Derivation and applications of human hepatocyte-like cells
Shuang Li,Shi-Qian Huang,Yong-Xu Zhao,Yu-Jie Ding,Dan-Jun Ma,Qiu-Rong Ding
Shuang Li,Shi-Qian Huang,Yong-Xu Zhao,Qiu-Rong Ding,CAS Key Laboratory of Nutrition,Metabolism and Food Safety,Shanghai Institute of Nutrition and Health,Shanghai Institutes for Biological Sciences,University of Chinese Academy of Sciences,Chinese Academy of Sciences,Shanghai 200031,China
Yong-Xu Zhao,Dan-Jun Ma,College of Mechanical Engineering,Dongguan University of Technology,Dongguan 523808,Guangdong Province,China
Yu-Jie Ding,Department of Pharmacy,Mudanjiang Kang'an Hospital,Mudanjiang 157011,Heilongjiang Province,China
Qiu-Rong Ding,Institute for Stem Cell and Regeneration,Chinese Academy of Sciences,Beijing 100101,China
Abstract
Key words:Hepatocyte-like cells; Human pluripotent stem cells; Hepatic differentiation;Biomedical application
INTRODUCTION
The liver represents one of the most pivotal organs of the human body in regulating glucose homeostasis,lipid metabolism,detoxification and many other physiological processes.As liver diseases,including fatty liver diseases,hepatic carcinoma,and viral hepatitis,continue to increase in prevalence,there is an urgent need for development of effective treatments,and sufficiently cell or tissue sources for transplantation.Primary human hepatocytes and liver donors offer immediate resources for studying liver diseases and transplantation.However,both primary cells and available donor transplants are in persistent shortage.Although different culture systems have been identified recently that enable long-term culture and expansion of both rodent and human primary hepatocytes[1-4],the capacity of expansion is still limited and has donor-dependent variability.As stem cells are known to have potent self-renewal ability as well as the capacity to differentiate into different somatic cell types,they have been proposed as an ideal alternative cell source for large or even unlimited supplies of hepatocytes and even liver tissues.Human hepatocytes can be derived from embryonic stem cells (ESCs),induced pluripotent stem cells (iPSCs),mesenchymal stem cells and hepatic progenitor cells[5].As the cells derived from stem cells often have incomplete function and exhibit characteristics of fetal liver cells,they are generally defined as hepatocyte-like cells(HLCs).The discovery made by Gurdon and Yamanaka that mature cells from individual patients can be reprogrammed to iPSCs,opened up the possibility that these cells can be applied to disease modeling and organ transplantation.Furthermore,intense efforts have been made in recent years in generating better HLCs and liver organoids from PSCs,and in applications of these cells in various fields.Therefore,in this review,we focus on HLCs derived from human pluripotent stem cells (hPSCs) and discuss recent progress in the derivation and applications of HLCs in biomedical research.
DERIVATION OF HUMAN HLCs
hPSCs include human ESCs,mostly derived from the inner cell mass of the fertilized eggs,and iPSCs reprogramed from terminally differentiated somatic cells.hPSCs promise an unlimited supply of human somatic cells,due to their theoretical capacity for self-renewal and differentiation into any kind of somatic cell types in human body.To date,many protocols have been established to generate human hepatocytes derived from hPSCs.Most induction methods are based on the understanding of the embryonic development processes of the liver,and aimed to imitate in Petri dishes the endoderm development,endoderm hepatic specification and hepatic maturation stages.The directed differentiation protocols either rely on the use of embryoid body(EB) formation[6,7]or start with monolayer culture,with the latter more frequently adapted currently in laboratories.EB formation means to mimic the blastocyst and epiblast architecture; however,it can be easily disturbed by suboptimal culture conditions and sources of reagents,for example,different batches of fetal bovine serum can affect to a large degree the quality of generated EBs.Most protocols currently in use apply similar strategies with contributions from individual laboratories by improving inducers of differentiation and optimizing their combinations (Table 1).These protocols can be largely specified to three consecutive steps:endoderm differentiation,hepatic induction,and liver maturation.
