功能纳米材料的“瘦肉精”传感检测技术研究进展
赵 杰,梁 刚,李 安,满 燕,靳欣欣,潘立刚
功能纳米材料的“瘦肉精”传感检测技术研究进展
赵 杰,梁 刚,李 安,满 燕,靳欣欣,潘立刚※
(1. 北京市农林科学院,北京农业质量标准与检测技术研究中心,北京 100097;2. 农业部农产品质量安全风险评估实验室(北京),北京 100097)
“瘦肉精”系一类具有相似结构的-肾上腺素受体激动剂化合物,曾被滥用作为动物生长促进剂,以提高胴体瘦肉率。中国虽自2010年起禁止其应用于动物养殖环节,但当前,“瘦肉精”类物质非法添加现象仍时有发生,且其替代品多、隐蔽性不断增强对畜产品安全和人类健康仍构成极大威胁。功能纳米材料所具有的特殊结构及性质,极大地提升了现有传感检测技术的性能,使得现有传感检测技术不断朝着灵敏、高效、简便、低成本及抗干扰能力不断增强等方向发展。该文分别从金纳米材料、碳质纳米材料、量子点以及其他新型纳米材料角度出发,总结了以上纳米材料与传感检测技术相结合在“瘦肉精”检测方面的研究进展,分析了各种检测方法的优缺点,并提出了未来功能纳米材料与传感检测技术相结合需要提升的地方,为下一步开发更灵敏准确、简便易行、高通量及低成本的检测方法提供参考。
传感器;纳米技术;无损检测;功能纳米材料;瘦肉精
0 引 言
“瘦肉精”是一类具有肾上腺素功能的苯乙醇胺类物质,临床上用于扩张支气管和增加肺通气量,治疗哮喘和肺炎等疾病[1]。当其使用剂量是临床治疗剂量的5~10倍时,可促进动物体内脂肪分解代谢,加快蛋白质合成速率,显著增加胴体瘦肉率、增质量和提高饲料转化率[2]。但是,由于其用量大、使用时间长、代谢慢,可长期存在于动物肌肉及肝脏中,人们食用被“瘦肉精”污染的食品会引起心悸、肌肉疼痛、头晕、呕吐等中毒症状,造成肾脏损害,甚至危及生命[3]。因此,世界许多国家,包括欧盟和中国均已明令禁止其作为生长促进剂应用于动物养殖环节[4]。然而,受经济利益驱使,动物养殖过程中非法使用“瘦肉精”的现象仍屡禁不止,且不断推陈出新以逃避监管[5],另外其应用范围也有所扩大[6]。为对动物及其产品实施更严格的监管,确保相应法律法规的有效实施,更加快速、灵敏及适于现场检测的方法仍为市场所急需。
目前,常见的“瘦肉精”类药物包括:克伦特罗(CLE)、莱克多巴胺(RAC)、沙丁胺醇(SAL)、苯乙醇胺A、赛庚啶、可乐定等。现有已开发出多种用于“瘦肉精”的检测方法,包括传统的基于大型仪器设备的检测方法,如高效液相色谱法[7-8]、气相色谱-质谱法[9-11]、液相色谱-串联质谱法[12-14]、毛细管电泳技术[15-17];基于免疫分析的相关技术,如酶联免疫相关方法[18-19]、免疫侧流层析法[20]、表面增强拉曼免疫法[21];以及分子印记聚合物法[22-23]等其他相关方法。尽管这些方法拥有检测灵敏度高、特异性理想,应用也最为广泛,但是由于对设备要求高、操作难度大、样本制备复杂耗时、需要专业的操作人员等原因还很难满足多种应用场景的要求。基于各种原理的传感检测技术是目前极具潜力的快速检测技术,具有简单快速、专一性高、选择性好、成本低、便携式及可实现实时分析等特点,目前在“瘦肉精”检测方面有许多报道。如,Wong等[24]开发了一种基于一次性丝网印刷碳电极的检测CLE的传感器,通过将丝网印刷电极预阳极化及利用射频氧等离子体进行处理,在不借助任何生物识别原件的情况下,实现对目标物的选择性检测,该传感器的线性响应范围为7~1 000 ng/mL,检测限为0.51 ng/mL,并在猪牛肉等实际样品中回收率介于94.92%~105.89%,而且该传感器分析时间短、成本低,重现性良好,有利于传感器的一次性的制造和使用。Feng等[25]将分子印迹技术与石英晶体微天平传感器阵列结合,实现了克伦特罗及其代谢产物的同时检测,该方法的检测限为3.0 ng/mL,低于食品法典委员会规定10g/L的残留限量。传感检测技术的快速发展,为“瘦肉精”更加高效、便捷的检测提供了可能。
纳米材料主要是指结构单元在纳米尺寸范围(1~100 nm)内的一类材料,自上世纪80年代初期被发现以来,其在光学、电学、磁学及力学等方面较普通材料更加优越的性能使其在诸多领域得到了广泛的研究和应用。因其结构的特殊性,在界面、表面效应、小尺寸效应上与传统材料具有明显差别,使其在光、电、热、磁、机械等各方面具有更加优越的性能,这些性能可以大大提高电子、光学和光电化学生物传感对识别事件的响应能力,以及提高生物传感接口的分析性能,如功能纳米材料在电化学传感领域的引入较传统分析方法在多种途径上促进了检测信号的放大,极大地提高了检测方法的性能[26]。纳米材料与各种原理的传感检测技术相结合,近年来在医药、食品安全、环境等领域研究应用广泛。目前,基于多种纳米材料的“瘦肉精”传感检测方法层出不穷,本文主要从金纳米材料、碳纳米材料、量子点以及其他新型纳米材料几个角度进行综述,以期为下一步开发更加简便、高效的检测方法提供参考。
1 基于金纳米材料的传感检测技术
金纳米材料是纳米科学和纳米技术研究的热点之一[27],也是当前研究较为深入和应用较为广泛的纳米材料之一。金纳米材料具有独特的光学、电学和催化性能以及良好的生物相容性。在物理、化学、催化、生物医学、材料科学以及电化学传感器等领域得到了广泛的应用。依赖于其独特的性质,使其成为晶体生长、电子转移机制、催化、DNA检测、生物成像及治疗等研究领域的理想候选材料[28]。同时,金纳米材料在研究光电化学性能、构建生物传感器、研究电化学催化等方面具有广阔的应用前景[29-30]。常见的金纳米材料依据其不同的形貌特征,包括球形[31]、棒形[32]、三角形[33]、链形[34]以及多态形[35]等。
