Enhanced Removal Ciprofloxacin by Self-assembled Hydrogel with Anatase Titanium Oxide Nanotubes and Grapheme
ZHENGKai(郑凯),ZHANGZizhen(张子贞),LIYuran(李雨然),XUQiuyi(徐秋旖),GUOTailin(郭泰麟),SUNXinpeng
School of Environmental Engineering, Nanjing Institute of Technology, Nanjing 211167, China
Abstract: A novel hydrogel, consisting of titanium dioxide nanotubes and graphene oxide (H-TN-GO), was fabricated to remove ciprofloxacin (CIP) from aqueous solution. The adsorbent was characterized by scanning electron microscope (SEM), transmission electron microscope (TEM), X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR) and Raman spectroscopy. Batch adsorption experiments systematically investigated the effect of contact time, temperature and pH on the capacity of H-TN-GO for CIP. Adsorption property of H-TN-GO and its regeneration performance was evaluated. H-TN-GO exhibited high adsorption capacity of 530 mg/g and Langmuir model was more suitable for fitting adsorption behavior. The removal ratio of CIP was more than 99% when initial concentrations were less than 100 mg/L. Besides, the initial pH of CIP solution had a distinct influence on the removal ratio, and adsorption capacity of adsorbance could be recovered by regeneration with mild alkaline condition. Large pore size and abundant oxygen-containing groups on the surface further improved its adsorption performance. The enhancing effect of H-TN-GO mainly attributed to the immobilization of TiO2 onto graphene oxide and π-π interactions stacking between H-TN-GO and graphene oxide. As potential adsorbents, H-TN-GO exhibited remarkable regeneration capacity for water treatment.
Key words: hydrogel; TiO2 nanotubes; graphene oxide; adsorption; ciprofloxacin
Introduction
Quinolones are the most commonly prescribed classes of antibiotics in the world and used to treat a variety of bacterial infections in humans[1-2]. All of the quinolone antibacterials, fluoroquinolones are generally used in the treatment of various diseases due to their broad spectrum of antimicrobial activity; they are not only effective against gram-negative bacteria but also moderately active against gram positive bacteria[3]. The use of fluoroquinolones in the United States increased about 40%[4], ciprofloxacin (CIP) is one member of the second generation of fluoroquinolone derivatives and another is widely used fluoroquinolone drug[4]. Therefore, CIP can represent drug-tainted wastewater well especially antibiotics and their metabolites, which are finally discharged into wastewater treatment plants (WWTPs) or soaked into the soil. As reported, the center of CIP reached to 10 μg/L in untreated hospital sewage[5]. The presence of CIP in water or living organisms mainly comes from pharmaceutical wastewater and residual antibiotics, which could cause great harm to organisms even human through food chain eventually. Scientists have conducted several methods about pharmaceutical wastewater such as photocatalysis[6-7], activated sludge process[8], ozone oxidation[9]and membrane processing[10]. CIP and its derivative are categorized as poorly biodegradable wastewater, so adsorption is a proper way to deal with this kind of wastewater. Furthermore, renewable absorbent seems to be an environmentally friendly material. Some researchers have focused on hotspot material graphene oxide (GO) as an adsorbent due to its high surface area[11-14]. GO has high concentration of the oxygen-containing functional groups and these groups can act as anchoring groups that bind GO is onto inorganic or organic materials[15]. Literatures have been reported that GO was used as a building block, thus it could be integrated with TiO2nanotube (TN) by self-assembly method[16-17].
The adsorption capacity of single GO or GO loaded with other substances rarely exceeds 350 mg/g for CIP[12]. Another paper reported that: modified graphene oxide as manganese oxide possessed high catalytic activity for the degradation/oxidation of biphenyl-A at ambient conditions[18], without light irradiation and/or the addition of oxidants, which was higher than that of the pure manganese oxides and can be attributed to the synergistic effect of the manganese oxide and the modified graphene oxide.
The paper reported[19]that nickel-graphene and nickel-graphene oxide composite electrodes was availed of electrocatalytic oxidation of urea, and the obtained composites have a higher activity than the nickel electrode in both alkaline solution and alkaline solution supplemented with urea.
Graphene hydrogels and aerogels, consisting of microporous and mesoporous networks, are widely used as adsorption material and photocatalysis[20-21].
The positive charged TiO2and negative GO sheets met together to form larger colloidal aggregation. The supported TN onto carbon-based materials makes photocatalyst be easily separated from aqueous solution and reused to the maximum extent. Unfortunately, little work has been done on adsorptive property of hydrogel TN-GO (H-TN-GO). Similar materials have been reported in the literature[22], which reported that the synergetic catalytic properties of gold nanoparticles planted on transparent titanium dioxide nanotube array bed.
