Tilapia processing waste silage (TPWS): An alternative ingredient for Litopenaeus vannamei(Boone,1931)diets in biofloc and clear-water systems
Joquim d Roch Sores Neto, Felipe de Azevedo Silv Rieiro, Alex Augusto Gonçlves,Murício Gustvo Coelho Emerencino
a Universidade Federal Rural do Semi-Árido (UFERSA), Departamento de Ciência Animal, Setor de Aquicultura, Mossoró, 59625-900, Brazil
b UFERSA, Departamento de Ciência Animal, Laboratório de Tecnologia e Controle de Qualidade do Pescado, Mossoró, 59625-900, Brazil
c Universidade do Estado de Santa Catarina(UDESC),Laboratório de Aquicultura(LAQ),88790-000,Laguna,SC,Brazil and Programa de Pós-Graduação em Zootecnia(PPGZOO/UDESC), Chapecó, 89815-630, Brazil
Keywords:Silage Nutrition Waste BFT Pacific white shrimp
ABSTRACT The aim of this study was to evaluate the growth performance of Litopenaeus vannamei juveniles reared under biofloc and clear-water conditions fed with different inclusion levels of tilapia processing waste silage (TPWS)based-diets. The experiment was performed in two individual systems: biofloc (BS) and clear-water systems(CWS). The trial used forty 40 L rectangular plastic bins (twenty per system) in a density of 63 shrimp/m2. The juveniles were distributed in a factorial completely randomized experimental design.The treatments were based on the percentage of silage inclusion (control or 0, 1.5%, 3.0%, 4.5% and 6.0% of inclusion) in BS or CWS,totalizing ten treatments and four replicates. Survival was above 80% in all treatments and was not affected by both systems and diet. Shrimp final weight and SGR were statistically influenced by system (P <0.05)but not by the diet; and presented high values in BS. The inclusion of TPWS in L. vannamei diets did not affect shrimp performance. In addition, shrimp raised in BS demonstrated better growth performance as compared to CWS.
1. Introduction
In recent years,studies approaching the production of Pacific white shrimp Litopenaeus vannamei in biofloc system desired great attention(Avnimelech, 2015; Lobato, Ribeiro, Miranda-Baeza, & Emerenciano,2019). The recent diseases outbreaks and low productivity lead the scientists to search for alternative systems to improve efficiently the shrimp growth and meet the market demand (Emerenciano, Ballester,Cavalli, & Wasielesky, 2012a; Vargas-Albores et al., 2019).
Biofloc system, also called as biofloc technology (BFT), has the advantage to allow the production of a great amount of shrimp per area or volume with limited or no water exchange.Such technology provides better biosecurity for the production, especially if the farm is located close to areas with high concentration of aquaculturists using the same water source. BFT has gained popularity because it offers a practical solution to maintaining water quality and recycle feed nutrients simultaneously (Xu & Pan, 2012). Another advantage of the biofloc system is the possibility to use alternatives low protein diets and consequently, decrease the production costs (Ballester et al., 2010; Scopel et al.,2011;Xu&Pan,2014);mainly due to the continuous availability of natural food source in a form of live microorganisms (Azim & Little,2008; Decamp, Conquest, Forster, & Tacon, 2002; Ray et al., 2010).
Fishmeal is an unsustainable and one of the most expensive ingredient used in aquaculture diets (Naylor et al., 2009). Therefore, the replacement or reduction of fishmeal presents a great interest for the aquaculture industry. On the other hand, problems related to the fishmeal replacement by alternative ingredients have been identifying including deficiency of some essential amino acids, the presence of antinutritional factors, palatability and digestibility (Forster, Dominy,Obaldo, & Tacon, 2003; Naylor et al., 2009). Although problems exist,many cases of success have been reported in L.vannamei diets(Amaya,Davis, & Rouse, 2007; Bauer, Prentice-Hernandez, Tesser, Wasielesky,& Poersch, 2012; Cruz-Suárez et al., 2007; Davis & Arnold, 2000;Forster et al.,2003;Hernández,Olvera-Novoa,Aguilar-Vejar,González-Rodríguez, & Parra, 2008; Samocha, Davis, Saoud, & DeBault, 2004;Suarez et al., 2009), including fishmeal replacement by plant protein sources (Moreno-Arias et al., 2018) also supported by relevant mineral supplementation (Huang, Wang, Zhang, & Song, 2017).