在电化学传感领域,金纳米颗粒(AuNPs)独特的光学特征,其颜色受尺寸、形状、配体结合及聚集状态等因素影响[36]。当AuNPs参与物质检测时,利用其的不同聚集程度,其光吸收峰变宽,导致颜色发生改变[37],从而实现目标物的可视化检测。如图1所示,Wang等[38]利用适配体对饲料中的RAC进行检测,当体系中不含目标物时,适配体包覆在金纳米粒子表面阻止氯化钠溶液作用导致的聚集,而当目标物存在时,适配体与目标物作用,金纳米粒子失去了适配体的保护后随即发生聚集,溶液的颜色也由红色变成蓝色,通过肉眼或借助分光光度计进行结果判断,操作十分简便。该方法的有效线性范围为10~400 ng/mL,检测限为10 ng/mL。在实际样品动物饲料和牛肉检测中,加标回收率分别为72.7%~87.3% 和78.2%~86.5%。Simon等[39]利用谷氨酸(Glu)和聚乙烯亚胺(PE)来修饰金纳米粒子,制备了在室温下极稳定的含有双官能团的PE-Glu-AuNPs复合物,利用AuNPs颜色的变化实现了对人尿样中CLE和RAC的可视化检测,其中CLE的线性检测范围为0~600 nmol/L,检测限为0.93 nmol/L,RAC的线性检测范围为0~1 000 nmol/L,检测限为0.98 nmol/L,二者在实际样品人体尿液中的加标回收率为91%~98.6%和87.5%~100.7%。该检测方法操作更加简便,成本更低,为更适于现场样品的检测。Peng等[40]开发了基于金纳米簇的多路并行的免疫侧流层传感器,通过合成高亮度的绿色发射金纳米簇实现CLE和RAC的同时检测,二者的可视检测限均为0.24 ng/mL,若借助便携式荧光检测器,二者的检测限分别为0.003 ng/mL和0.023 ng/mL,用于实际样品猪尿的检测,二者的添加回收率分别为89.2%~111.4%和93.3%~105.6%。该方法与LC-MS/MS方法相比,显示出良好的一致性,同时该方法在对30个样本进行分析时仅需18 min,为实际样品的检测提供了更加高效便捷的方法。另外,该方法中制备的纳米粒子粒径更小、水溶性及生物相容性更好,这就为其进一步的开发应用奠定了基础。
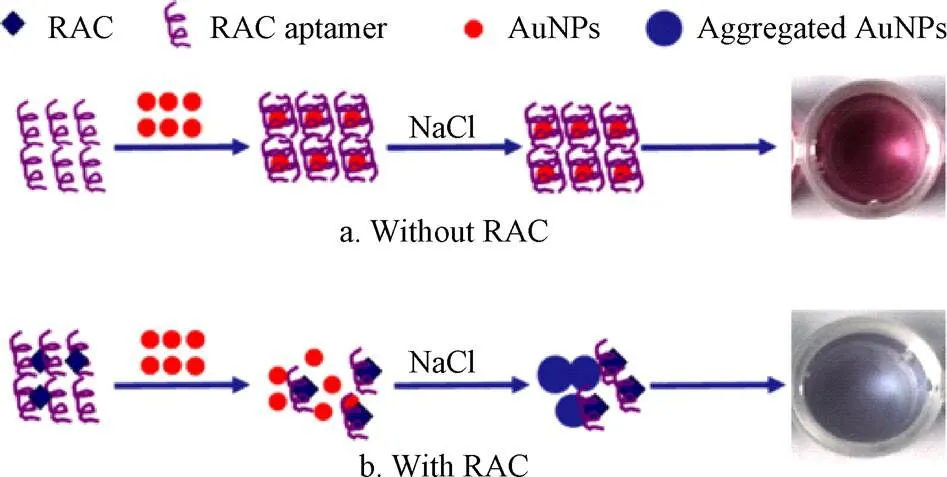
图1 基于金纳米粒子的比色传感器检测原理[38]
由于金属颗粒体系具有不寻常的拉曼散射电磁增强现象,AuNPs及金纳米簇经可见光激发后表现出表面等离子激元共振(LSPR)的特性,特别是金纳米粒子具有可调谐的纵向LSPR特性和对激发极化高度敏感特性[41],基于这一性质,AuNPs在表面增强拉曼散射传感器中有较多的报道。如图2所示,Zhu等[42]将AuNPs标记上4,4′-联吡啶和CLE抗体,作为表面增强拉曼散射探针,建立了竞争性表面增强拉曼散射免疫传感器实现了对CLE高灵敏的检测,该方法的线性范围为0.1~100 pg/mL,检测限为0.1 pg/mL。该方法用于猪尿样本的检测,回收率介于76.7%~135.8%。该方法的特别之处在于将CLE的人工抗原而不是CLE抗体固定在底物上,这样可以极大地降低检测成本,该方法在食品安全和激动剂控制方面具有广阔的应用前景。Cheng等[43]利用石墨烯的氧化产物和AuNPs作为表面增强拉曼基底实现了动物尿液中克罗特罗的表面增强拉曼光谱检测,该方法的线性范围为1~20 ng/mL,检测限和定量限分别为0.5和1 ng/mL。利用该方法对30份动物尿液进行检测,其检测结果与LC-MS/MS结果100%一致,而检测时间仅需8 min/个样品。该方法操作简单,特别适用于定期监测在畜牧业中CLE的非法使用。
金纳米粒子还具有优异的电子传递效能,同时较大的比表面积使其可以负载更多的目标捕获分子,同电化学检测技术相结合,既可发挥电化学检测方法的仪器设备简单、方法特异性和灵敏度高,且试验操作简单、成本低廉等特点[44],也可将生物反应的特异性同电化学分析方法的灵敏性结合在一起,进一步提高传感器的检测性能,也拓展了电化学传感技术的应用领域[45]。Yan等[46]将AuNPs作为反应基底和电子传递加速器,以克伦特罗的多克隆抗体为目标物的识别原件,借助于量子点构建了竞争性的电化学发光免疫传感器,实现了对盐酸克伦特罗的超灵敏检测,方法有效线性范围为0.02~50 ng/mL,检测限为0.008 4 ng/mL。该传感器表现出良好的稳定性、特异性和重现性。并将该传感器用于猪肉和猪肝样品的检测,其添加回收率为76%~122%,进一步证明了免疫传感器在实际样品中的适用性较好。如图3所示,Yang等[47]利用AuNPs/PPDA/GR复合材料修饰玻碳电极,以此来增加电极表面固定的RAC适配体的量,并提高生物传感器的信号传导性能,采用示差脉冲伏安法()检测适配体与靶标的结合前后电流信号变化,从而实现了对RAC的超灵敏检测。该传感器的线性范围为1.0×10-12~1.0×10-8mol/L,检测限为5.0×10-13mol/L。在动物尿液检测中表现出良好的稳定性和重现性。同样,Zhu等[48]采用AuNPs/PDA/GR复合材料作为信号放大器,同时增大抗原和抗体负载量,并结合量子点电化学发光法来检测RAC,该方法的线性范围为0.01~1 000 ng/mL,最低检测限为2.6 pg/mL。Li等[49]将AuNPs、壳聚糖(Chitosan)及抗原溶液按1:1:1比例固定在阵列丝网印刷工作电极上,利用自制的单孔四掷开关,实现了针对多种“瘦肉精”成分近同时的电化学发光检测,以RAC和SAL为检测对象,二者的检测限分别为8.5和17 pg/mL。该方法由于采用低成本的丝网印刷电极,成本低廉,适合大量样本的快速筛查,应用前景广阔。表1整理了以上基于金纳米材料的“瘦肉精”检测方法的相关文献。
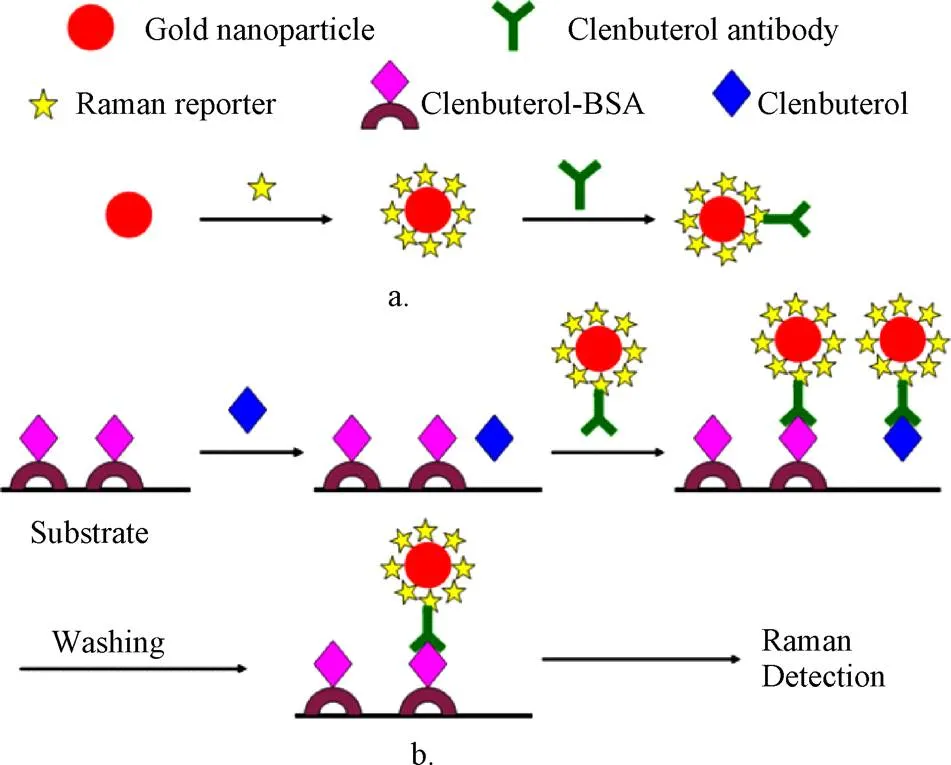
图2 基于金纳米粒子的竞争性表面增强拉曼散射免疫传感器检测克伦特罗原理图[42]
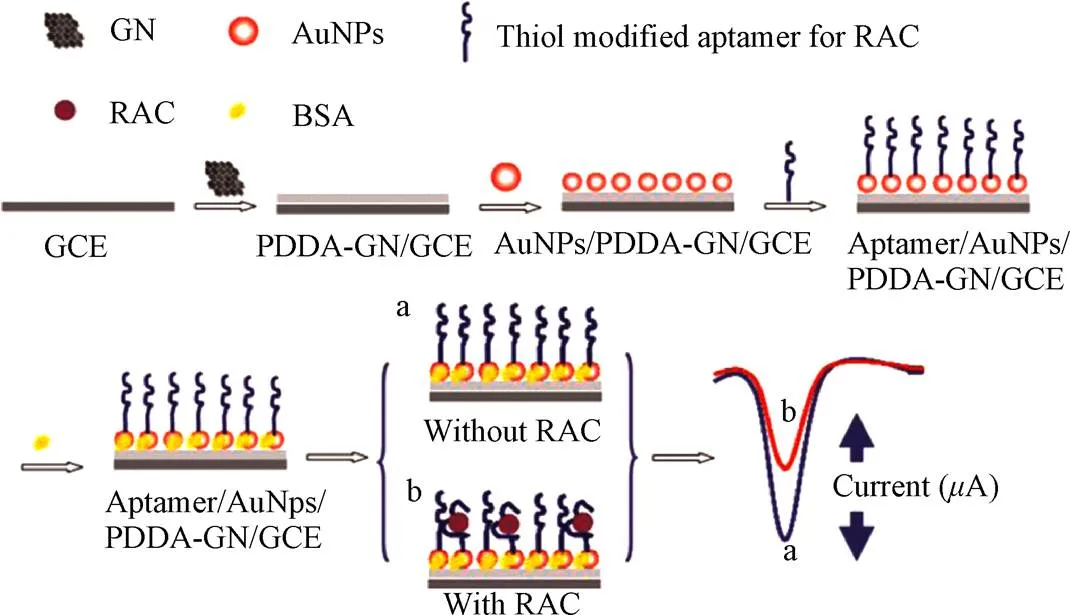
图3 基于金纳米粒子的电化学适配体传感器检测莱克多巴胺原理图[47]
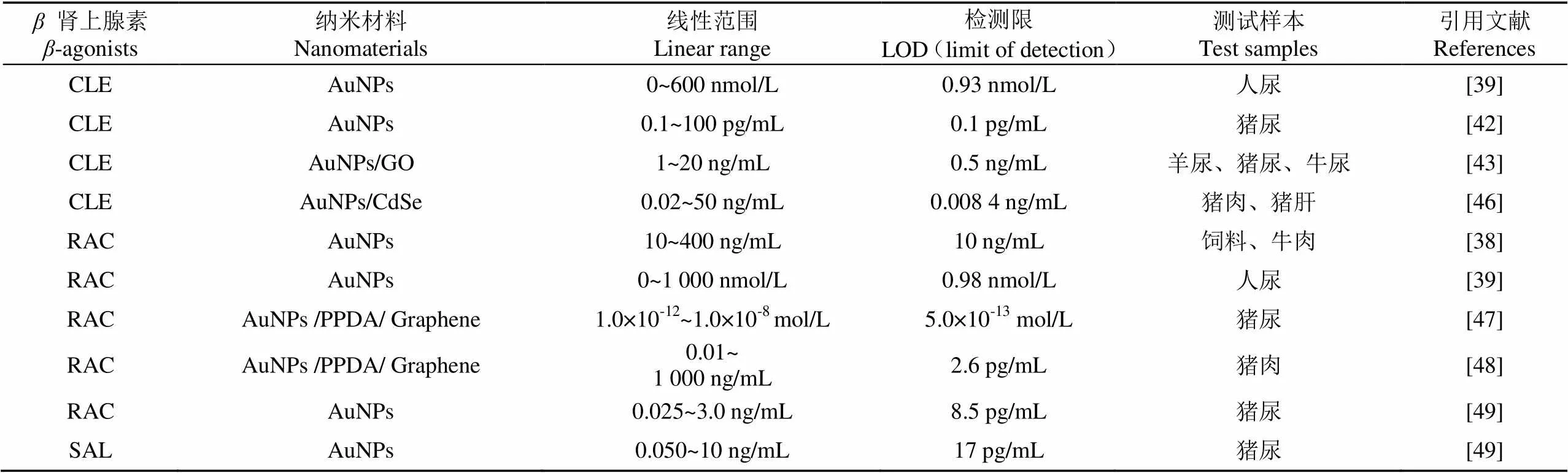
表1 基于金纳米材料的“瘦肉精”检测方法对比
2 基于碳纳米材料的传感检测技术
在功能纳米材料中,碳纳米材料也是研究及应用最广泛的纳米材料之一。依据不同的维度,碳纳米材料可划分为:零维结构的富勒烯、碳点;一维结构的碳纳米管、碳纳米纤维;二维结构的石墨烯等[50-52]。碳纳米材料具有独特的化学/物理稳定性、高耐热性和耐腐蚀性、大的比表面积、超高的导电性、催化活性、光学性能、强机械强度和良好的生物相容性等优异性能,自问世以来就被认为是开发高性能传感器的良好候选材料[53-54]。