Endoderm formation
Transforming growth factor (TGF) β family member Nodal is vital in endoderm formation,based on studies in developmental biology in models including frogs,zebrafish,and mice[8-10].Although Nodal is an attractive candidate for inducing hPSCs to differentiate into definitive endoderm (DE),it is difficult to get highly active protein.Activin is another TGFβ family member,which mimics Nodal activity in triggering similar intracellular signaling events[11],thus is often used as a substitution of Nodalin vitro[12].In 2005,D'Amouret al[12]demonstrated efficient endoderm induction from monolayers of hPSCs by applying activin A,which was subsequently reproduced by many other groups.The monolayer culture here seems important to the endoderm differentiation in that cells can be exposed evenly to the endodermal inducer,activin A,and can better synchronize development of the endodermal cell fate[13].Levels of Nodal signaling comprise key elements in cell fate determination,with high level promotes endoderm differentiation,whereas low level initiates mesoderm specification[14-17].Therefore,high concentrations of activin A are now widely utilized for endoderm induction in hPSC culture[18-22].Besides,activation of fibroblast growth factor (FGF),bone morphogenetic protein (BMP) and Wnt signaling pathways also promote endoderm development[7,19,23].Phosphatidylinositol 3-kinase(PI3K) inhibitors,such as LY 294002 and AKT1-II,also promote activin-A-induced endoderm development[17].Several studies have shown that low doses of serum are necessary for activin A to induce an efficient endoderm program[12,17,24].
Hepatic specification
In early embryo development,FGF signals and BMP signals initiate the liver gene program and simultaneously block that for pancreas development[25].Consistent with thein vivodiscoveries,the signaling molecules FGF and BMP have also been demonstrated to be important in generating hepatic cells from DE cellsin vitro.The combination of FGFs and BMPs are thus widely used to induce hepatic endoderm programs[18,21,23].Dimethylsulfoxide (DMSO) can assist in promoting hPSC differentiation and specific generation of hepatic progenitors,and is usually used in hepatic differentiation[19,22,26,27].
Liver maturation
As for further liver maturation,hepatic progenitors are mostly treated by hepatocyte growth factor (HGF),oncostatin M (OSM),and glucocorticoid dexamethasone (Dex).HGF binds to its tyrosine kinase receptor c-Met,promoting hepatoblast proliferation,increasing cell migration and improving cell survival[28,29].OSM produced by hematopoietic cells is an interleukin(IL)-6 family cytokine,which induces hepatic maturation by the phosphorylation of signal transducer and activator of transcription[28,30].The glucocorticoid dexamethasone has also been implicated in the maturation of the hepatocytes[31,32].After the maturation stage,obtained HLCs display many hepatocyte features,such as albumin expression and secretion,urea secretion,low-density lipoprotein (LDL) uptake,indocyanine green (ICG) uptake,and glycogen storage (Table 1).However,those cells express fetal liver markers,such as αfetoprotein (AFP),and have lower activities of CYP450 enzymes when compared to primary liver tissue.With comparison of a set of human adult and fetal liver markers,it is roughly estimated that the HLCs have the characteristics of fetal hepatocytes at <20 wk gestation[33].
Protocol optimization
Different strategies have been adopted with the aim to promote maturation and to reduce the large heterogeneity of HLCs.One strategy is to use 3D culture,mimicking liver development in the body,thus promoting further maturation.Indeed,it has been shown that cells demonstrate more matured phenotypes in 3D than other culture systems.For example,it has been demonstrated that cAMP signaling within the 3D hepatoblast aggregates can promote further maturation of HLCs that display comparable metabolic enzyme levels to those of primary human hepatocytes[34].The other main strategy is to optimize the current protocols through screening for molecules that can improve differentiation,and to understand better the molecular mechanisms underlying liver development.Towards this aim,by screening 4000compounds,the Melton group identified IDE1 and IDE2,which can efficiently promote differentiation of mouse and human ESCs into DE cells[35].Other groups have also identified other small molecules,and demonstrated their effects in improving hPSC differentiation toward endoderm[36].In 2015,the Silleret al[37]group developed a new method for HLC differentiation with a combination of small molecules without the inclusion of growth factors in a defined minimum medium.Shanet al[38]developed a high-throughput chemical screening platform and identified two different classes of small molecules,which are able to induce functional proliferation of human primary hepatocytesin vitroand improve HLC maturation.By utilizing an established hepatic lineage hPSC reporter line,our laboratory performed genetic and chemical screenings,and identified several modulators involved in hepatic differentiation,and CI-994 compound (histone deacetylase 3 inhibitor) that can promote HLC differentiation at a late stage[39].