Fish silage can be produced using fisheries and aquaculture processing residues. Fish silage is an alternative protein source to the fishmeal (Vidotti, Viegas, & Carneiro, 2003) and posses a simpler and cheaper production method (Gallardo et al., 2012). Furthermore, the use of fish silage as a substitute for protein ingredients in aquafeeds emerges as an alternative to solve environmental and sanitary problems caused by the lack of use and/or inadequate disposition of fish residues.Besides, it is also a way of decreasing the feeding costs, and, consequently, the production costs since feeding corresponds around of 60%of the overall expenses (Arruda, Borghesi, & Oetterer, 2007).
On this context, tilapia is a worldwide relevant species for aquaculture industry and has demonstrated positive results as a fish silage incorporated into diets (Carvalho, Pires, Veloso, Silva, & Carvalho,2006; Fernandes, Bueno, Rodrigues, Fabregat, & Sakomura, 2007) due to its nutritional quality (Oliveira, Pimenta, Camargo, Fiorini, &Pimenta, 2006). Thus, the aim of this study was to evaluate the inclusion of tilapia processing waste silage (TPWS) in diets for L. vannamei juveniles reared under clear-water and biofloc conditions.
2. Material and methods
2.1. Experimental design and culture conditions
The study was carried-out in the Aquaculture Sector,Department of Animal Sciences,Universidade Federal Rural do Semi-Árido(UFERSA),RN, Brazil. The postlarvae of Pacific white shrimp L. vannamei (PL20)were supplied by a local commercial hatchery.
Before the experiment, PLs were stocked in a 15 m3fiberglass circular tank (called as “macrocosm”) aiming to an acclimation and prior biofloc formation. Water was vigorously aerated using one air diffuser(composed by ¾” PVC pipe with several 1 mm holes) located in the center of the macrocosm tank. In order to maintain biofloc culture medium, shrimp were stocked at a density of 200 shrimp/m2and maintained until the end of the experimental period. Shrimp feeding was carried-out at 08:00 am and 6:00 pm, with 35% crude protein commercial feed(Aquabalance 35,Presence Animal Nutrition,Paulínia,SP, Brazil) in two feed trays in order to monitor the food consumption.The sugar cane molasses as a carbon source was daily added after the feed addition in order to maintain a high C:N ratio(20:1)(Avnimelech,1999) aiming to ensure optimal heterotrophic bacteria growth (Crab,Kochva, Verstraete, & Avnimelech, 2009). The vertical substrates(polyethylene 1.0 mm mesh) were placed in the center of the tank to provide an additional area of 30% of the tank. This experiment was performed in euryhaline conditions (~5).Limited water exchange was carried out not exceeding 0.5% daily by a central drain to prevent accumulation of sludge throughout the experimental period. Dechlorinated freshwater was added to compensate evaporation losses and sludge removal.
Two individual systems were set-up according to Emerenciano et al.(2007): biofloc (BS) and clear-water systems (CWS). The trial was initiated stocking L. vannamei juveniles (1.43 ± 0.33 g) in forty(20+20)40 L rectangular plastic bins(27 cm×37 cm×54 cm)in a density of 63 shrimp/m2(12 juveniles per bin). The juveniles were distributed in a factorial completely randomized experimental design(water type and%of tilapia waste silage inclusion as the main factors)and reared during 45 days. Four replicate tanks were randomly assigned to each treatment.The treatments were based on the percentage of TPWS inclusion (0 or control, 1.5%, 3.0%, 4.5% and 6.0% of inclusion)in BS or CWS system,totalizing ten treatments.The formulation of diets was described below (section 2.4).
For both treatments, the water was pumped from the macrocosm tank to the experimental units by a submerged pump(¾HP pumps)and returned by gravity. Water flow in all experimental units was checked two times per day in order to maintain uniformity between units as much as possible (~500%/d of water circulation between macrocosm and bins). The experimental units were also siphoned once a week aiming to remove faeces and any other residues.