Another paper[23]reported the fact that enhanced photocatalytic activity of hierarchical titanium dioxide microspheres with combining carbon nanotubes as “e-bridge, The as-prepared three-dimensional TiO2microspheres covered by intercrossing lamellar crystals are abundant in pores and sharp edges, forming an ideal interface with large surface area and numerous active sites for photocatalysis. Combined with carbon nanotubes (CNTs), the TiO2microspheres are connected and stabilized. Moreover, the CNTs serve as pathways for electrons, benefiting the effective separation of e--h+pairs and accounting for the superior photocatalytic activity.
We use facile way to fabricate macroscopic graphene-based hydrogels and focus on the explanation of macromolecular adsorption by polymeric hydrogels. The mixture of TiO2nanomaterials and GO nanosheets could form new nanocomposite hydrogel during thermal reduction without using surfactant and crosslinking agent. Some kinds of adsorbents may have good and steady performance for CIP, however treatment of desorption solution and recycling of adsorbents still remain problems. TN as an essential ingredient can be utilized to implement both mineralization of the pollutants and regeneration of adsorbent.
2 Material and Methods
2.1 Material and chemicals
TiO2(P25 analytical standard) used in this study was purchased from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China without further purification. Then P25 was subjected to the following procedures: stirring, ion exchange, centrifugation and freeze-drying, and its detailed synthetic method can be found in our previous work[24]. Finally, TN can be synthesized. Pure GO was purchased from XFNANO Co., Ltd., Nanjing, China, FeSO4·7H2O,HCl,NaOH,CIP and other chemicals were of analytical grade and purchased from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China. HCl and NaOH (0.5 mol/L) solutions were prepared for adjusting acidity.
2.2 Synthesis of H-TN-GO
A mixture of GO suspension (2.5 mg/mL) and TN (1 mg/mL) was diffused fully into the deionized water and helped settle down an ultrasound bath pot for 24 h. The concentration of FeSO4·7H2O solution was chosen as 140 g/L, and then the acidity was adjusted equivalent to 3. Then 0.5 mL above-mentioned FeSO4solution was added into TiO2/GO mixture (8 mL).During the whole process,the trivalent iron oxide and reduced graphing were produced by the redox reaction between Fe2+and grapheme oxide. The obtained mixture solution was processed with ultrasonic for 10 min. Then the suspension was immersed into a water bath pot at 358 K and the time lasted for 8 h. After the reaction was finished, the product was dumped out from vessel; it was transferred into deionized water, then rinsed 10 times with enough distilled water regularly. Finally, cylindroid hydrogel with 10 mm height and 12 mm diameter could be achieved. In order to analyze their morphology and structure, the H-TN-GO was made as an aerogel by freeze-drying.
2.3 Batch adsorption experiments
Batch adsorption experiments were accomplished in 50 mL flasks. During the batch test, 0.02 g H-TN-GO was slowly put in glass tubes. Adsorption experiments were carried out at ambient conditions (293 K).The supernatants were taken out regularly and filtered with 0.45 μm filter membrane for analysis after interval. And higher concentrations of CIP were diluted by the background solution for precise measurement.
The adsorption isotherm was drawn under the condition of concentration CIP, 200, 400, 600, 800 and 1 000 mg/L at pH=4.86 (initial pH of solution). Adsorption experiments were conducted in flasks, which were shaken on a temperature-controlled water bath shaker at 125 r/min. The concentration of CIP adsorbed onto H-TN-GO was calculated according to the difference value between the initial concentration and the concentration at time t by following equations:
(1)
(2)
(3)
whereC0was the initial concentration of CIP in aqueous solution (mg/L),Ctwas the concentration solution at timet(mg/L),Cewas concentration of CIP at equilibrium (mg/L),mrepresented mass of adsorbent (mg) andVrepresented volume of solution (L).qtwas the adsorption capacity of CIP in solution at timet(mg/g),qewas the equilibrium adsorption capacity of CIP in solution (mg/g) andrrepresented the removal ratio of CIP.
The calibration curve was established by choosing standard solution ranging from 0 to 10 mg/L and theR2value was more than 0.999,the relationship between absorbance and concentration is:y= 11.117x+ 0.138 2(xstands for absorbance andyrepresents concentration of CIP, mg/L). The concentration of CIP in the solution was accurately measured by UV-vis spectrophotometer at wavelength of 275 nm (Unico UV2100).
The kinetics studies were conducted to measure the time dependent uptake of CIP. Kinetic studies were conducted with different concentrations equivalent to 200 and 400 mg/L of CIP at different time intervals.
2.4 Characterization of H-TN-GO
The concentration of CIP in the solution was accurately measured by UV-vis spectrophotometer at wavelength of 275 nm (Unico UV2100). Powder X-ray diffraction (XRD) patterns were recorded on an ARL X′TRA diffractometer (40 kV, 30 mA) using Ni-filtered Cu/K radiation. SEM analysis was carried out (Hitachi S-4800). TEM images and Energy-dispersive X-ray spectra (EDX) were obtained using a JEM-200CX at 200 kV (JEOL Co. Ltd.). The FTIR spectra were recorded on the NEXUS870 spectrometer. The spectra were recorded using KBr wafers containing 1% of the sample at ambient conditions with a resolution of 4 cm-1in the region ranging from 400 to 4 000 cm-1.
