsorbed by activated charcoal than monosaccharides did. In addition, the monosaccharides composition of oligosaccharides seemed to play an important role in the adsorption process. In this study, the chitooligosaccharides used for the adsorption experiments have a relatively high degree of deacetylation (93.7%), and are mainly composed of GlcN but GlcNAc. In combination with the adsorption results of monosaccharides, GlcN seems to play a pivotal role in the chitooligosaccharides adsorption process.
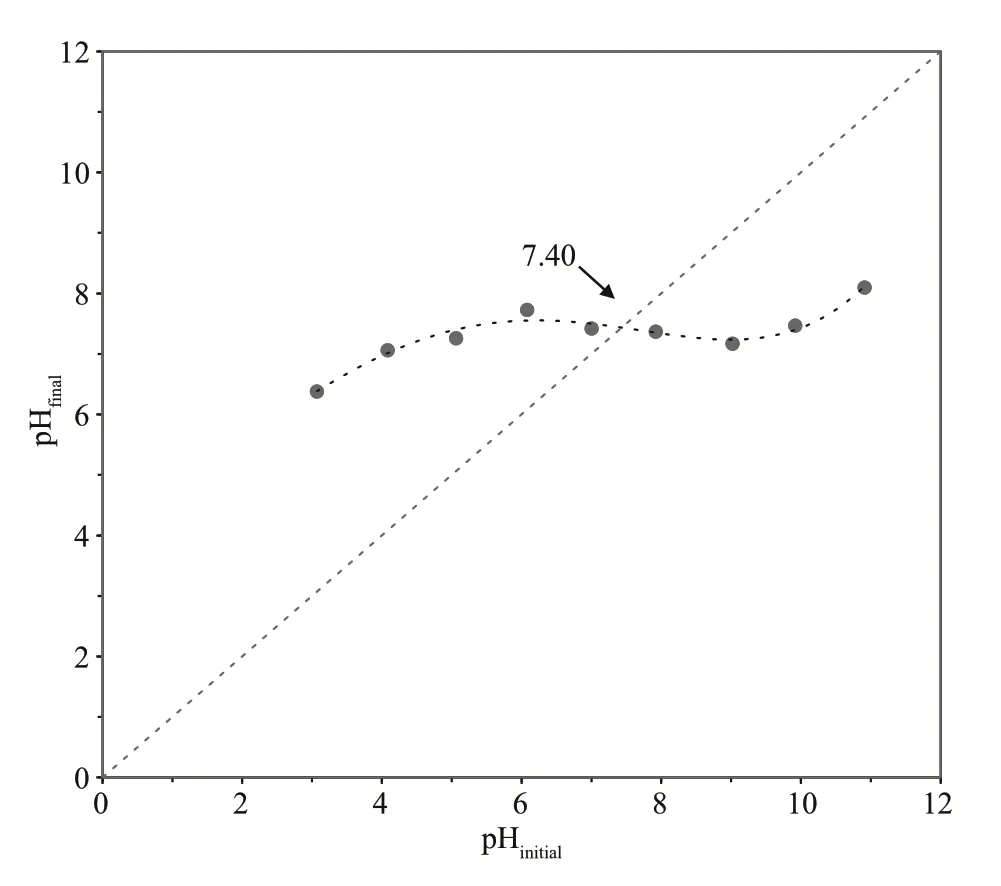
Fig.3 pH titration curve of activated charcoal for determining its point of zero charge
3.3 Eff ect of solution pH on the adsorption process
It has been reported that the adsorption behavior of activated charcoal in liquid is closely related to pH condition (Canilha et al., 2004). In this case, the eff ect of pH on the adsorption was studied by varying the solution pH from 2 to 14, in an initial chitooligosaccharides concentration of 4.0 g/L. As illustrated in Fig.2, the activated charcoal showed a higher chitooligosaccharides uptake at pH ranging from 6 to 12 and presented decreasing adsorption in more acidic and basic solutions. The maximum adsorption was observed at pH 8-9, and slightly shifted to the basic condition. However, neutral or acidic solutions were always used in previous separation and purif ication of chitooligosaccharides using activated charcoal (Xiong et al., 2009; Li et al.,2012a, 2013a & b). Improper pH condition seems be a critical reason for the sample loss in these applications.Hydrogen ions and OH-ions could aff ect the adsorption process through dissociation of the functional groups on the adsorbent and adsorbate (Ma et al., 2010). As shown in Fig.3, the point of zero charge (pHpzc) of the charcoal adsorbent used in this case was pH 7.40, which is similar to that of a ligninbased activated carbon reported previously (Fu,2018). pHpzcis the pH at which the net surface charge of an adsorbent is zero. When solution pH is lower than pHpzc, the adsorbent surface charge is positive, orotherwise, pH > pHpzc, surface charge is negative. It was reported that the adsorption of inulin oligosaccharides, a neutral saccharide, on Darco G-60 activated charcoal reached its highest uptake in a nearly neutral solution at pH 6 to 8 (Li et al., 2015).This probably suggests that, in terms of Darco G-60 charcoal adsorbent, a neutral solution around pHpzcis