碳基纳米材料,尤其是碳纳米管(CNTs)、石墨烯(GR)、有序介孔碳(OMCs)等,由于具有高表面积、可接受的生物相容性、化学和电化学稳定性以及良好的导电性,在电极设计的生物分析领域极具吸引力,基于碳纳米材料的传感器通常具有比传统传感器更高的灵敏度和更低的检测限[55]。近年碳纳米材料在“瘦肉精”的传感检测中应用也十分广泛。如图4所示,Liu等[56]借助多壁碳纳米管(MWNTs)建立了一种新的蛋白传感层的方法,首先利用MWNTs将羊抗鼠二抗固定于电极表面,然后通过抗体间的特异性识别作用将CLB的单抗组装于电极表面,利用辣根过氧化物酶(HRP)-CLB复合物与样品中游离的CLB竞争结合该单克隆抗体,测定HRP催化底物产生的电流信号变化来实现对目标物的检测,线性范围为0~10 ng/mL,检测限为0.1 ng/mL。该蛋白的组装方法与化学共轭法相比,具有较高的灵敏度和较好的重现性,响应时间短,且由于该传感装置携带了一次性丝网印刷电极,可满足现场快速检测的要求。
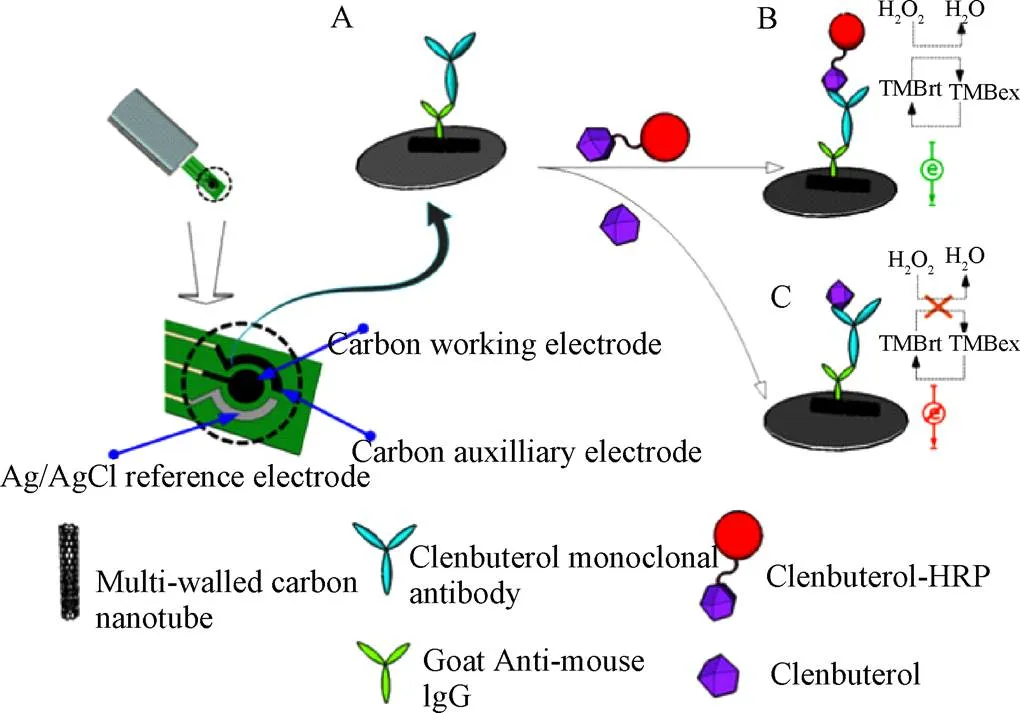
图4 基于多壁碳纳米管的电化学免疫传感器检测克伦特罗原理图[54]
陈昌云等[57]建立了一种基于碳纳米管和离子液体复合物修饰电极的免疫传感器检测莱克多巴胺的方法,将MWNTs与室温离子液体1-丁基-3-甲基咪唑四氟硼酸盐([BMIM]BF4)复合物同偶联了牛血清蛋白(BSA)的RAC抗原,使用Nafion一同修饰在玻碳电极表面,利用RAC抗体和抗原之间特定反应的竞争模式,以K3Fe(CN)6为探针, 通过循环伏安法和差分脉冲伏安法监测反应信号,实现对溶液中RAC的浓度的检测.,该方法的线性范围1~1 500 ng/mL,检测限为0.3 ng/mL,对猪饲料样本进行检测,回收率在85%~99%之间,该免疫传感器的重复性、稳定性和再生良好。张媛媛等[58]利用改性的壳聚糖来包埋固定羧基化的MWNTs和RAC抗体,同样以K3Fe(CN)6为探针,构建了RAC的免疫传感检测方法,该方法的线性范围0.05~4.05 ng/mL,最低检测限为0.08 ng/mL。该法用于猪肉、羊肉等样品的检测,其结果与采用高效液相色谱法的国标方法一致,效果良好。由于MWNTs具有较小的粒径、大的比表面积以及表面的功能基团,它的应用一方面可以显著的提高特异性识别分子(如抗体、适配体等)的固载量;另一方面,多孔结构的碳纳米管良好的三维微环境也不会破坏抗体等的免疫活性;同时,适当的分子间的间隔也有助于提高抗体对目标物的选择性,最终提高了传感器的信噪比。但是,碳纳米管的自组装性能有时不够理想,稳定性还有待提高。
近年,单原子厚度的二维石墨烯因其机械强度高、光学性能可调、比表面积大、高电导性等特点引起了人们的广泛关注。石墨烯家族还包括结构和化学衍生物,如多层石墨烯、氧化石墨烯(GO)及还原石墨烯(rGO)等[59]。由于GO和rGO更易于表面修饰和可调节的导电性能,在生物传感领域应用更多[60-61]。Wu等[62]通过对比发现在玻碳、石墨烯、还原石墨烯及氧化石墨烯中,氧化石墨烯表现出更强的信号增强效应,可以大大提高RAC和CLE的氧化信号,进而利用GO建立了对RAC和CLE的电化学检测方法,通过检测脉冲伏安(DPV)信号获知目标物的浓度,二者的线性范围25~1 000 ng/mL,对应的检测限分别为17和15 ng/mL。该方法用于猪肉样本的检测,其添加回收介于90.1%~98.6%之间,效果较好。Lin等[63]在修饰有石墨烯-Nafion膜的玻碳电极上电聚合酸性铬兰K,实现了8种“瘦肉精”成分的高灵敏检测,方法的检测范围为1.0~36.0 ng/mL,检测限为0.58~1.46 ng/mL,对实际样品猪肉添加CLE进行检测,添加水平为0.01和0.02g/g,对应的回收率为89.2%和91.3%。结果表明,该方法能较好地测定猪肉中克伦特罗的含量。Wang等[64]制备了异丙醇-Nafion膜-4-乙烯苯磺酸钠-石墨烯纳米复合材料用以修饰玻碳电极,构建了用于猪肉中CLE检测的灵敏伏安传感器,该方法的线性范围为7.5×10-8~2.5×10-5mol/L,检测限为2.2×10-8mol/L。该方法中制备的修饰电极,稳定性和重现性良好,4 ℃条件下可以保存一周,这在实际应用过程中将提供极大便利。如图5所示,Wang等[65]报道了一种利用竞争策略快速同时分析RAC、CLE和SALSAL的多路电化学生物传感器。试验首先将rGO固定在丝网印刷工作电极表面,然后将3种物质的人工抗原分别固定在3个电极表面,采用银钯合金来标记RAC、CLE和SAL的对应抗体,利用竞争策略,同时检测RAC、SAL和CLE,相邻电极之间不存在干扰,实现了对猪肉中3种物质的同时检测,三者的检测范围介于0.01~100 ng/mL之间,对应的检测限分别为1.52、1.44及1.38 pg/mL。该方法消除了酶和介质可能带来的负面影响,降低了检测成本。此外,丝网印刷电极价格低廉,易于实现商业化,有望成为一种新的食品安全快速检测方法。Bai等[66]制备了分散性良好的石墨烯,并将金纳米棒自组装在石墨烯表面,将二者的复合物修饰在玻碳电极表面,构建了RAC电化学传感器,该传感器检测RAC的线性检测范围为1×10-9~2.7×10-6mol/L,检测限为5.1×10-10mol/L。该方法灵敏度高,操作简便,对猪尿样本进行检测其回收率介于99.2%~107.3%之间,效果良好。Jin等[67]将rGO电沉积在玻碳电极上,借助上转化纳米粒子(UCNPs),构建了超灵敏的分子印迹电化学发光传感器检测克伦特罗,该传感器具有良好的灵敏度、选择性和稳定性,方法的线性范围为10~100mol/L,最低检测限6.3 nmol/L。在对实际样本猪肉,肝和肾的检测中,其回收率介于89.0%~100.4%,在食品安全检测领域具有广阔的应用前景。同时,在该研究中,rGO不仅作为固定UCNPs的载体,而且由于其高导电性、优越的电子传输速率和较大的比表面积,对提高UCNPs的电化学发光响应具有显著的影响。Li等[68]开发了基于纳米金修饰的Fe3O4/GR分子印迹聚合物电化学传感器,用于水中痕量RAC的高选择性、高灵敏的检测,该方法的线性检测范围为0.002~0.1mol/L,最低检测限可达 0.02 nmol/L。Zhai等[69]将GR与MWNTs联合应用,采用水分散磺化石墨烯片(SGSs)和氧功能化多壁碳纳米管(MWCNS-COOH)组成的复合纳米材料修饰玻璃碳电极,制备了一种新型灵敏的电化学传感器检测肝组织样本中的克伦特罗,该传感器的线性检测范围为0.01~5.0mol/L,检测限为4.6 nmol/L。基于以上研究表明,rGO和GO是理想的石墨烯类似物,可用于生物传感器的制备,并且研究发现由于石墨烯的表面官能团能够参与生物识别元素的共价固定,还可用于ssDNA、适配体和抗体的优良载体[70]。