3 Results and Discussion
3.1 Characterization of H-TN-GO
Particle average size is an important physical parameter for adsorbent since it directly affects the specific surface area. GO can introduce a huge amount of —COOH and —OH functional groups over its surface, which makes GO become hydrophilic in aqueous solution[25]. It can be seen from Table 1, the characteristic of porous structure (0.127 cm3/g) and higher specific surface area (40.44 m2/g) made H-TN-GO become a good adsorbent, providing large amount of activated sites. In Table 1, SSA represents specific surface area, ESA represents external surface area,and PV represents pore volume.
Table 1Specific surface area of H-TN-GO calculated on the basis of the standard BET methods
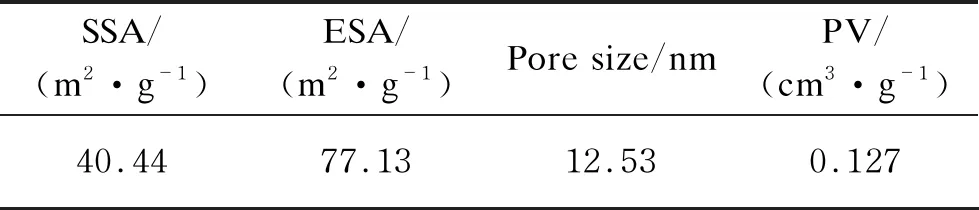
SSA/(m2·g-1)ESA/(m2·g-1)Pore size/nmPV/(cm3·g-1)40.4477.1312.530.127
Pure GO has an obvious peak at 10.6° according to standard XRD spectrum[26]. There were no observable peaks of GO in Fig. 1, which suggested that GO had successfully been reduced into rGO (reduced graphene oxide). This phenomenon was probably induced by destruction of the regular stacking of GO. Some peaks in Fig. 1 were belonged to a tetragonal α-FeOOH phase (JCPDS file No.34-1266), indicating the existence of FeOOH in the composite,and α-FeOOH was produced by the redox reaction between Fe2+and grapheme oxide. H-TN-GO exhibited the characteristic (101), (103), (200), (211) and (213), which corresponded to the anatase crystal phase (JCPDS file No. 21-1272). The observable peaks of anatase implied high TiO2content.

Fig. 1 XRD patterns of H-TN-GO
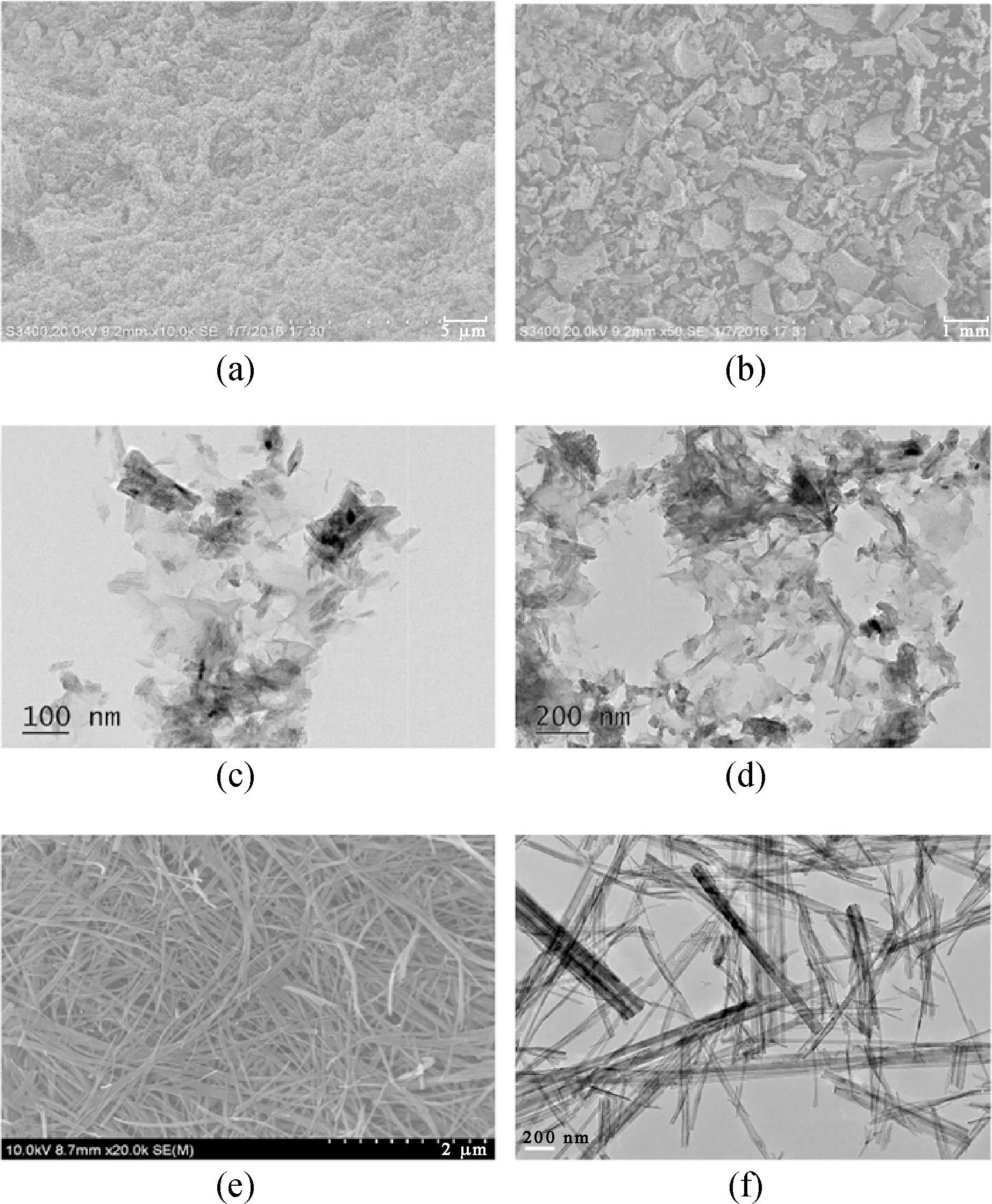
Fig. 2 SEM images of (a), (b) H-TN-GO; (c), (d) TEM images of H-TN-GO; (e) (f) TN, TEM images of TN
The SEM image showed that H-TN-GO had uniform structures under different magnifications (Figs. 2(a)-(b)). Each graphene sheet interacted with another sheet to form hydrogels through self-assembly. In order to verify the combination of GO and TN, microstructure was investigated with TEM. It can be observed from Figs. 2(c)-(d) that the TN has the following characteristics: a diameter of 10 nm, length of 8 nm and agglomeration of Fe3O4, Fe3O4was produced by the redox reaction between Fe2+and grapheme oxide. It can be observed that TN had much higher surface area and thus could bear out a number of active sites. TiO2had successfully been embedded into several stacks of graphene; it is likely that TN grew onto the GO surface. This phenomenon indicated that strong forces of attraction existed between rGO and TN. Compared to pure TN (Figs. 2(e)-(f)), TiO2nanoparticles would anchor on rGO sheets during hydrothermal process. Ultimately, nanometer-sized particles were evenly dispersed onto the walls of the pores.