more favorable for oligosaccharides adsorption than acid or alkaline solutions. However, the maximum adsorption pH of chitooligosaccharides, an alkaline saccharide, on activated charcoal shifted to basic conditions at pH 8-9. This was likely caused by the property of chitooligosaccharides.Chitooligosaccharides (degree of deacetylation 93.7%) used in this study consists mainly of glucosamine that contains exchangeable amine protons. The amine groups in chitooligosaccharides molecules may exist in the form of -NH2or -in aqueous solutions. Basic conditions would result in less allocation of -on chitooligosaccharides and thus weaker electrostatic repulsion between chitooligosaccharides and activated charcoal. Very weak or no electrostatic repulsion would be more favorable to the adsorption of chitooligosaccharides onto activated charcoal. On the other hand, for activated charcoal adsorbent, high pH resulted in low oligosaccharides adsorption capacity as stated above.Therefore, as the result of the properties of both activated charcoal (adsorbent) and chitooligosaccharides (adsorbate), the maximum adsorption of chitooligosaccharides on charcoal occurred at pH 8-9. pH 9 was used for the following batch mode experiments.
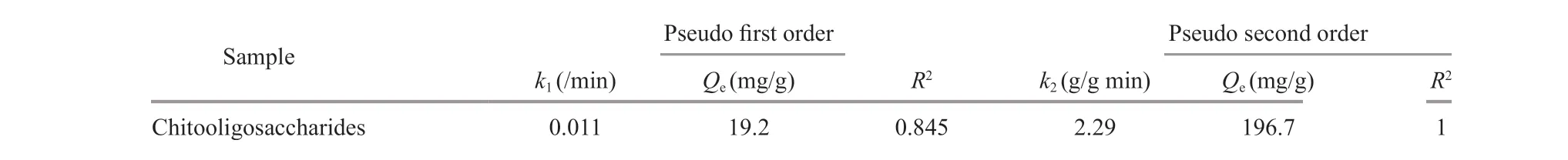
Table 1 Kinetic parameters for the adsorption of chitooligosaccharides onto activated charcoal

Fig.4 Eff ect of contact time on the adsorption of chitooligosaccharides on activated charcoal
3.4 Adsorption kinetics
Adsorption kinetics was investigated varying the shaking time from 5 to 300 min in the solution of pH 9 at 298 K. It is shown in Fig.4 that the adsorption amount of chitooligosaccharides reached 0.161 g/g at 5 min, and then continuously increased until 60 min,after which the adsorption equilibrium was established and the adsorption amount remained constant at 0.195 g/g. After the equilibrium, 81.3% of chitooligosaccharides in the solution was adsorbed onto activated charcoal. It is observed that the adsorption process consists of two stages. The f irst stage was the f irst f ive minutes, during which the adsorption rate was very fast, and 67.0% of chitooligosaccharides in the solution was adsorbed by activated charcoal. This stage corresponded to the surface adsorption. In the second stage (5-60 min),the adsorption amount still increased but the growth rate slowed down. Intra-particle diff usion occurred in this stage (Qadeer, 2012).