For the CWS treatments, the same scheme described above in BS was performed,except by the macrocosm tank(i)was not stocked with animals, (ii) didn't receive carbon addition in order to maintain the water clear.In addition,the aeration was supplied by two 4 HP blowers connected to an emergency diesel electric generator to keep optimum dissolved oxygen levels in both systems.
Again, young writers need to be encouraged. Because of Miss Byrne’s influence, I have enjoyed a lifetime writing books, songs, and TV scripts. And guess what? I haven’t plagiarized13 a single word of any of it.
Temperature, salinity, pH (YSI-100, Yellow Springs Instruments Inc., OH, USA) and dissolved oxygen (YSI-55, Yellow Springs Instruments Inc., OH, USA) concentration were monitored twice daily.Settling solids (Imhoffcones) was monitored daily (08:00am). Total ammonia nitrogen(NH4-N)and nitrite(NO2-N)were measured once a week(UNESCO,1983).All shrimps were weighed to the nearest 0.1 g at the beginning and the end of the experiment. Final body weight (g),specific growth rate (SGR=[(ln final body weight - ln initial body weight)/experimental time]×100),feed conversion ratio(FCR=total feed intake/total weight gain) and survival (final number of live shrimp/total number of shrimp×100) were assessed.
2.2. Fish silage production
The TPWS used in this study was produced in the Laboratory of Seafood Technology and Quality Control (LAPESC/UFERSA) using filet residues of Nile tilapia (Oreochromis niloticus) processing including head, bones, skin, fins and viscera. The acid silage was produced using the methodology described by Arruda, Borghesi, Brum, D′Arce, and Oetterer (2006) with some modifications: 2% Formic acid (purity≥95%, Sigma-Aldrich) and 3% Phosphoric acid (purity 85%-90%,Sigma-Aldrich),and 1%Sorbic acid(purity ≥99.0%,Sigma-Aldrich)as antifungal.The silage was dried in the oven at 60°C for 24 h(to obtain moisture below 13%), ground in a Rotor Mill (Rotating Knives and Swing Hammer MA900, Marconi Equip. Lab. Ltda., Brazil) and homogenized (500-μm mesh). Before formulation of the experimental diets,fish silage was neutralized by adding 1.6% calcium hydroxide to raise the silage pH from 2.8 to 7.1.
2.3. Diet formulation and feeding
Five experimental diets were formulated to be isocaloric and isoproteic and to attend the nutritional requirements of the species(Table 1).The TPWS inclusion ranged from zero to 6% of the diet. The overall low level of inclusion was due to the high level of crude lipid presented in the silage (37.4%). All diets were processed in the Laboratory of Aquatic Animal Nutrition of the Universidade Federal do Ceará (UFC) using the method described by Nunes, Sa, and Sabry-Neto(2011), with some modifications (i.e., inclusion of sardine hydrolyzed as EAA source; and reduced amount of salmon oil due to the high lipid content of the TPWS). All diets were kept frozen at - 20°C until use.For both treatments, the juveniles were fed twice a day (08:00 am and 06:00 pm) using a feed tray to monitor feed consumption.
2.4. Statistical analysis
After a check for homoscedasticity and normality (Zar, 1996),shrimp performance data were analyzed using a two-way ANOVA and Tukey's test to compare the means with α fixed in 0.05 (Zar, 1996)using the software R (version 3.0.2). Survival data in percentage was transformed using the arcsine transformation in order to normalize the data before the analysis; however, the original means and standard deviation are presented.
3. Results
In regards to proximate analysis, the results demonstrated that TPWS contained 83.8% dry matter, 33.7% crude protein, 37.4% crude lipid, and 21.5% ash based on dry matter (Table 2). Water quality parameters maintained in the recommended ranges for L. vannamei in both systems with temperature ranging from 24 to 32°C, pH ranging from 6.7 to 8.7 and salinity 4 to 5. The dissolved oxygen always was kept >3.7 mg/L, total ammonia nitrogen <0.52 mg/L and nitrite <0.25 mg/L stayed in safe concentration to the animals during all the experiment. Settling solids increased over time although levels maintained below 15 mL/L.