The chemical reduction of GO was also confirmed with Raman spectroscopy. D band is attributed to the presence of defects within the hexagonal graphitic structure and G band confirms the presence of sp2carbon-type structure. The Raman spectrum of the aerogel TN-GO(A-TN-GO) (A respresented aerogel) composite showed D peak at 1 333 cm-1and G peak at 1 591 cm-1, which attributed to the well-known bands for rGO[27]. The intensity ratio of the D band to the G band can reflect the order of defects in GO or graphene. The measured ratio of ID/IG (1.16) was much higher than that of original GO, suggesting a successful reduction from GO to rGO in A-TN-GO[28].

Fig. 3 Raman spectra of H-TN-GO

Fig. 4 FTIR spectra of H-TN-GO (before adsorption and after adsorption)
3.2 Effect of contact time and initial concentration
Equilibrium adsorption capacity on H-TN-GO increased by two times as the initial concentration (C0) whose range of change was from 200 mg/L to 1 000 mg/L. After the adsorption reached 24 h, the adsorption capacity reaches 65% of the maximum. Moreover, removal ratio nearly reached equilibrium after approximately 55 h of duration in the experiment. The adsorption rate of CIP on H-TN-GO was fast at the initial stage, but it became slower and slower while the equilibrium reached before the time change from 55 h to 96 h. Besides, Fig. 6(a) also indicated 66% of CIP was removed at an initial concentration of 200 mg/L, 49% at 400 mg/L, 38% at 600 mg/L, 29% at 800 mg/L and 25% at 1 000mg/L in the event that the equilibrium is reached.
It is well known that conduction band electrons (e-) and valence band holes (h+) are generated when the irradiated energy to TN with UV light was greater than the band gap energy between conduction band and valence band[29]. Besides, TiO2has the intrinsic capacity to absorb contaminants including CIP in previous work and also contributes to the photocatalytic activity[30]. By comparison sorption performance between H-TN-GO and H-GO in Fig. 6(b), it is obvious that capacity of H-TN-GO was 1.3 times as that of H-GO.