Adsorption kinetics studies can provide valuable information to elucidate the nature of adsorption process. The experimental data obtained from the contact time experiment were f itted with both the pseudo-f irst-order and pseudo-second-order model to reveal the kinetic mechanisms of the adsorption process. The pseudo-f irst-order Lagergren model assumes that the adsorption rate is proportional to the number of unoccupied sites of adsorbent. This model for solid/liquid system is written as
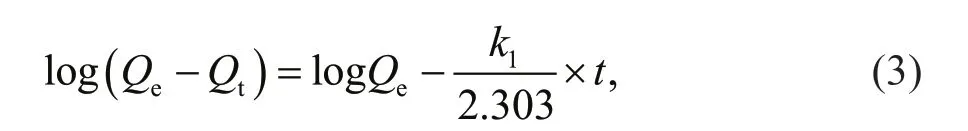
whereQeandQt(g/g) are the amounts of chitooligosaccharides adsorbed at equilibrium and at timet, respectively.k1is the pseudo-f irst-order adsorption rate constant (/min) (Acemioğlu, 2005).The plot of log(Qe-Qt) versustis illustrated in Fig.5a,andQeand rate constantk1were calculated from the linear regression, whose values are given in Table 1.The determination coeffi cient (R2)of this plot is0.85,suggesting that the adsorption did not f it with the pseudo-f irst-order model well. Additionally, the calculatedQe(19.2 mg/g) did not agree with the experimental value of 195.1 mg/g.
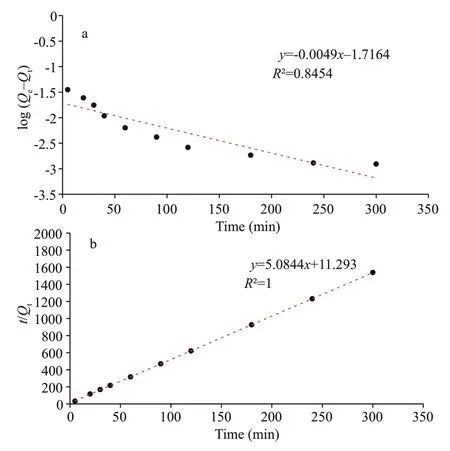
Fig.5 Plots of the pseudo-f irst-order kinetics model (a) and the pseudo-second-order model (b) for adsorption of chitooligosaccharides onto activated charcoal
The adsorption kinetics data were further f itted with pseudo-second-order model developed by Ho and McKay (1999). This model assumes that the adsorption rate is proportional to the square of the number of unoccupied sites, which can be given by the following equation:
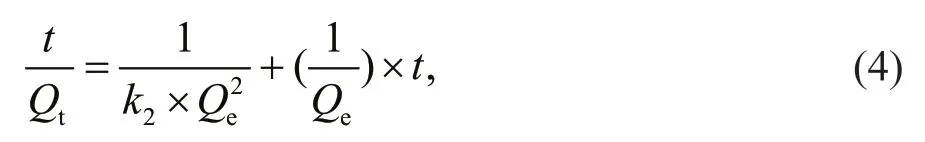
wherek2is pseudo-second-order rate constant of adsorption (g/g min). The plot oft/Qtversustare shown in Fig.5b, andk2andQewere calculated from the slope and intercept of the plot (Table 1). The linear correlation coeffi cient is nearly 1. This suggests that the adsorption system studied here belongs to the second order kinetic model that expresses the nature of chemisorption in an adsorption process (Skodras et al., 2008). Furthermore, the calculatedQe(196.7 mg/g)is in good accordance with the experimental value of 195.1 mg/g.