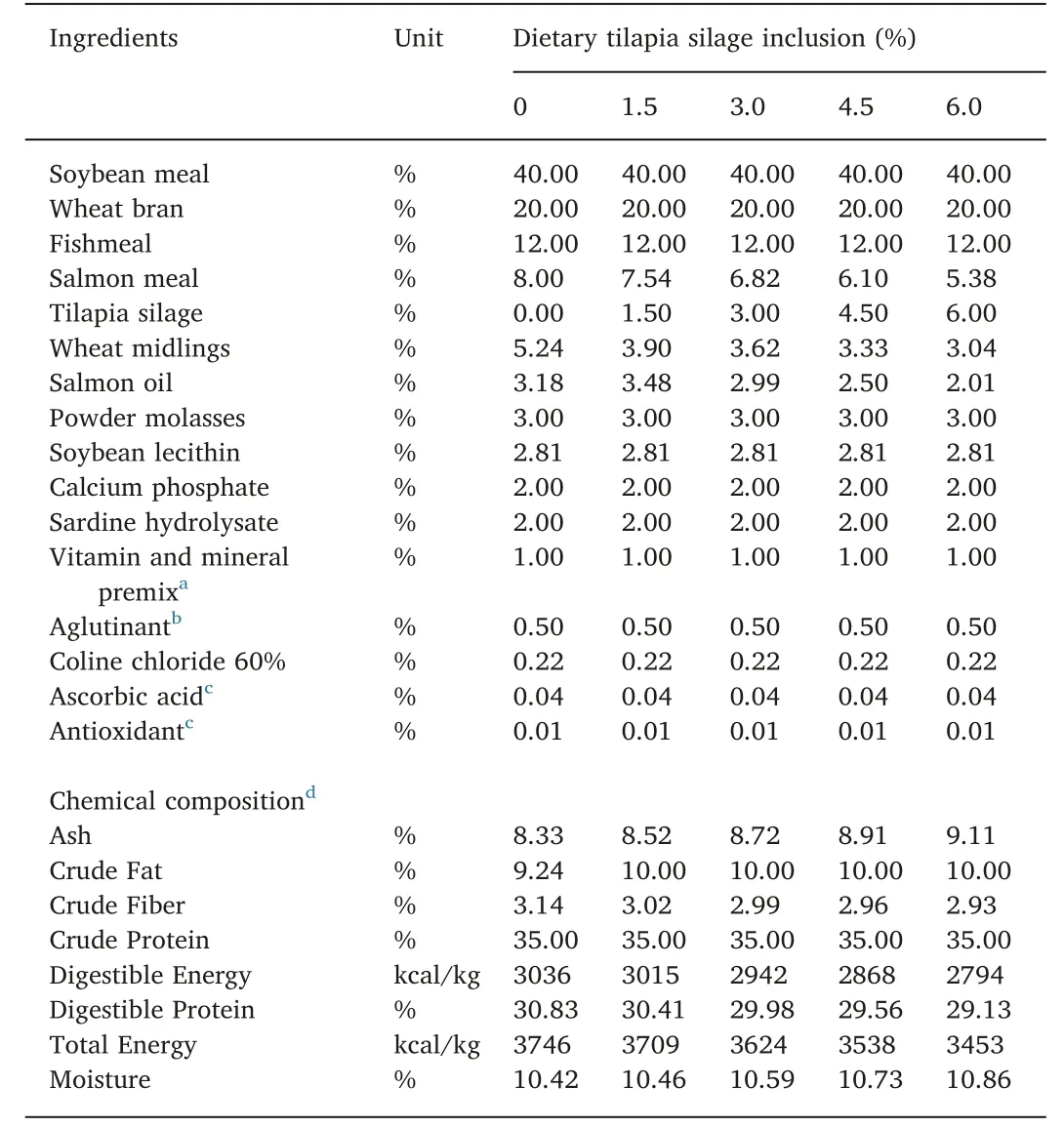
Table 1 Ingredients and chemical composition of diets used in the present study.

Table 2 Proximate composition of tilapia processing waste silage (TPWS - acid silage)using residues of Nile tilapia (Oreochromis niloticus) produced using the methodology described by Arruda et al. (2006).