在碳纳米材料中,孔径在2~50 nm的有序介孔碳(OMCs)具有比表面积高、颗粒外形丰富、孔径均一可调,化学稳定性好、以及导电性良好在生物传感器、电容器电极、催化载体及吸附材料等方面有着良好的应用前景[71-72]。Yang等[73]利用OMCs修饰玻璃碳电极,研制了一种灵敏的电化学传感器来检测RAC。试验结果表明OMCs可以显著提高电催化活性,使峰值电流显著增加,进而实现对RAC的检测,该方法的线性检测范围为0.085~8.0mol/L,检测限为0.06mol/L,且对RAC具有良好的灵敏度和选择性。对实际样品猪肉进行检测,回收率在96.6%~104.5%之间,对传感器的进一步开发具有重要的实用价值。Ma等[74]利用OMCs和AuNPs修饰丝网印刷电极,并结合免疫印迹技术制备了一次性电化学传感器,用于RAC的测定,该方法的线性检测范围为5.0×10-11~1.0×10-9mol/L,检测限为4.23×10-11mol/L。试验结果表明,该传感器具有快速的平衡孵育时间(100 s),对RAC有高亲和力以及选择性,实际样品猪尿的回收率介于95.7%~99.3%。表2整理了以上基于碳纳米材料的“瘦肉精”检测方法的相关文献。
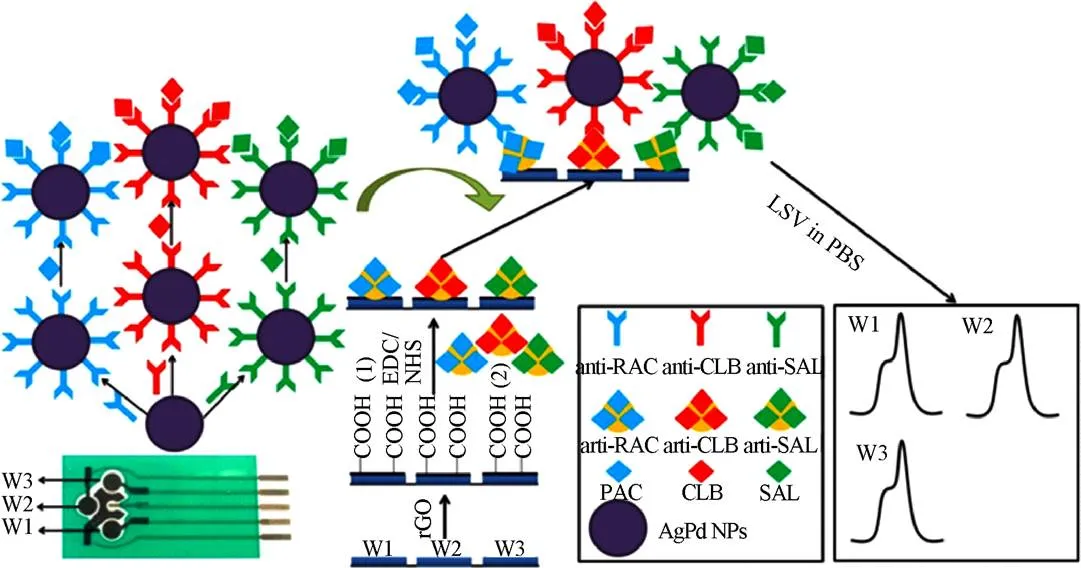
图5 基于还原氧化石墨烯的电化学生物传感器同时检测RAC、克伦特罗和沙丁胺醇原理图[63]
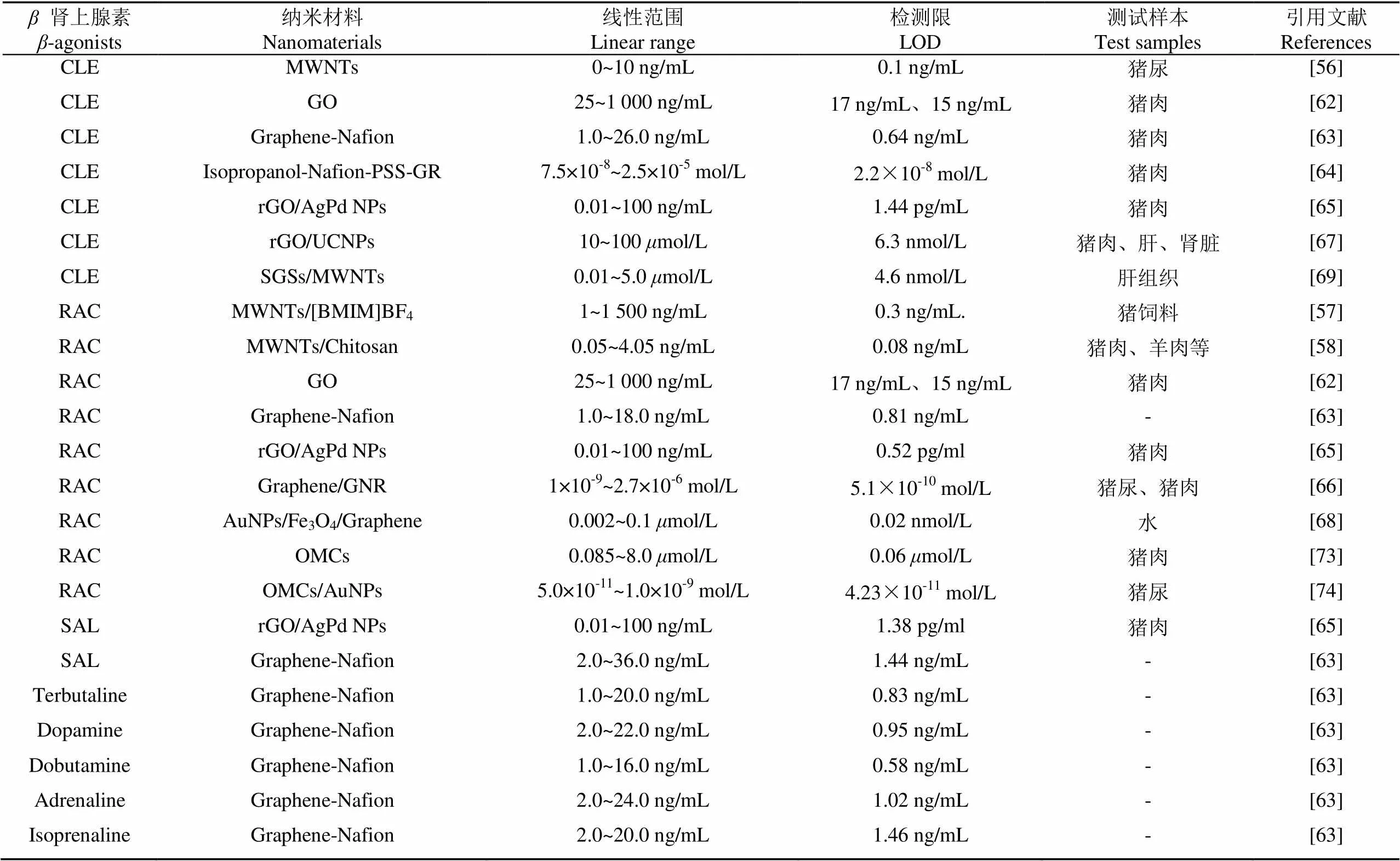
表2 基于碳纳米材料的“瘦肉精”检测方法对比
3 基于量子点的传感检测技术
量子点(quantum dots, QDs),也称半导体纳米晶体,直径约为2~10 nm,具有光稳定性好、宽激发谱和窄发射谱,以及生物相容性好、荧光寿命长等独特的性质,在物理、化学、生物等诸多领域得到了广泛的应用[75-76]。量子点组成元素在元素周期表中通常可以划分为II-VI、III-V或IV-VI族,同时也可以由2种或2种以上的复合材料形成核壳结构,如CdTe、CdSe/ZnS以及CdSe/ZnSe/ZnS等[77-78]。目前,在“瘦肉精”检测领域中与电化学发光方法联合应用组成高灵敏度的量子点-电化学发光传感器应用较多,建立了诸多基于CdSe量子点与其他纳米材料联合应用的传感检测技术。如图6所示,Yao等[79]建立了基于CdSe QDs的盐酸克伦特罗的ECL免疫传感器。通过层层组装的方式将量子点、人工抗原及抗体组装至电极表面,进步一步结合酶的放大作用大大提高了传感器的检测灵敏度,方法的线性范围介于0.05~1 000 ng/mL之间,最低检出限为0.02 ng/mL。该传感器具有良好的稳定性和重现性,应用于实际样品猪肉和肝脏的检测,回收率为82.6%~108.3%,表明该免疫传感器在实际样品的分析中具有一定的适用性。Yan等[46]以AuNPs作为反应基底,将CLE抗体固定于CdSe量子点上,通过竞争法实现对克伦特罗的超灵敏检测,该方法线性范围为0.02~50 ng/mL,检测限为0.008 4 ng/mL。Dong等[80]同样利用AuNPs和CdSe量子点,建立了检测新型“瘦肉精”溴布特罗的电化学发光免疫传感器,方法的线性范围为0.01~1 000 ng/mL,检测限为0.003 ng/mL。同时,该传感器的特异性和稳定性良好,将其应用于实际样品中猪肉和饲料的测定,回收率为87%~111%,结果令人满意。Zhu等[48]将AuNPs、石墨烯复合同量子点CdSe进行组合,构建了电化学发光免疫传感器实现RAC的检测,该方法的线性范围为0.01~1 000 ng/mL,最低检测限为2.6 pg/mL。Raksawong等[81]利用杂交分子印迹聚合物(MIP)包覆CdTe量子点,开发了检测SAL荧光免疫传感器,实现了动物饲料和肉中SAL的高灵敏和特异性的检测,方法的线性范围为0.10~25.0g/L,检测限为0.034g/L。在测试的3种动物饲料(猪、牛、禽)和2种肉类(猪肉和牛肉)的所有样本的添加回收介于85.1%~98.0%,为复杂样本中SAL的检测提供了一种可靠的方法。Tang等[82]利用CdSe量子点,并借助HRP的放大作用,建立了苯乙醇胺A的电化学发光免疫检测方法,该方法的线性范围0.05~1 000 ng/mL,最低检测限为15 pg/mL,在实际样本猪肉和猪肝检测中,加标回收率为85.6%~110.4%,并与HPLC的检测结果进行了对比,结果表明两者间的检测结果无显著差别,可以满足实际样本的检测需求。Dong等[83]利用AuNPs及经SiO2包覆的CdSe量子点建立了双重信号放大的电化学发光免疫传感器实现了对猪肉和饲料中SAL的超灵敏检测,方法的线性范围是0.001~1 000 ng/mL,检测限为0.17 pg/mL,在实际样品猪肉和饲料中,回收率为83%~116%。该方法操作稳定,灵敏度高,选择性好,同时为其他小分子的分析检测提供了技术参考,具有更广泛的应用价值。Hu等[84]将一种新型的锌基有机框架(ZnMOFs)同rGO及CdTe量子点结合,将CdTe量子点固定于rGO上,用于提高反应信号,构建电化学发光传感器实现了克伦特罗的高灵敏检测,该方法的线性范围为3.0×10-13~6.0×10-10mol/L,最低检测限为1.0×10-13mol/L。该传感器具有良好的重复性和稳定性,在猪肉样本的检测中,添加回收为94.0%~102%。同时,该研究中采用的ZnMOF-rGO-CdTe QDs杂化技术为CLB检测提供了新的方法。表3整理了以上基于量子点纳米材料的“瘦肉精”检测方法的相关文献。
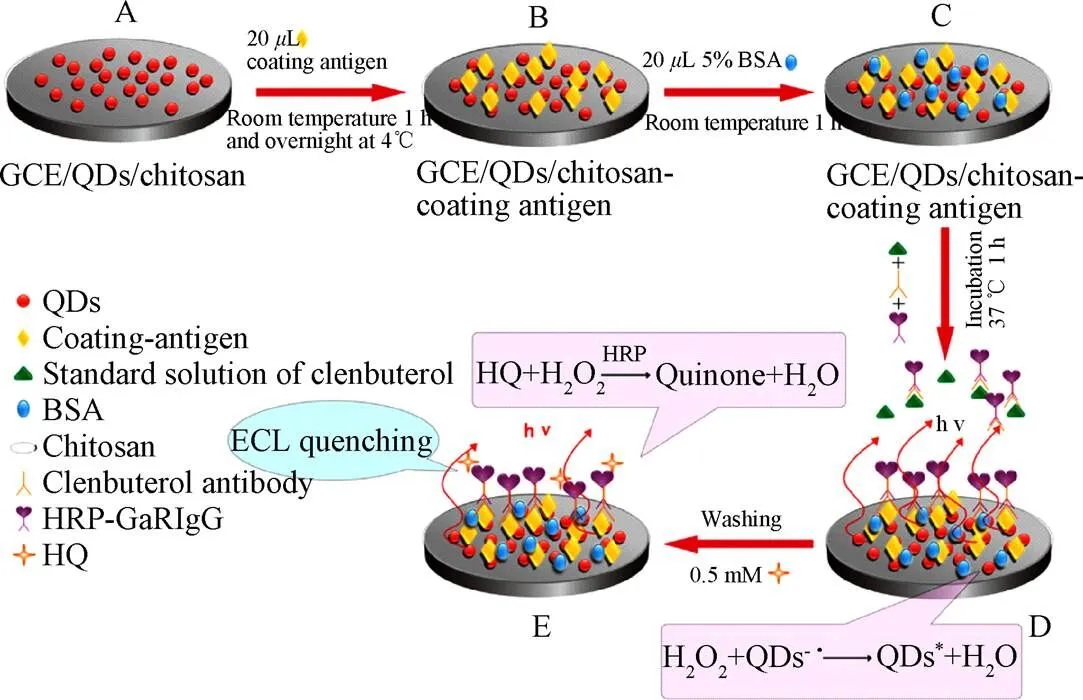
图6 基于量子点的电化学发光免疫传感器检测克伦特罗原理图[77]
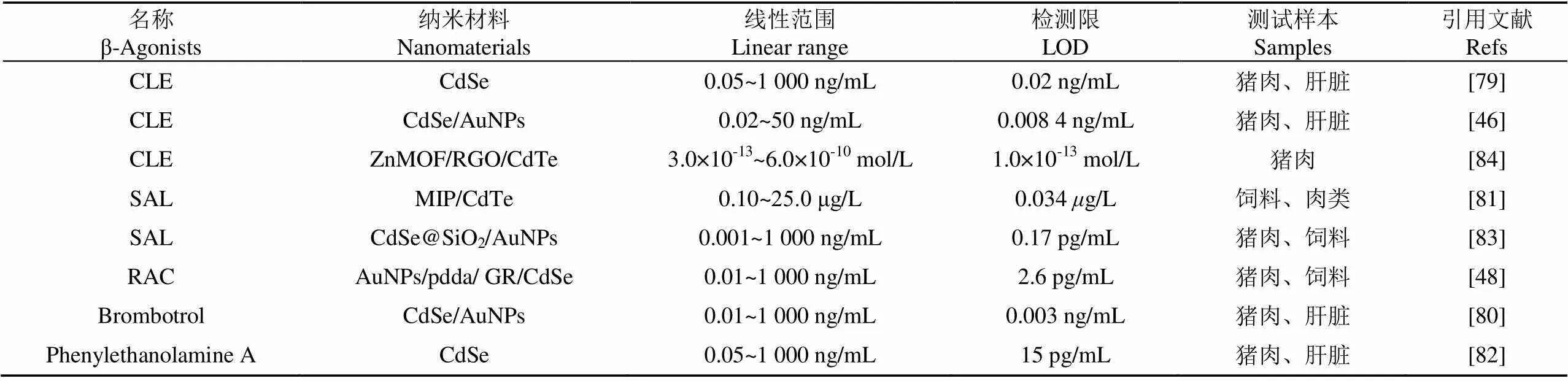
表3 基于量子点纳米材料的“瘦肉精”检测方法对比
4 其他新型纳米材料