3.5 Eff ect of temperature and adsorption isotherms
Adsorption behavior of activated charcoal may be inf luenced by temperature depending on the property of the adsorbate, adsorbent, and the medium (Qadeer,2012; Li et al., 2015). Eff ect of temperature on the adsorption and the adsorption isotherms were obtained by batch adsorption experiments at 298, 308, and 318 K with initial chitooligosaccharides concentrations of 0.5, 1.0, 2.0, 3.0 and 4.0 g/L,respectively. As is presented in Fig.6, the adsorption capacity of activated charcoal did not show signif icant diff erence with increasing temperature. This suggests that the adsorption system in this case is temperatureinsensitive, unlike the adsorption of neutral inulin oligosaccharides on activated charcoal which showed decreasing adsorption capacity with increasing temperature (Li et al., 2015).
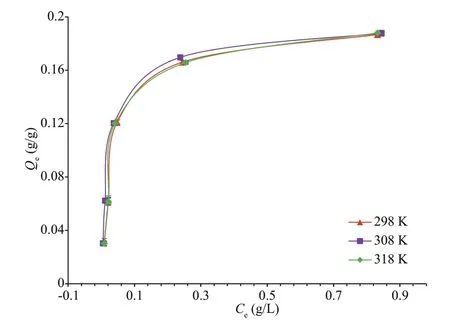
Fig.6 Adsorption isotherms of chitooligosaccharides onto activated charcoal
Adsorption isotherms describe the relationship between adsorbed amount and equilibrium concentration of the adsorbate. To reveal the controlling mechanisms of the adsorption, the isotherms data in this study were f itted with two commonly used adsorption isotherms models: the Langmuir and the Freundlich equations. The Langmuir model is suitable to monolayer adsorption and the adsorption sites are energetically identical,while the Freundlich model describes multilayer adsorption with heterogeneous active sites (Langmuir,1916; Fytianos et al., 2000).
The Langmuir model can be given as:

whereQeis the amount of adsorbed chitooligosaccharides at equilibrium (g/g) andCeis the equilibrium concentration of chitooligosaccharides(g/L).Qmax(g/g) andKL(L/g) are constants related to the adsorption capacity and energy of adsorption,respectively. They can be calculated from the linear plot ofCe/QeversusCe.
The Freundlich model is expressed as
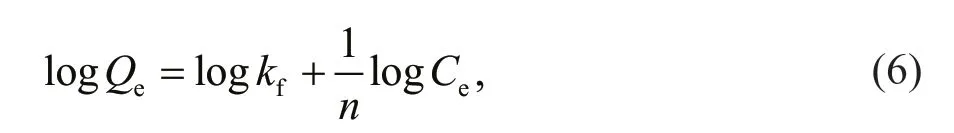
wherekf(L/g) andnare the Freundlich constants thatindicate the adsorption capacity and intensity,respectively. Values ofkfandnare calculated from the plot of logQeversus logCe.