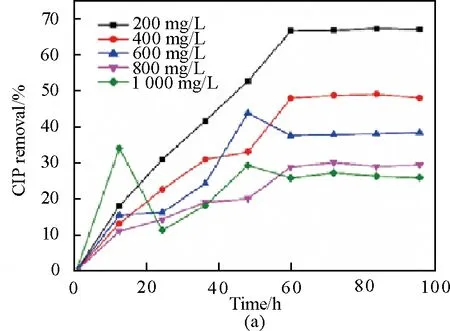
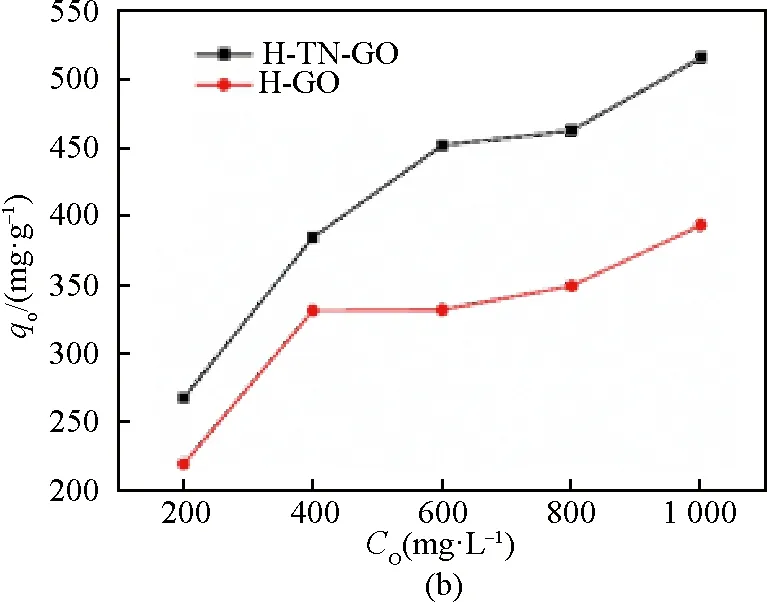
Fig. 5 Influence of (a) contact time; (b) comparative adsorption capacity between H-TN-GO and H-GO (concentration of CIP: 200, 400, 600, 800 and 1 000 mg/L, temperature =29 K, pH=4.86)
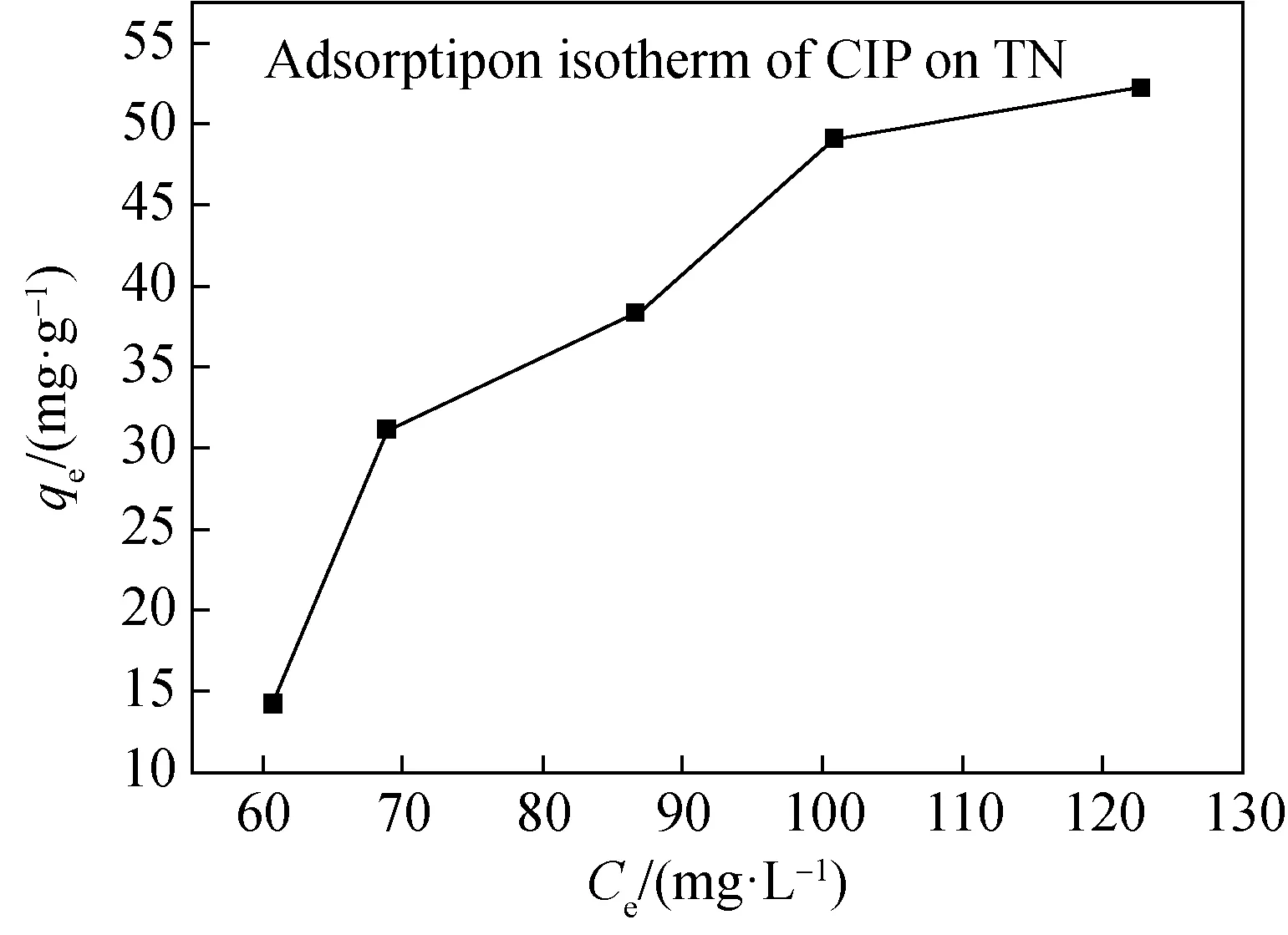
Fig. 6 Adsorption isotherm of CIP on TN
From Fig.6 the adsorption isotherm of CIP on TN indicated that the equilibrium adsorption capacity of CIP on TN was about 50 mg/g.