磷烯(Phosphorene)又称黑磷烯或二维黑磷,是由块体黑磷剥离成的具有原子层厚度的二维层状新型半导体材料,具有能带可调的直接带隙结构和高载流子迁移率以及良好的导电导热能力等独特的性质,在电子和光电应用领域具有独特的优势,现已在晶体管、光通讯、能源、生物医学等领域显示出极大的应用潜力,自2014年出现以来就备受关注[85-88]。如图7所示,Ge等[89]制备了磷烯-Nafion的纳米复合材料,并将其修饰于玻碳电极表面,构建了检测CLE伏安传感器,方法的线性范围为0.06~24mol/L,检测限3.7 nmol/L,该传感器应用于牛肉和牛血清中CLB的测定,当添加量为9mol时,二者的添加回收分别为102.22%和100.33%。该研究中制备的纳米复合材料与以往报道的磷烯不同,该纳米复合材料对水和氧具有很好的稳定性,使其可以成功地应用于电化学生物传感器领域。

图7 基于黑色磷烯纳米复合材料的伏安传感器检测克伦特罗原理图[89]
Janus粒子是表面由2个或2个以上不同物理性质的成分构成的特殊类型的纳米颗粒,可以将2种具有相反作用功能基团组装于表面,如两亲性Janus粒子是在对立面分别含有亲水基、疏水基2种不同化学基团[90]。Janus粒子独特的结构和组成多样性使其表现出特殊的性能,这种非对称材料可以提高表面活性、催化或生物传感器的性能,作为一种新型纳米材料受到广泛的关注[91]。如图8所示,Zhou等[92]建立了基于适配体和Janus粒子的电化学传感器,该传感器首先将通过Janus粒子的疏水结构将其固定于玻碳电极表面,另一侧的亲水基团用于金纳米粒子的组装,进而与目标物的适配体连接,实现对RAC的检测。在此过程中,Janus粒子可以增加电极上生物分子的负载量,从而放大电化学反应的信号。采用DPV法对RAC浓度进行定量分析,根据RAC的浓度建立起2条检测曲线,其检测范围分别是1.0×10−13~1.0×10−11mol/L及1.0×10−11~1.0×10−7mol/L,方法的检测限为3.3×10−14mol/L,与其他方法相比,该检测限是目前报道的有关RAC功能化电极的最低检测限。该方法用于人尿样本的检测,添加回收率为94.3%~103.0%,与LC-MS/MS的结果有很好的一致性,说明所建立的方法可用于测定人体尿液中RAC的含量。同时,该研究中对于Janus粒子的开发和应用也为其他分析检测提供一种通用的思路参考。
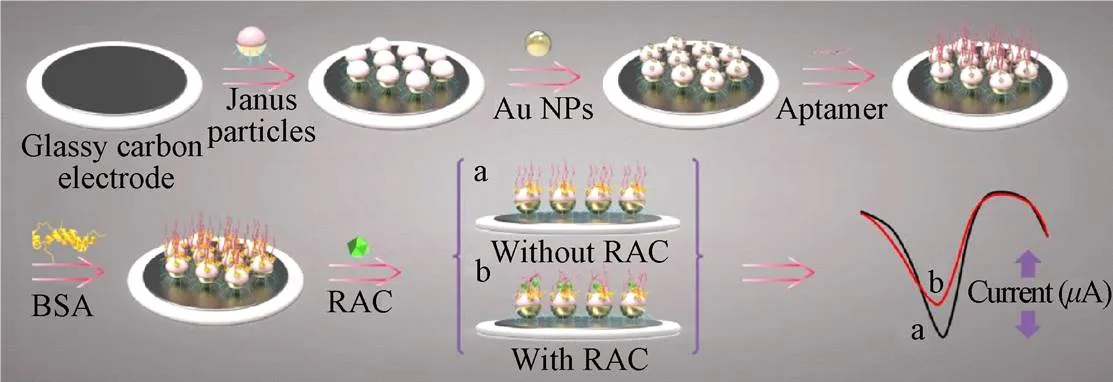
图8 基于Janus颗粒的电化学适配体传感器检测莱克多巴胺原理图[90]
铈(Ce)元素是稀土元素中含量最丰富的元素,二氧化铈(CeO2)是铈最稳定的氧化物。CeO2纳米晶体为萤石状结构,晶格内部的氧原子极易脱出形成氧空位,随着氧空位的生成,与其相连的2个Ce+被还原成Ce3+[93],因此,CeO2具有非常强大的储氧和放氧能力,且在该过程中,其晶体结构保持不变,是一种非常理想的循环材料[94-95]。同时,CeO2超强的氧释放能力也使其成为优秀的催化材料,在催化剂、新能源电池、传感器、半导体等行业有着广泛应用。Xiao等[96]采用热处理方法合成了具有明显电催化活性的多层结构的CeO2纳米颗粒,该纳米颗粒经nafion修饰后固定于玻碳电极表面,建立了克伦特罗的电化学传感器,该方法的线性范围0.39~2.79 mmol/L,检测限为0.08 mmol/L,添加试验结果表明,在猪尿样本中回收为率91.59%~102.53%,适于实际样本的检测。同时,该传感器具有良好的稳定性和重现性,在食品安全分析和药物残留微量分析领域具有广阔的应用前景。表4整理了基于其他新型纳米材料的“瘦肉精”检测方法的相关文献。
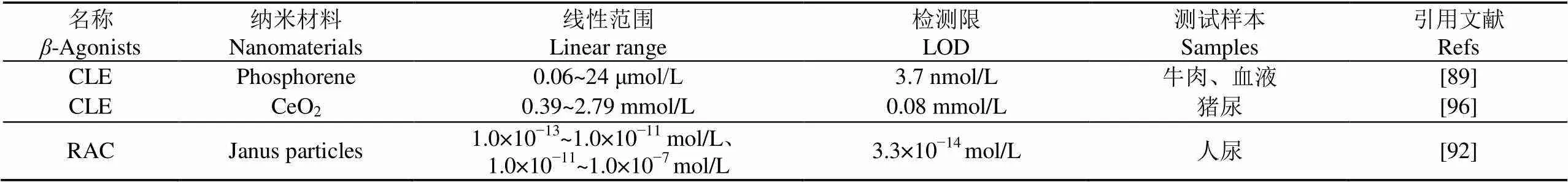
表4 基于其他新型纳米材料的“瘦肉精”检测方法对比
5 结论与展望
本文综述了近年来基于纳米材料的“瘦肉精”传感器技术的研究进展,重点围绕金纳米材料、碳质纳米材料、量子点以及其他新型纳米材料进行了介绍。总体来说,通过纳米材料及复合纳米材料的使用,在作为反应基底、装载特异性识别分子、提高导电性能、表面增强效应、信号传导性能、催化性能及良好的生物相容性等方面对传感器的灵敏度、重现性以及响应时间等方面具有巨大的提升作用。
未来,为使传感检测技术朝着更加灵敏、选择性更好、操作更简便、成本更低的方向发展,对于纳米材料的种类及其应用方式还有待进一步提高:
1)现有纳米材料性能需要提升,特别是对于无标签的纳米传感器,其对目标物的特异性还有待提升,否则很难适应实际检测的需要。
2)开发、筛选新的功能纳米材料,一方面开发全新种类的新型纳米材料,扩大传感领域可供选择纳米材料的范围,另一方面,对现有纳米材料进行改进,合成包含2种或2种以上元素种类的纳米材料以及具有特殊结构的纳米材料,获得具有优越性能的复合纳米材料,以金纳米粒子为例,如金-银双金属纳米粒子、金-金属氧化物复合物,金-碳纳米管纳米复合材料,金-石墨烯/氧化石墨烯复合材料、核-壳金包覆磁性纳米粒子(如Fe3O4-AuNPs)等,从而获得具有全新性能的纳米材料。
3)研究纳米材料的增强机制,并对识别分子的修饰方法进行创新,现有已明晰的纳米材料对信号的增强放大效应包括其良好的生物相容性、表面效应、宏观量子隧道效应、高比表面积和高表面能等,未来不断探索新的增强机制有利于纳米材料性能的进一步提升,同时,新的具有潜力的修饰方法如层层自组装法、等离子体聚合膜技术以及利用蛋白质工程技术直接实现抗体等的定向固定,这些方法的应用必将为传感器的性能提升提供巨大的空间。
今后,随着纳米技术不断取得新的进展,传感器将朝着以下几个方面发展:更加集成及微型化,便于携带,以利于现场和野外等场景的特殊需求;自动化程度更高,可以实现实时在线的检测,及自动化分析;高通量及多组分并行检测,以利于规模化的筛查应用。同时,将纳米材料同新型检测技术相结合,不断满足质检领域的新要求。
[1] Blanca J, Munoz P, Morgado M, et al. Determination of clenbuterol, ractopamine and zilpaterol in liver and urine by liquid chromatography tandem mass spectrometry[J]. Analytica Chimica Acta, 2005, 529(1): 199-205.