4. Discussion
The water quality parameters remained within the recommended range for L. vannamei culture (Van Wyk & Scarpa, 1999), includingsettling solids that maintained below to 15 mL/L. Schveitzer et al.(2013)demonstrated that high concentrations of settling solids and TSS appear to be more harmful for culture of L. vannamei.
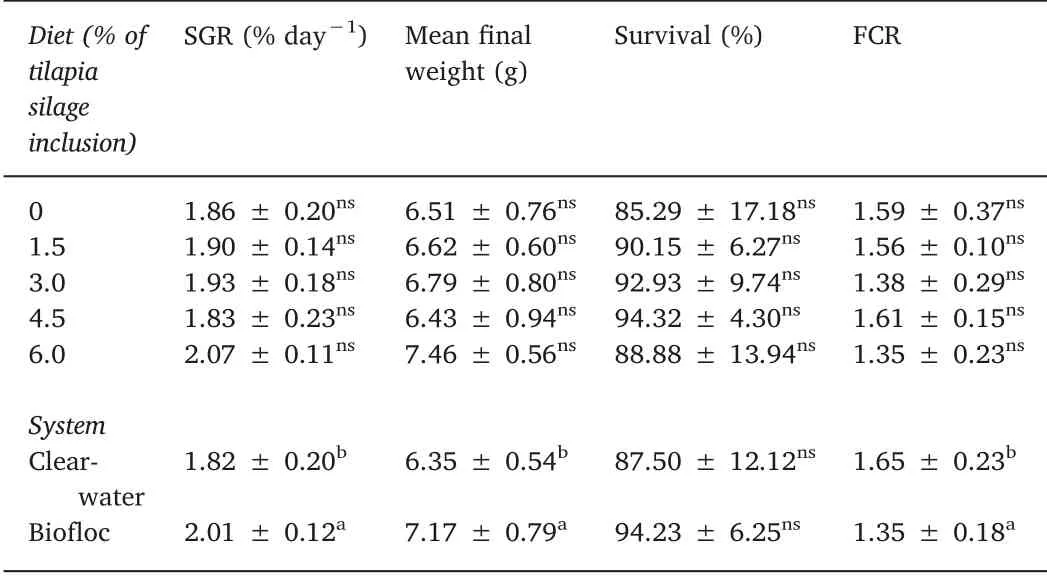
Table 3 Growth performance of L. vannamei fed increasing percentages of tilapia processing waste silage(TPWS)inclusion in clear-water and biofloc systems during 45-d.
In our experimental conditions, both biofloc (BS) and clear-water(CWS) systems, tilapia processing waste silage (TPWS) could be included at the highest level(6.0%)without losses in growth performance and survival. On the other hand, in BS condition shrimp presented the best performance as compared to CWS,probably due to the continuous availability of natural food. This natural productivity is normally present in a form of bacteria, microalgae, protozoa, nematodes, copepods and rotifers (Azim & Little, 2008; Ballester et al., 2010; Decamp et al.,2002;Ray et al.,2010).These microorganisms are a rich source of lipids(Maica,Borba,&Wasielesky,2012),vitamins and essential amino acids(Ju, Forster, Conquest, & Dominy, 2008), as well as highly diverse“native protein”. The concept of “native protein” is related to protein source without previous treatment mainly including live food(Emerenciano, Cuzon, Goguenheim, Gaxiola, & Aquacop, 2012b). Is important to note that bacteria protein-source plays an important role in the equilibrium and re-ingestion of particulate organic matter and faeces (coprophagia) left by shrimp results in a form of the constant food supply.The colonization of shrimp gut by bacteria had been shown positive effects such as improvement of shrimp digestive enzymes activity (Xu, Pan, Sun, & Huang, 2012) and increasing the availability of extracellular enzymes (Xu & Pan, 2012) acting as “natural probiotic”(De Schryver, Boon, Verstraete, & Bossier, 2012).