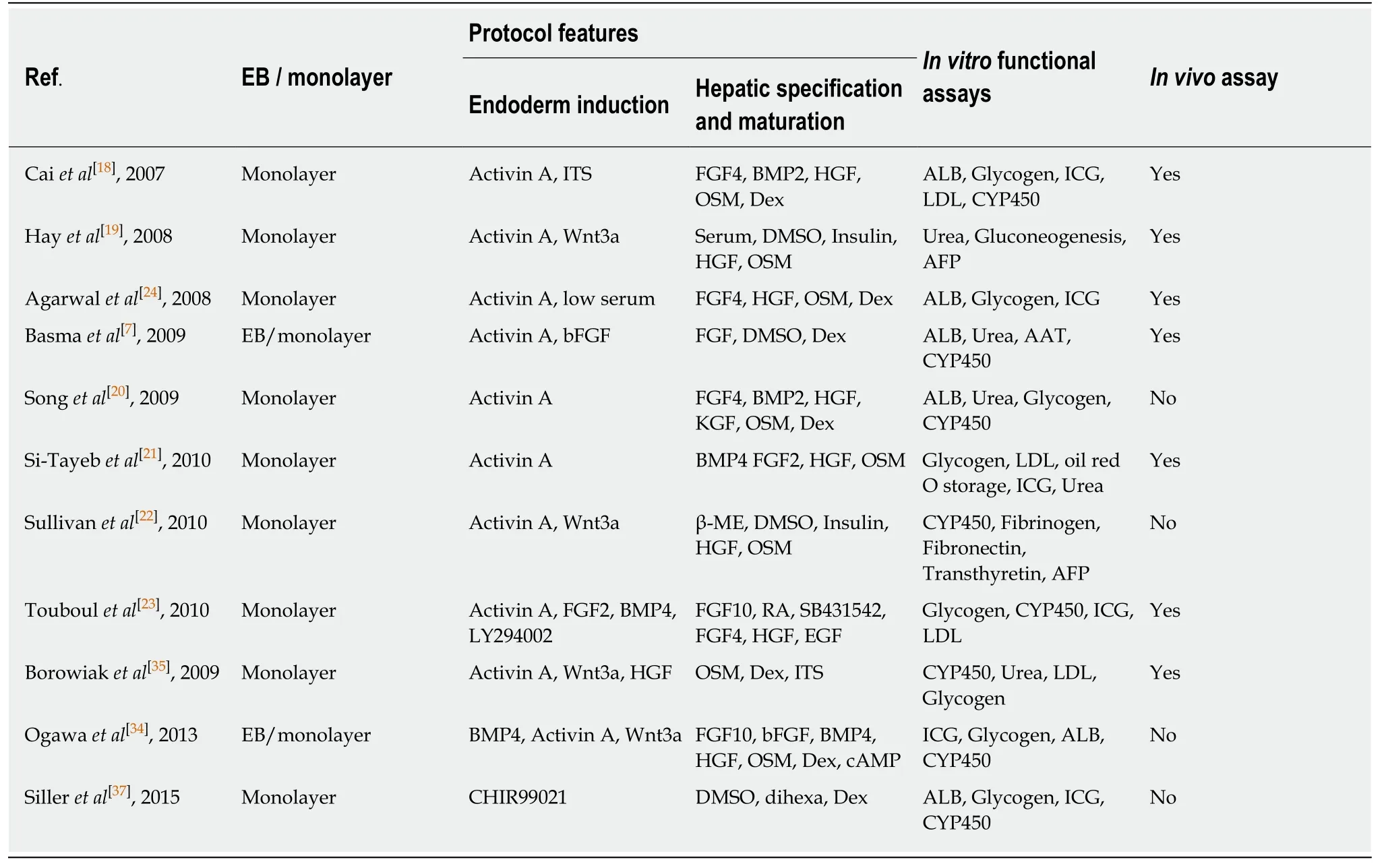
Table1 Summary of hepatocyte-like cells differentiation protocols
APPLICATIONS OF HLCs
Disease models
Human PSCs offer a uniquein vitrocellular model system for disease modeling.Induced PSCs derived from patients or hPSCs engineered with specific diseasecausing mutations using genome editing technologies allow researchers to study the consequences of genetic mutations with a human- and patient-specific genetic background; whereas the differentiation processesin vitrooften recapitulate aspects of normal development,thus providing the opportunity to investigate the developmental and degenerative processes of certain human diseases.Furthermore,as hPSCs possess great capacity in self-renewal,they can offer large-scale cellular materials with identical genetic background for disease modeling and for possible compound screenings to develop potential treatments.