3.3 Adsorption isotherms of CIP on H-TN-GO
Adsorption isotherms are important for the description of how adsorbates interacted with adsorbents. The adsorption curves were fitted with both the Freundlich and the Langmuir models. And the results were shown in Table 2.
Langmuir isotherm:
(4)
Freundlich isotherm:
(5)
whereqerepresented the amount of CIP adsorbed at equilibrium in unit mass of H-TN-GO,Cerepresented the concentration of CIP in aqueous phase at equilibrium,nandKFwere Freundlich coefficients,qmandKLwere Langmuir coefficients.
The Langmuir model assumes monolayer adsorption over the surface of adsorbent without attraction between adsorbed molecules. The isotherms fitted better with Langmuir model than the Freundlich model, suggesting the sorption on H-TN-GO may be controlled by heterogeneous chemisorption.
An ideal adsorbent should efficiently remove toxic contaminants from environments to meet a criterion. Only very faint yellow color is recognized in the solution ultimately. It should be noted that H-TN-GO can maintain good performance of 530 mg/g for CIP removal, higher than most reports. The comparison of maximum adsorption capacities of H-TN-GO with other various adsorbents is represented in Table 3. Figure 7 indicated that the whole adsorption process was of endothermic.

Table 2 Adsorbent Langmuir model and Freundlich model
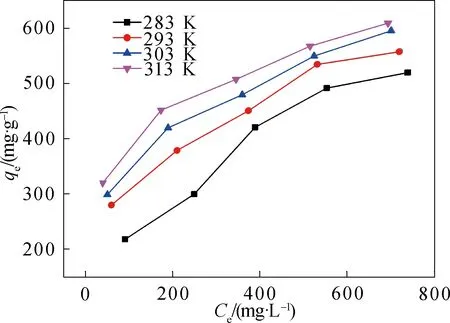
Fig. 7 Adsorption isotherms of CIP on H-TN-GO
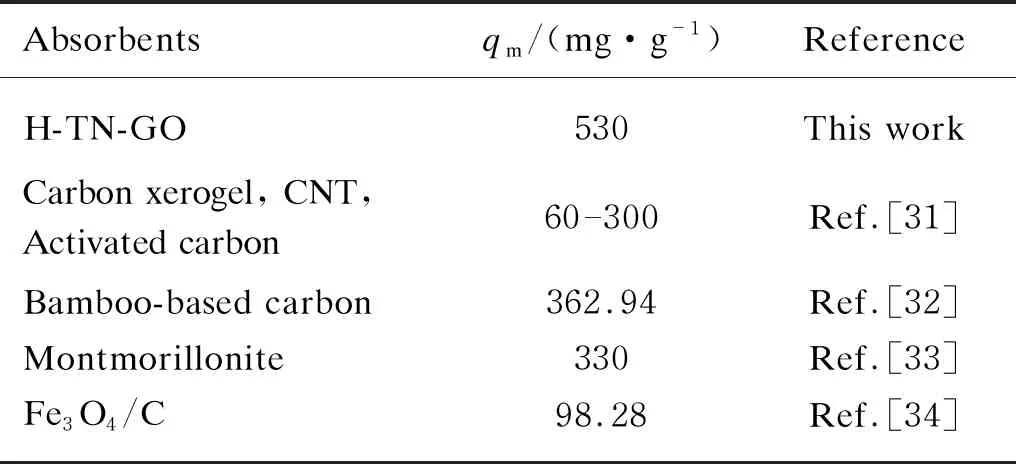
Absorbentsqm/(mg·g-1)ReferenceH-TN-GO530This workCarbon xerogel, CNT, Activated carbon60300Ref.[31] Bamboo-based carbon362.94Ref.[32]Montmorillonite330Ref.[33]Fe3O4/C98.28Ref.[34]
3.4 Adsorption kinetic of CIP onto H-TN-GO
Pseudo-first-order and pseudo-second-order kinetics model were used to fit the experimental data and to assess adsorption kinetics of CIP. The pseudo-first-order:
ln(qe-qt)=lnqe-k1t.