In the recent years, the interests on alternative dietary protein sources such as vegetable grains and terrestrial animal industry byproducts has increased. On the other hand, special attention on palatability, digestibility, deficiency of essential amino acids, and the presence of anti-nutritional factors still need to be done (Forster et al.,2003; Naylor et al., 2009). Although problems exist, many cases of success have been reported using alternative dietary protein such as cattle by-product and a mixture of canola and soya(Forster et al.,2003;Suarez et al., 2009), poultry by-product (Amaya et al., 2007; Cruz-Suárez et al., 2007; Samocha et al., 2004), swine by-product(Hernández et al.,2008),and soy protein concentrate(Paripatananont,Boonyaratpalin, Pengseng, & Chotipuntu, 2001). Bauer et al. (2012)suggested that a mixture of soy protein concentrate and microbial floc meal can be utilized as a substitute for fishmeal in diets for L.vannamei juveniles. These studies have been carried out in clear-water condition and few efforts have been done to investigate alternative sources in biofloc conditions. Scopel et al. (2011) evaluated the replacement of fishmeal (0, 12.5% and 21.0%) by a combination of soy and animal terrestrial by-products. The authors found that 12.5% of replacement did not affect shrimp growth, resulting in growth rates of 0.7 g/week similar to those found in our study in clear-water condition, but less than 0.9 g/week observed in biofloc.
No literature was found related to the use of TPWS in L. vannamei diets under biofloc condition. Although low levels of silage were included in the diets due to the high lipid content in the fish silage, the highest level of 6% still could represent a significant reduction in costs in shrimp formulations. In a study using clear-water, Gallardo et al.(2012)evaluated in L.vannamei juveniles feeds containing(i)fish waste silage,(ii)fish waste silage with soybean meal and(iii)fish waste meal as a protein source. The authors reported that shrimp fed with diets containing fish waste silage combined with soybean meal gained 0.7 g/week higher than those fed with fish waste silage or fish waste meal(0.3 g/week). It is important to note that these values are lower than observed in our study e.g. with biofloc conditions (0.9 g/week). Additionally,in our study values of FCR were 1.3 and 1.6 for BS and CWS,respectively, lower than 2.8 and 2.5 observed by Ray et al. (2010) and Xu et al. (2012) using soy protein-based diets and low protein content diets, respectively, both in biofloc conditions for L. vannamei.
In contrast with our work, Costa, Portz, Hisano, Druzian, and Ledo(2009) did an interesting study evaluating shrimp silage in juvenile tilapia (O. niloticus) diets. The authors concluded that it is possible to include 2.75% of shrimp silage, reducing the diets costs in 3.3%without losses in fish performance. In a similar work, Cavalheiro,Souza, and Bora (2007) tested shrimp head silage (approximately 40%protein) as a substitute for fishmeal in tilapia diets at 0, 33.3%, 66.6%and 100% dietary levels. The results indicate that the shrimp silage could replace fishmeal at 100% level with economic advantages and without sacrificing the quality of the feed.
5. Conclusion
In our experimental conditions, the inclusion of tilapia processing waste silage(TPWS)in L.vannamei diets was possible up to 6%without compromise shrimp performance and survival. In addition, shrimp raised in BS demonstrated better growth performance as compared to CWS.
Acknowledgements
The authors would like to thankthe Funding of Studies and Projects FINEP-Brazil (1550/10) and the Brazilian National Council for Scientific and Technological Development CNPq Brazil (475609/2010-7) for the financial support received, Larvi and Aquatec for the shrimp post-larvae supply, Darlimeire Dantas de Aquino (Scholarship PIVIC -CNPq/UFERSA) and Professor Alberto J. P. Nunes for the contribution in the diet formulation and manufacture.
 Aquaculture and Fisheries2019年5期
Aquaculture and Fisheries2019年5期
- Aquaculture and Fisheries的其它文章
- Management of China's capture fisheries: Review and prospect
- Vulnerability of inland and coastal aquaculture to climate change:Evidence from a developing country
- Biological manipulation of eutrophication in West Yangchen Lake
- Changes in the phytoplankton community structure of the Backshore Wetland of Expo Garden, Shanghai from 2009 to 2010
- Community structure of benthic macroinvertebrates in reclaimed and natural tidal flats of the Yangtze River estuary