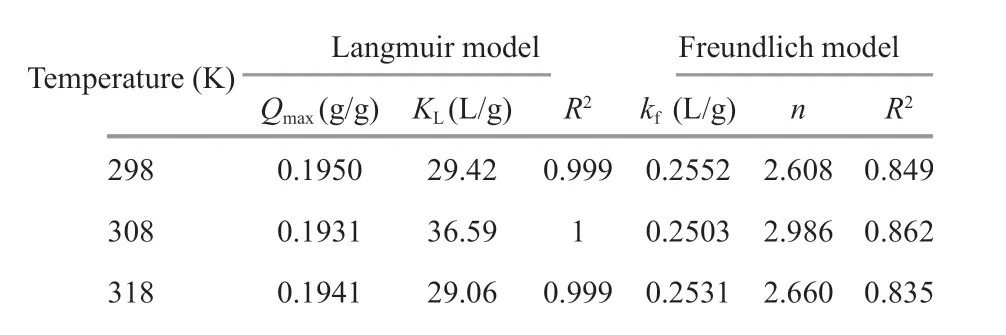
Table 2 Parameters for Langmuir and Freundlich adsorption model
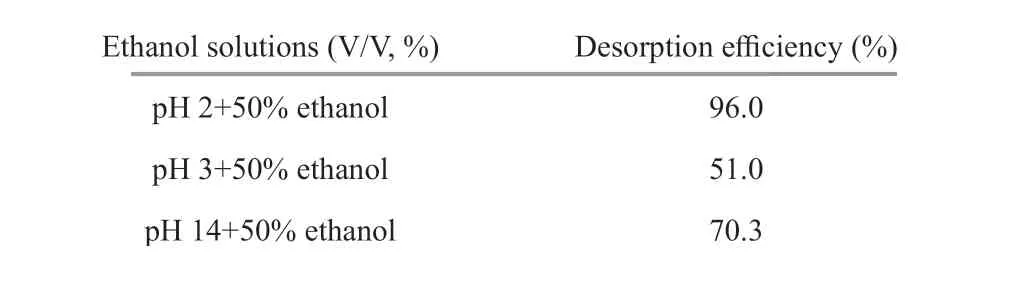
Table 3 Desorption effi ciency of chitooligosaccharides from activated charcoal by ethanol solutions at diff erent pHs
The constants of both isotherms models and the correlation coeffi cients were collected using leastsquare linear regression and are presented in Table 2.It is shown that theR2values of all the Langmuir model were >0.99, while those of Freundlich model ranged between 0.83-0.86. Thus, the adsorption could be best described by the Langmuir model rather than the Freundlich model. Additionally, adsorption capacity (Qmax) at 298 K calculated in the Langmuir model was 0.195 g/g, which agrees precisely with the experimental data (195.1 mg/g). Therefore, the adsorption of chitooligosaccharides on activated charcoal was related mainly to monolayer adsorption in uniform surface sites.
3.6 Desorption studies
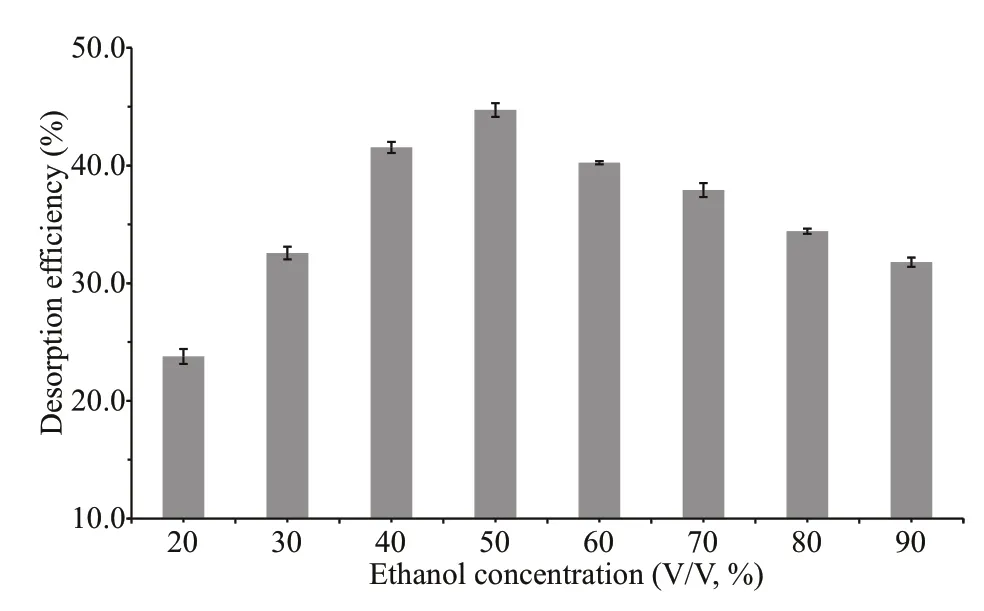
Fig.7 Desorption effi ciency of activated charcoal with solutions of diff erent ethanol concentrations
Polar molecules adsorbed by activated charcoal could be eluted by adjusting the polarity of solutions using ethanol. Gradient elution by ethanol is one of the most widely used methods for the separation of various oligosaccharides by activated charcoal chromatography (Wei et al., 1997; Kittur et al., 2005;Fujimoto et al., 2009). In general, the higher the ethanol concentration of the eluent solution, the weaker the interaction between chitooligosaccharides and activated charcoal. However, the solubility of chitooligosaccharides decreased with increasing ethanol concentration, which might aff ect the desorption effi ciency. Hence, a batch experiment was conducted to study the desorption effi ciency of activated charcoal with eluents of diff erent ethanol concentrations (Fig.7). It is shown that the desorption effi ciency reached its maximum at the ethanol concentration of 50% (V/V), and decreased again when the ethanol concentration increased. Fifty percent ethanol solution seems to be the optimal ethanol eluent. However, only less than a half of chitooligosaccharides (44.7%) was desorbed by 50%ethanol solution. This should be another critical reason for the sample loss in previous practices on the separation and purif ication of chitooligosacchaides using activated charcoal (Xiong et al., 2009; Li et al.,2012a, 2013a & b). Other common solvents, such as DMSO, methanol, acetonitrile, and acetone all have stronger polarity than ethanol, and thus are expected to have lower desorption effi ciency. In order to resolve this problem, considering