(6)
(7)
wherek1(min-1) andk2[g·(mg·min)-1] are the pseudo first-order and pseudo second-order rate constants, respectively. From Table 4, experimental results showed that the pseudo-first order model could better fit the behavior of CIP onto H-TN-GO.
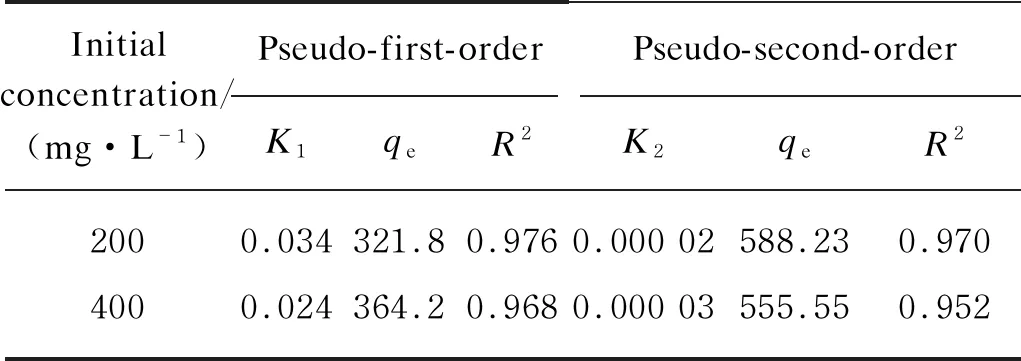
Table 4 Kinetics parameters for CIP adsorption
3.5 Effect of pH
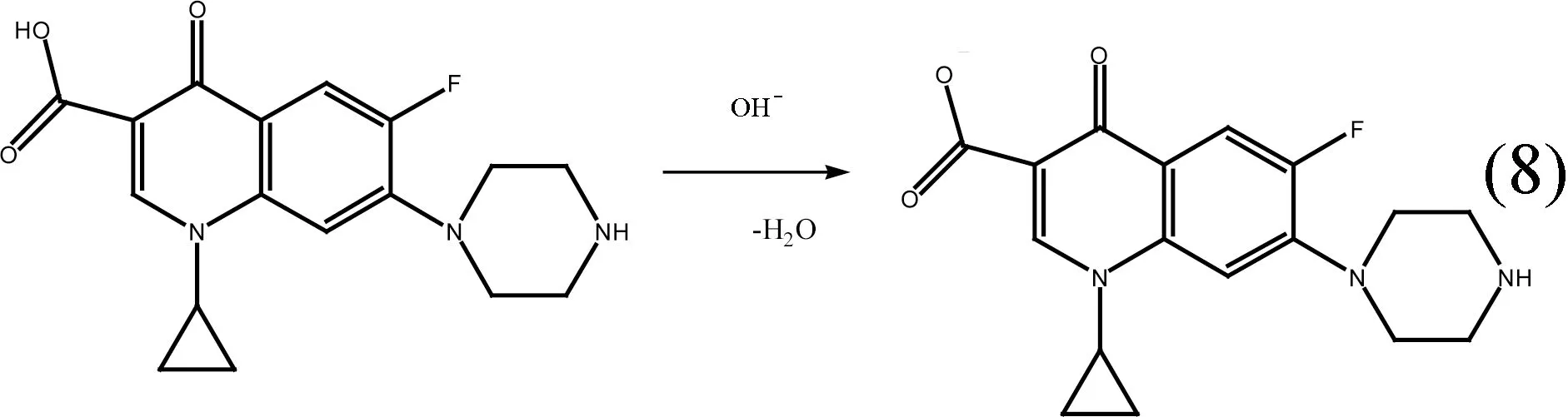
pH is an important factor to affect form of CIP[35]. It exists as cationic, zwitterionic and anionic forms under different aqueous circumstance conditions with pKa1being 6.1 and pKa2being 8.7. The different state of CIP was listed in formulas (8)-(9). CIP is present of cationic state form at low pH (pH less than 6.1) due to protonation of the amine group, of zwitterion state when pH range change was from 6.1 to 8.7, and of anionic state at high pH (pH being greater than 8.7) due to loss of a proton from the carboxylic group[36]. Experiments were conducted at acidic or alkaline conditions,whose values of pH were equivalent to 2, 4, 6, 8, 10, and 12, respectively. Those tasks could be accomplished by availing of 0.50 mol/L HCl or NaOH solution. The initial concentration of CIP was selected at 1 000 mg/L. The capacity of CIP adsorption slightly varied between 300 and 380 mg/g when the pH changed from 4 to 6. The effect of pH on the adsorption capacity of CIP was listed in Fig. 8. Although the pKa1value of CIP is equivalent to 6.1, the adsorption capacity of CIP kept basically constant when pH was greater than the pKa1, which suggested that the ammonium group existing in the zwitterionic form could be beneficial to the adsorption by means of exchange mechanism of cation. As pH increased, the adsorption capacity of CIP increased sharply to 500 mg/g when pH range change was from 8 to 10. Thus, ion exchange between zwitterionic and anionic ion played an important role during the whole adsorption process when solution pH was close to or more than its pKa2. The adsorption capacity reached the maximum 530 mg/g while the pH was equivalent to 8.0. Under the strong acidic condition, the surface of carbon atom could be protonated by the large numbers of H+, which was in favor of the electrostatic action. When the solution pH was more than 8.7, there was a negative charge on the surface of H-TN-GO; thus the oxygen-containing functional groups deprotonated with the increase of pH, which strongly repelled CIP existing in anionic form and resulted in lower adsorption.