[2] Strydom P E, Frylinck L, Montgomery J L, et al. The comparison of three beta-agonists for growth performance, carcass characteristics and meat quality of feedlot cattle[J]. Meat Science, 2009, 81(3): 557-564.
[3] Zhang Y T, Zhang Z J, Sun Y H, et al. Development of an analytical method for the determination of2-agonist residues in animal tissues by high-performance liquid chromatography with on-line electrogenerated [Cu(HIO6)2]5--luminol chemiluminescence detection[J]. Journal of Agricultural and Food Chemistry, 2007, 55(13): 4949-4956.
[4] Pulce D, Lamaison G, Keck C. Collective human food poisonings by clenbuterol residues in veal liver, Vet. Hum[J]. Toxicol. 1991, 33: 480-481.
[5] Courtheyn D, Bizec B L, Brambilla G, et al. Recent developments in the use and abuse of growth promoters[J]. Analytica Chimica Acta, 2002, 473(1/2): 71-82.
[6] Barbosa J, Cruz C, Martins J, et al. Food poisoning by clenbuterol in Portugal[J]. Food Additives and Contaminants, 2005, 22(6): 563-566.
[7] Morales-Trejo F, León S V Y, Escobar-Medina A, et al. Application of high-performance liquid chromatography-UV detection to quantification of clenbuterol in bovine liver samples[J]. Journal of Food and Drug Analysis, 2013, 21(4): 414-420.
[8] Yan K, Zhang H, Hui W, et al. Rapid screening of toxic salbutamol, ractopamine, and clenbuterol in pork sample by high-performance liquid chromatography-UV method[J]. Journal of Food and Drug Analysis, 2016, 24(2): 277-283.
[9] He L M, Su Y J, Zeng Z L, et al. Determination of ractopamine and clenbuterol in feeds by gas chromatography–mass spectrometry[J]. Animal Feed Science and Technology, 2007, 132(3/4): 316-323.
[10] Daniele D C, Veronica M, Marco P, et al. Simultaneous determination of2-agonists in human urine by fast-gas chromatography/mass spectrometry: Method validation and clinical application[J]. Biomedical Chromatography BMC, 2010, 24(4): 358-366.
[11] Gallo P, Brambilla G, Neri B, et al. Purification of clenbuterol-like2-agonist drugs of new generation from bovine urine and hair by alpha1-acid glycoprotein affinity chromatography and determination by gas chromatography- mass spectrometry[J]. Analytica Chimica Acta, 2007, 587(1): 67-74.
[12] Guo C C, Shi F, Gong L P, et al. Ultra-trace analysis of 122-agonists in pork, beef, mutton and chicken by ultrahigh- performance liquid-chromatography-quadrupole-orbitrap tandem mass spectrometry[J]. Journal of Pharmaceutical and Biomedical Analysis, 2015, 107: 526-534.
[13] Mastrianni K R, Metavarayuth K, Brewer W E, et al. Analysis of 10 beta-agonists in pork meat using automated dispersive pipette extraction and LC-MS/MS[J]. Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences, 2018, 1084: 64-68.
[14] Xiong L, Gao Y Q, Li W H, et al. Simple and sensitive monitoring of2-agonist residues in meat by liquid chromatography-tandem mass spectrometry using a QuEChERS with preconcentration as the sample treatment[J]. Meat Science, 2015, 105: 96-107.
[15] Us M F, Alshana U, Lubbad I, et al. Dispersive liquid-liquid microextraction based on solidification of floating organic drop combined with field-amplified sample injection in capillary electrophoresis for the determination of beta(2)-agonists in bovine urine[J]. Electrophoresis, 2013, 34(6): 854-861.
[16] Jin W, Xu X, Jiang L. Determination of-agonists in pig feed, pig urine and pig liver using capillary electrophoresis with electrochemical detection[J]. Meat Science, 2010, 85(2): 302-305.
[17] Ji X, He Z, Ai X, et al. Determination of clenbuterol by capillary electrophoresis immunoassay with chemiluminescence detection[J]. Talanta, 2006, 70(2): 353-357.
[18] Jiang D N, Cao B Y, Wang M Y, et al. Development of a highly sensitive and specific monoclonal antibody based enzyme-linked immunosorbent assay (ELISA) for the detection of a new-agonist phenylethanolamine a in food samples[J]. Journal of the Science of Food & Agriculture, 2016, 97(3): 1001-1009.
[19] Ma L, Nilghaz A, Choi J R, et al. Rapid detection of clenbuterol in milk using microfluidic paper-based ELISA [J]. Food Chemistry, 2018, 246: 437-441.
[20] Zhang M Z, Wang M Z, Chen Z L. et al. Development of a colloidal gold-based lateral-flow immunoassay for the rapid simultaneous detection of clenbuterol and ractopamine in swine urine[J]. Analytical & Bioanalytical Chemistry, 2009, 395(8): 2591-2599.
[21] Zhu G, Hu Y, Gao J, et al. Highly sensitive detection of clenbuterol using competitive surface-enhanced Raman scattering immunoassay[J]. Analytica Chimica Acta, 2011, 697(1/2): 61-66.
[22] Du W, Lei C M, Zhang S R, et al. Determination of clenbuterol from pork samples using surface molecularly imprinted polymers as the selective sorbents for microextraction in packed syringe[J]. Journal of Pharmaceutical and Biomedical Analysis, 2014, 91: 160-168.
[23] Peñuela-Pinto O, Armenta S, Esteve-Turrillas F A, et al. Selective determination of clenbuterol residues in urine by molecular imprinted polymer-Ion mobility spectrometry[J]. Microchemical Journal, 2017, 134: 62-67.
[24] Wong C S, Chen Y D, Chang J L, et al. Biomolecule-free, selective detection of clenbuterol based on disposable screen-printed carbon electrode[J]. Electrochemistry Communications, 2015, 60: 163-167
[25] Feng F, Zheng J, Qin P, et al. A novel quartz crystal microbalance sensor array based on molecular imprinted polymers for simultaneous detection of clenbuterol and its metabolites[J]. Talanta, 2017, 167: 94-102.
[26] Liu Y J, Liu Y X, Qiao L, et al. Advances in signal amplification strategies for electrochemical biosensing[J]. Current Opinion in Electrochemistry, 2018, 12: 5-12.
[27] Xiao T, Huang, J S, Wang, D W, et al. Au and Au-Based nanomaterials: Synthesis and recent progress in electrochemical sensor applications[J/OL]. Talanta, 2020, 206: 120-210.
[28] Wang P L, Lin Z Y, Su X O, et al. Application of Au based nanomaterials in analytical science[J]. Nano Today, 2017, 12: 64-97.
[29] Cobley C M, Chen J, Cho E C, et al. Gold, nanostructures: a class of multifunctional materials for biomedical applications[J]. Chemical Society Reviews, 2010, 40(1): 44-56.
[30] Beloglazkina E K, Majouga A G, Romashkina R B, et al. Gold nanoparticles modified with coordination compounds of metals: Synthesis and application[J]. Russian Chemical Reviews, 2012, 81(1): 65-90.
[31] Kim Z H, Leone S R. High-resolution apertureless near-field optical imaging using gold nanosphere probes[J]. The Journal of Physical Chemistry B, 2006, 110(40): 19804-19809.
[32] Zhang L, Xia K, Lu Z, et al. Efficient and facile synthesis of gold nanorods with finely tunable plasmonic peaks from visible to Near-IR range[J]. Chemistry of Materials, 2014, 26(5): 1794-1798.
[33] Bhattarai S R, Derry P J, Aziz K, et al. Gold nanotriangles: scale up and X-ray radiosensitization effects in mice[J]. Nanoscale, 2017, 9(16): 5085-5093.