that pH is an important factor aff ecting the adsorption of chitooligosaccharides on activated charcoal, we further investigated the desorption effi ciency of 50% ethanol solution under very acidic and basic conditions. The results were given in Table 3. As expected, more chitooligosaccharides were desorbed from activated charcoal at pH 2 and 14, with the desorption effi ciency of 96.0% and 70.3%, respectively. Thus, 50% ethanol solution at pH 2 is supposed to be an eff ective eluent for the desorption of chitooligosaccharides from activated charcoal.
4 CONCLUSION
Adsorption characteristics of chitooligosaccharides onto activated charcoal were investigated. The results showed that particle size of activated charcoal, the solution pH, contact time and initial chitooligosaccharides concentration all could inf luence the adsorption process, but the adsorption was temperature-insensitive. The activated charcoal with smaller particle size has higher adsorption capacity of chitooligosaccharides and the adsorption capacity reached its maximum at pH 8-9. The adsorption equilibrium was attained after 60 min, and the kinetic process followed the pseudo-second-order model and expressed the chemisorption’s nature.Adsorption capacity showed no signif icant diff erence at varied temperature. The adsorption isotherms were best f itted to the Langmuir equation, suggesting monolayer adsorption of chitooligosaccharides onto activated charcoal with homogeneous active sites.Chitooligosaccharides on activated charcoal could be eff ectively desorbed by 50% ethanol solution at pH 2,in desorption effi ciency of 96%. These f indings are of great signif icant for the production and purif ication of chitooligosaccharides and other amino oligosaccharides using activated charcoal.
5 DATA AVAILABILITY STATEMENT
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
 Journal of Oceanology and Limnology2020年2期
Journal of Oceanology and Limnology2020年2期
- Journal of Oceanology and Limnology的其它文章
- Contribution of surface wave-induced vertical mixing to heat content in global upper ocean*
- Upper ocean response to typhoon Kujira (2015) in the South China Sea by multiple means of observation*
- Inf luence of simulating deep-sea environmental factors on cathodic performance of seawater battery*
- Eff ects of hypoxia on survival, behavior, and metabolism of Zhikong scallop Chlamys farreri Jones et Preston 1904*
- Distinct inf luence of trimethylamine N-oxide and high hydrostatic pressure on community structure and culturable deep-sea bacteria*
- Microbial communities present on mooring chain steels with diff erent copper contents and corrosion rates*