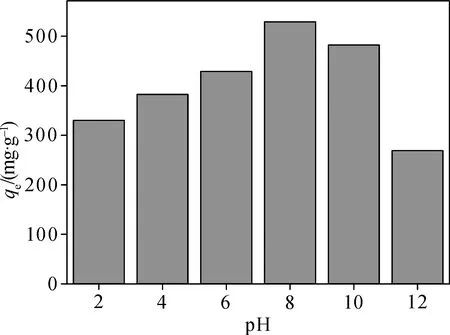
Fig. 8 Effect of pH on the adsorption capacity

3.6 Regeneration of H-TN-GO
To evaluate recycling potential, H-TN-GO was reused in the next cycle of adsorption experiment when it reached the adsorption equilibrium. The sorption performance of used H-TN-GO was recovered by eluting with ethanol and 0.5 mol/L NaOH as desorption reagent and followed with an excess of deionized water in order to remove ethanol or NaOH. Batch static adsorption experiments were repeated in the same circumstances. As shown in Fig. 9, adsorption capacity maintained 85% of total efficiency under visible light irradiation. Figure 9 displayed no significant changes could be found from the 1st to 5th cycles, which indicated the adsorption capacity kept within a good range. It is observed that the adsorption capacity of CIP decreased slightly from 520 mg/g to 480 mg/g with increasing times of reuse. H-TN-GO showed extremely high sorption capacity and good stability, which is significant to environmental applications as remediation agents.
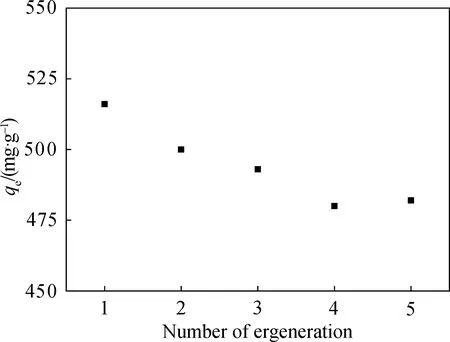
Fig. 9 Regeneration of H-TN-GO
4 Conclusions
A graphene oxide-assisted (H-TN-GO) porous hydrogel was prepared via a one-step hydrothermal method and then used as an adsorbent for the removal of ciprofloxacin. The experimental results indicated graphene aerogel exhibited good hydrophilicity and large specific surface area (40.44 m2/g) with 3D structure. CIP strongly deposited on the H-TN-GO surface via π-π interaction and cation-π bonding. Equilibrium adsorption experiment showed that adsorption capacity of the H-TN-GO can reach 530 mg/g. The enhancement of photocatalytic activity can be attributed to the existence of TN. The synthesized H-TN-GO composite hydrogel showed excellent removal capabilities for CIP, good stability and reliable reusability.
 Journal of Donghua University(English Edition)2019年4期
Journal of Donghua University(English Edition)2019年4期
- Journal of Donghua University(English Edition)的其它文章
- Parameter Estimation for Complex Ornstein-Uhlenbeck Processes
- Elastic Predictions of 3D Orthogonal Woven Composites Using Micro/meso-scale Repeated Unit Cell Models
- Sound Character Calculation and Analysis of Sound Barrier Based on Acoustoelectric Analogy
- Tool Health Condition Recognition Method for High Speed Milling of Titanium Alloy Based on Principal Component Analysis (PCA) and Long Short Term Memory (LSTM)
- Upper Bound Solution of Soil Slope Stability under Coupling Effect of Rainfall and Earthquake
- Exploring the Performance of Magnetic Immobilized Lysozyme on Sludge Hydrolysis and Mechanism of Improving Dewaterability of Excess Sludge