[34] Gong S, Schwalb W, Wang Y, et al. A wearable and highly sensitive pressure sensor with ultrathin gold nanowires[J]. Nature Communications, 2014, 5: 3132-3141.
[35] Thiele M, Soh J.Z.E, Knauer A, et al. Gold nanocubes-Direct comparison of synthesis approaches reveals the need for a microfluidic synthesis setup for a high reproducibility[J]. Chemical Engineering Journal, 2016, 288: 432-440.
[36] Ghosh S K, Pal T. Interparticle coupling effect on the surface plasmon resonance of gold nanoparticles: From theory to applications[J]. Chemical Reviews, 2007, 107(11): 4797-4862.
[37] 王慎. 基于金纳米材料传感器的构建及应用研究[D]. 新乡:河南师范大学,2018.
Wang Shen. Preparation of Gold Nanomaterials and Their Analytical Applications[D]. Xinxiang: Henan Normal University, 2018. (in Chinese with English abstract)
[38] Wang P L, Su X O, Shi L Y, et al. An aptamer based assay for the-adrenergic agonist ractopamine based on aggregation of gold nanoparticles in combination with a molecularly imprinted polymer[J]. Microchimica Acta, 2016, 183(11): 2899-2905.
[39] Simon T, Shellaiah M, Steffi P, et al. Development of extremely stable dual functionalized gold nanoparticles for effective colorimetric detection of clenbuterol and ractopamine in human urine samples[J]. Analytica Chimica Acta, 2018, 1023: 96-104.
[40] Peng T, Wang J Y, Zhao S J, et al. Highly luminescent green-emitting Au nanocluster-based multiplex lateral flow immunoassay for ultrasensitive detection of clenbuterol and ractopamine[J]. Analytica Chimica Acta, 2018, 1040: 143-149.
[41] Li L, Steiner U, Mahajan S. Single nanoparticle sers probes of ion intercalation in metal-oxide electrodes[J]. Nano Letters, 2014, 14(2): 495-498.
[42] Zhu G C, Hu Y J, Gao J, et al. Highly sensitive detection of clenbuterol using competitive surface-enhanced Raman scattering immunoassay[J]. Analytica Chimica Acta, 2011, 697(1): 61-66.
[43] Cheng J, Wang S, Zhang S, et al. Rapid and sensitive determination of clenbuterol residues in animal urine by surface-enhanced Raman spectroscopy[J]. Sensors and Actuators B: Chemical, 2019, 279: 7-14.
[44] Varmira K, Saed-mocheshi M, Jalalvand A R. Electrochemical sensing and bio-sensing of bisphenol A and detection of its damage to DNA: A comprehensive review[J]. Sensing and Bio-Sensing Research, 2017, 15: 17-33.
[45] Ju H X. Functional nanomaterials and nanoprobes for amplified biosensing[J]. Applied Materials Today, 2018, 10: 51-71.
[46] Yan P P, Tang Q H, Deng A P, et al. Ultrasensitive detection of clenbuterol by quantum dots based electrochemiluminescent immunosensor using gold nanoparticles as substrate and electron transport accelerator[J]. Sensors and Actuators B: Chemical, 2014, 191: 508-515.
[47] Yang F, Wang P L, Wang R G, et al. Label free electrochemical aptasensor for ultrasensitive detection of ractopamine[J]. Biosensors and Bioelectronics, 2016, 77: 347-352.
[48] Zhu Q, Liu H X, ZHang J, et al. Ultrasensitive QDs based electrochemiluminescent immunosensor for detecting ractopamine using AuNPs and Au nanoparticles@ PDDA-graphene as amplifier[J]. Sensors and Actuators B: Chemical, 2017, 243: 121-129.
[49] Li Z Y, Wang Y H, Kong W J, et al. Highly sensitive near-simultaneous assay of multiple “lean meat agent” residues in swine urine using a disposable electrochemiluminescent immunosensors array[J]. Biosensors and Bioelectronics, 2013, 39(1): 311-314.
[50] 唐伟. 基于碳纳米材料的柔性气体传感器的研究进展[J]. 硅酸盐通报,2019,38(2):398-409.
Tang Wei. Research progress of flexible gas sensors based on carbon nanomaterials[J]. Bulletin of the Chinese Ceramic Society, 2019, 38(2): 398-409. (in Chinese with English abstract)
[51] Angione M D, Pilolli R, Cotrone S, et al. Carbon based materials for electronic bio-sensing[J]. Materials Today, 2011, 14(9): 424-433.
[52] Muniandy S, Teh S J, Thong K L, et al. Carbon nanomaterial-based electrochemical biosensors for foodborne bacterial detection[J]. Critical Reviews in Analytical Chemistry, 2019: 1-24. DOI: 10.1080/10408347.2018. 1561243.
[53] Yao Y, Ping J F. Recent advances in graphene-based freestanding paper-like materials for sensing applications[J]. TrAC Trends in Analytical Chemistry, 2018, 105: 75-88.
[54] Zhao F N, Wu J, Ying Y B, et al. Carbon nanomaterial-enabled pesticide biosensors: Design strategy, biosensing mechanism, and practical application[J]. TrAC Trends in Analytical Chemistry, 2018, 106: 62-83.
[55] Zhu Z, Garcia-Gancedo L, Flewitt A J, et al. A Critical review of glucose biosensors based on carbon nanomaterials: Carbon nanotubes and graphene [J]. Sensors, 2012, 12(5): 5996-6022.
[56] Liu G, Chen H D, Peng H Z, et al. A carbon nanotube-based high-sensitivity electrochemical immunosensor for rapid and portable detection of clenbuterol[J]. Biosensors and Bioelectronics 2011, 28(1): 308-313.
[57] 陈昌云,张红琳,柳闽生,等. 基于碳纳米管和离子液体复合物修饰电极的免疫传感器检测莱克多巴胺[J]. 化学学报,2011,69(23):2865-2869.
Chen Changyun, Zhang Honglin, Liu Minsheng, et al. An electrochemical immunosensor for the determination of ractopamine based on multi-walled carbon nanotubes and ionic liquid composite[J]. Acta Chimica Sinica, 2011, 69(23): 2865-2869. (in Chinese with English abstract)
[58] 张媛媛,王瑞鑫,李秋薇,等. 基于多壁碳纳米管免疫传感器法快速测定食品中的莱克多巴胺[J]. 食品科学,2016,37(8):170-175.
Zhang Yuanyuan, Wang Ruixin, Li Qiuwei, et al. An Immunosensor for rapid determination of ractopamine in foods based on multi-walled carbon nanotubes[J]. Food Science, 2016, 37(8): 170-175. (in Chinese with English abstract)
[59] Tiwari J N, Vij V, Kemp K C, et al. Engineered carbon-nanomaterial based electrochemical sensors for biomolecules[J]. Acs Nano, 2015, 10(1): 46-80.
[60] Zhang J, Zhang F, Yang H, et al. Graphene oxide as a matrix for enzyme immobilization[J]. Langmuir, 2010, 26(9): 6083-6085.
[61] Kang S M, Park S, Kim D, et al. Simultaneous reduction and surface functionalization of graphene oxide by mussel-inspired chemistry[J]. Advanced Functional Materials, 2011, 21(1): 108-112.
[62] Wu C, Sun D, Li Q, et al. Electrochemical sensor for toxic ractopamine and clenbuterol based on the enhancement effect of graphene oxide[J]. Sensors and Actuators B: Chemical, 2012, 168: 178-184.
[63] Lin X Y, Ni Y N, Kokot S. A novel electrochemical sensor for the analysis of-agonists: The poly (acid chrome blue K)/ graphene oxide-nafion/glassy carbon electrode[J]. Journal of Hazardous Materials, 2013, 260: 508-517.
[64] Wang L, Yang R, Chen J, et al. Sensitive voltammetric sensor based on Isopropanol-Nafion-PSS-GR nanocomposite modified glassy carbon electrode for determination of Clenbuterol in pork[J]. Food Chemistry, 2014, 164: 113-118.
[65] Wang H, Zhang Y, Li H, et al. A silver-palladium alloy nanoparticle-based electrochemical biosensor for simultaneous detection of ractopamine, clenbuterol and salbutamol[J]. Biosensors and Bioelectronics, 2013, 49: 14-19.
[66] Bai W Q, Huang H Y, Li Y, et al. Direct preparation of well-dispersed graphene/gold nanorod composites and their application in electrochemical sensors for determination of ractopamine[J]. Electrochimica Acta, 2014, 117: 322-328.
[67] Jin X C, Fang G Z, Pan M F, et al. A molecularly imprinted electrochemiluminescence sensor based on upconversion nanoparticles enhanced by electrodeposited rGO for selective and ultrasensitive detection of clenbuterol[J]. Biosensors and Bioelectronics, 2018, 102: 357-364.
[68] Li Y, Xu W K, Zhao X R, et al. Electrochemical sensors based on molecularly imprinted polymer on Fe3O4/graphene modified by gold nanoparticles for highly selective and sensitive detection of trace ractopamine in water[J]. The Analyst, 2018, 143: 5094-5102.
[69] Zhai H Y, Liu Z P, Chen Z G, et al. A sensitive electrochemical sensor with sulfonated graphene sheets/oxygen-functionalized multi-walled carbon nanotubes modified electrode for the detection of clenbuterol[J]. Sensors and Actuators B: Chemical, 2015, 210: 483-490.
[70] Muniandy S, Teh S J, Thong K L, et al. Carbon nanomaterial-based electrochemical biosensors for foodborne bacterial detection[J]. Critical Reviews in Analytical Chemistry, 2019: 1-24.
[71] Ma T Y, Liu L, Yuan Z Y. Direct synthesis of ordered mesoporous carbons[J]. Chemical Society Reviews, 2013, 42(9): 3977-4003.
[72] 康洪权. 有序介孔碳的功能化修饰及其电化学性能研究[D]. 青岛:青岛科技大学,2018.
Kang Hong Quan. Functional Modification of Ordered Mesoporous Carbon and Its Electrochemical Performance[D]. Qingdao: Qingdao University of Science & Technology, 2018. (in Chinese with English abstract)
[73] Yang X, Feng B, Yang P, et al. Electrochemical determination of toxic ractopamine at an ordered mesoporous carbon modified electrode[J]. Food Chemistry, 2014, 145: 619-624.
[74] Ma M, Zhu P, Pi F W, et al. A disposable molecularly imprinted electrochemical sensor based on screen-printed electrode modified with ordered mesoporous carbon and gold nanoparticles for determination of ractopamine[J]. Journal of Electroanalytical Chemistry, 2016, 775: 171-178.
[75] Cunha C R A, Oliveira A, Firmino T V C, et al. Biomedical applications of glyconanoparticles based on quantum dots[J]. Biochimica et Biophysica Acta (BBA) - General Subjects, 2018, 1862(3): 427-439.
[76] Molaei M J. A review on nanostructured carbon quantum dots and their applications in biotechnology, sensors, and chemiluminescence[J]. Talanta, 2019, 196: 456-478.
[77] 张殿伟,王金菊,王艳萍. 量子点传感体系在有机磷农药残留检测中的应用[J].食品研究与开发,2019,40(1):214-219.
Zhang Dianwei, Wang Jinju, Wang Yanping. Application of sensing system based on quantum dots on the detection of organophosphate pesticide residues[J]. Food Research And Development, 2019, 40(1): 214-219. (in Chinese with English abstract)
[78] Probst C E, Zrazhevskiy P, Bagalkot V, et al. Quantum dots as a platform for nanoparticle drug delivery vehicle design[J]. Advanced Drug Delivery Reviews, 2013, 65(5): 703-718.
[79] Yao X, Yan P P, Tang Q H, et al. Quantum dots based electrochemiluminescent immunosensor by coupling enzymatic amplification for ultrasensitive detection of clenbuterol[J]. Analytica Chimica Acta, 2013, 798: 82-88.
[80] Dong T T, Yang X K, Zhao K, et al. Signal amplification strategy for highly sensitive detecting brombuterol with electrochemiluminescent immunoassay by using CdSe QDs as label and gold nanoparticle as substrate[J]. Electroanalysis, 2016, 28(8): 1847-1855.
[81] Raksawong P, Chullasat K, Nurerk P, et al. A hybrid molecularly imprinted polymer coated quantum dot nanocomposite optosensor for highly sensitive and selective determination of salbutamol in animal feeds and meat samples[J]. Analytical and Bioanalytical Chemistry, 2017, 409(20): 4697-4707.
[82] Tang Q H, Cai F D, Deng A P, et al. Ultrasensitive competitive electrochemiluminescence immunoassay for the-adrenergic agonist phenylethanolamine A using quantum dots and enzymatic amplification[J]. Microchimica Acta, 2014, 182(1/2): 139-147.
[83] Dong T T, Tang Q H, Zhao K, et al. Ultrasensitive electrochemiluminescent salbutamol immunoassay with dual-signal amplification using CdSe@SiO2as label and gold nanoparticles as substrate[J]. Microchimica Acta, 2017, 184(3): 961-968.
[84] Hu X X, Zhang H, Chen S H, et al. A signal-on electrochemiluminescence sensor for clenbuterol detection based on zinc-based metal-organic framework–reduced graphene oxide-CdTe quantum dot hybrids[J]. Analytical and Bioanalytical Chemistry, 2018, 410(30): 7881-7890.
[85] 赵岳涛,王怀雨,喻学锋. 二维黑磷的制备及表面修饰技术研究进展[J]. 科学通报,2017,62(20):102-111.
Zhao Yuetao, Wang Huaiyu, Yu Xuefeng. Progress of fabrication and surface modification of 2D black phosphorus[J]. Chinese Science Bulletin, 2017, 62(20): 102-111. (in Chinese with English abstract)
[86] 贾蕾,雷天民. 二维黑磷物理性质及化学稳定性的研究进展[J]. 材料导报,2018,32(7):1100-1106.
Jia Lei, Lei Tianmin. Research progress on physical properties and chemical stability of two-dimensional black phosphorus[J]. Materials Review, 2018, 32(7): 1100-1106. (in Chinese with English abstract)
[87] Xia F N, Wang H , Jia Y C. Rediscovering black phosphorus as an anisotropic layered material for optoelectronics and electronics[J]. Nature Communications, 2014, 5: 4458-4464.
[88] Woomer A H, Farnsworth T W, Hu J, et al. Phosphorene: synthesis, scale-up, and quantitative optical spectroscopy[J]. ACS Nano, 2015, 9: 8869-8884.
[89] Ge Y, Camarada M B, Xu L J, et al. A highly stable black phosphorene nanocomposite for voltammetric detection of clenbuterol[J]. Microchimica Acta, 2018, 185(12): 566-576.
[90] 张立平. Janus颗粒的制备与应用研究进展[J]. 山东化工,2018,47:56-60.
Zhang Liping. Preparation and application of Janus particles[J]. Shandong Chemical Industry, 2018, 47: 56-60. (in Chinese with English abstract)
[91] Wang F, Paulettg M, Wang J T, et al. Dual surface- functionalized Janus nanocomposites of polystyrene/ Fe3O4@SiO2for simultaneous tumor cell targeting and stimulus-induced drug release[J]. Advanced Materials, 2013, 25(25): 3485-3489.
[92] Zhou Y, Yang Y J, Deng X, et al. Electrochemical sensor for determination of ractopamine based on aptamer/ octadecanethiol Janus particles[J]. Sensors and Actuators B: Chemical, 2018, 276: 204-210.
[93] Karl M, Chinnu A B, Vijai k, et al. Formation and characterisation of CeO2and Gd:CeO2nanowires/rods for fuel cell applications[J]. Journal of Experimental Nanoscience, 2015, 10(7): 520-531.
[94] Chinnu M K, Anand K V, Kumar R M, et al. Synthesis and structural, optical and thermal properties of ceria and rare earth doped Ceria nanocrystals[J]. 2013, 7(11/12): 976-979.
[95] Huang K, Lei M, Wang Y J, et al. Green hydrothermal synthesis of CeO2NWs-reduced graphene oxide hybrid with enhanced photocatalytic activity[J]. Powder Diffraction, 2014, 29(1): 8-13.
[96] Xiao X, Wang Y H, Tan W, et al. Simple synthesis of multilayer-shaped CeO2nanomaterial and its electrochemical detection of clenbuterol[J]. Electroanalysis, 2018, 30: 2744-2749.
Review on sensing detection progress of “lean meat agent” based on functional nanomaterials
Zhao Jie, Liang Gang, Li An, Man Yan, Jin Xinxin, Pan Ligang※
(1.,100097,;2.(),100097)
The “lean meat agents” is a class of-agonists with a similar structure, which had been abused as an animal growth promoter to improve carcass lean meat rate. However, the drug residue accumulation in meat and body tissues would cause acute poisoning after eating, which gave rise to muscular pain, dizziness, cardiacpalpitation and vomiting, so China has banned its application for growth promotion in animal breeding processes since 2010. But the illegal abuse of “lean meat agents” still frequently occurs in some animal farms. Besides, the plenty of substitutes and increasingly concealing performance still pose a great threat to the safety of animal products and human health. The frequently abused ‘‘lean meat agents’’ include clenbuterol (CLE), ractopamine (RAC), salbutamol (SAL), terbutaline, cimaterol, phenylethanolamine A, etc. At present, various analytical methods have been developed to detect the drug residue, including high performance liquid chromatography (HPLC), gas chromatography mass spectrometry (GC-MS), liquid chromatography mass spectrometry (LC-MS), capillary electrophoresis (CE) and enzyme-linked immunoassay (ELISA), lateral flow chromatography, surface-enhanced Raman immunity, and molecular imprinting polymers, and so on. While these chromatographic methods all require complicated sample pre-treatments, which is not only laborious and time consuming but also sophisticated and large apparatus. Thus, they are most often used as precise quantitative and confirmatory methods, and not fit to the rapid screening. The ELISA and lateral flow chromatography are suitable for field analysis and extensive screening, but the sensitivity is not unsatisfactory in most time. So the routine methods have been unable to meet the requirements of multiple application scenarios and the complex sample substrates. In the past decades, the nanotechnology has made great progress. The functional nanomaterials possess a lot of extraordinary property, such as large surface-to-volume ratio, excellent electrical conductivity, high chemical stability, good biological compatibility, etc. At the time, sensors have interdisciplinary applications in many fields, including chemistry, biology and electronics, industry, agriculture, clinical medicine, environmental protection, food safety jaince and the other fields. The special structure and properties of functional nanomaterials have greatly improved the performance of the existing sensing technologies, making the sensing technologies develop towards the direction of sensitivity, efficiency, simplicity, low cost and increasing anti-interference ability. In addition, the sensing instrument is easier to be miniaturized, portable and automatic, combining with the functional nanomaterials, which is expected to achieve real-time, online, simple, sensitive, high-flux and portable drug residue detection, having a promising application prospects. So far, the wide and common use functional nanomaterials in sensing detection include gold nanomaterials, carbon nanomaterials, quantum dots and other new nanomaterials (such as Phosphorene, Janus nanoparticles, CeO2nanoparticles), so the above-mentioned functional nanomaterials were summarized with the various detection principle of sensing test, such as colorimetric methods, surface enhanced Raman scattering, immunoassay, electrochemical and electrochemiluminescence methods in this review. In generally, functional nanomaterials and composite nanomaterials usually improve sensor performance from the following aspects, as a reaction substrate, load the specific molecular recognition, improve electrical conductivity, surface enhancement effect, signal conduction properties, catalytic properties and good biocompatibility, and so on. In the future, in order to improve the performance of sensor, the functional nanomaterials can be improved in the following aspects. Firstly, the specificity of the nanomaterials in free-label sensor should be enhanced to ensure the detection methods suitable to the needs of actual detection. Secondly, development and screening of new kind of functional nanomaterial, or synthesis the nanomaterial contains two or more elements or has special structure, to obtain the superior performance. Thirdly, make further study of the strengthening mechanism of nanomaterial, and innovate the modification methods for identifying molecules. With the rapid development in nanotechnology, the functional nanomaterials in sensing technology will make greater function to develop more sensitive, accurate, simple, high throughput and low-cost detection methods for drug residue detection.
sensors; nanotechnology; non destructive inspection; nanomaterials; lean meat agents
赵 杰,梁 刚,李 安,满 燕,靳欣欣,潘立刚. 功能纳米材料的“瘦肉精”传感检测技术研究进展[J]. 农业工程学报,2019,35(18):255-266.doi:10.11975/j.issn.1002-6819.2019.18.031 http://www.tcsae.org
Zhao Jie, Liang Gang, Li An, Man Yan, Jin Xinxin, Pan Ligang. Review on sensing detection progress of “lean meat agent” based on functional nanomaterials[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2019, 35(18): 255-266. (in Chinese with English abstract) doi:10.11975/j.issn.1002-6819.2019.18.031 http://www.tcsae.org
2019-05-25
2019-08-26
北京市农林科学院博士后科研基金(2018-ZZ-020);北京市农林科学院科技创新能力建设专项(KJCX20170420);北京市自然科学基金(6194038、L182031)
赵杰,博士,研究方向为农产品质量安全。Email:zhaojie20090127@126.com
潘立刚,博士,研究员。研究方向为农产品质量安全。Email:panlg@brcast.org.cn
10.11975/j.issn.1002-6819.2019.18.031
S879.2
A
1002-6819(2019)-18-0255-12