Studying rare genetic variants
For modeling liver diseases with rare mutations in Mendelian diseases,patientspecific iPSCs carrying certain genetic mutations are often derived and differentiated to HLCs.Many disease models of inborn liver metabolic disorders,such as α1-antitrypsin deficiency,familial hypercholesterolemia,glycogen storage disease type 1a,and Wilson's disease,have been generated[40-42].Upon differentiation to HLCs,these cells with genetic mutations displayed certain disease phenotypes that are reflected in patients,highlighting potential utility of these models for studying diseases or screening for therapeutic interventions.In situations in which patients are not available,disease mutations of interest can be engineered using genome editing technologies into wild-type hPSCs to create mutant hPSCs for disease study[43,44].Drug screening with these disease models can highlight novel discoveries for disease treatment.In a study by the Duncan and Rader groups[45],HLCs derived from familial hypercholesterolemia iPSCs were applied to drug screening to identify potential LDL-cholesterol (LDL-C)-lowering drugs,which has successfully revealed cardiac glycosides as a candidate treatment for hypercholesterolemia.Other than studying diseases harboring genetic mutations,hPSC-derived HLCs are also powerful in providing cellular models for studying the lifecycle of hepatitis viruses.hPSC-derived HLCs have be used in hepatitis C virus (HCV) infection and screening for anti-HCV drugs[46],as well as modeling hepatitis B virus infection[47].
Studying common genetic variants
As a remarkable improvement in the recent iPSC disease modeling fields,large,diverse population cohorts of iPSCs have been generated and differentiated in parallel to HLCs as well as other cell types,offering valuable tissue substitutes for studies to reveal the relationship between genotype and phenotype; for example,expression quantitative trait locus (eQTL) analysis[48,49].Two independent cohorts of iPSCs have been generated from healthy donors (68 iPSC lines from 34 donors in one study and 91 iPSC lines from 91 donors in the other study) and used for subsequent hepatic differentiation and genetic analysis.Studies either successfully confirmed eQTLs previously characterizedin vivo[49],or identified a number of loci controlling hepatic gene expression with thesein vitroHLCs[48].In one study,the cohort of iPSC-derived HLCs were also subjected to metabolite abundance quantitative trait locus (mQTL)analysis,leading to the discovery of a strong association between a lipiddysregulating phenotype and the minor allele at the 1p13 locus[49].For the first time,these two studies demonstrated the capacity for iPSCs-derived cells to reproducein vivophenotypes driven by common genetic variants,and uncovered a potentially unlimited supply of human cells that allow to discover cell-type-specific QTL phenotypes (eQTL,mQTL and potentially others) that would be inaccessible usingin vivotissues.Together with several other studies that have performed genome-wide QTL analyses and identified a number of loci that contribute to interline heterogeneity using hundreds of undifferentiated iPSC lines[50-52],these studies have offered a new paradigm for human research,with iPSC-driven disease modeling being applied to study population geneticsin vitro.
In vitro pharmacogenomics
Aside from drug discovery with iPSC-derived disease models with small cohorts,large cohorts of iPSCs and iPSC-derived cells have been proposed to perform trials-indish,to assist in translating the discoveries of genome-wide association studies(GWASs) into improved treatment regimens and drug discovery; that is,to apply genotype analysis to patient stratification and design of individual treatment plans[53].In possible scenarios,iPSC-derived cells may provide an important link between drug development and Phase I trials,where iPSC-derived hepatocytes,cardiomyocytes or neurons can be used for preliminary safety screens with candidate drugs that might induce hepatotoxicity,cardiotoxicity,neurotoxicity or other off-target effects.Furthermore,between Phase I and Phase II trials,drug target cells derived from large cohorts of iPSCs can serve as the surrogate human population and be used in testing for drug efficacy; results from which can be applied to classify patients into responder and non-responder groups,thus increasing the relevance and successfully rate of further Phase 2 and 3 trials.Altogether,small or large cohorts of iPSCs and iPSCderived function cell types are revolutionizing the field of drug discovery.
Making liver organoids
The liver is a highly specialized organ consisting of mostly hepatocytes,but also several other cell types,such as Kupffer cells,endothelia cells,bile duct cells,and hepatic stellate cells.These cells all contribute to the highly organized architecture and functions of liver tissue.Compared to HLCs in 2D culture,liver tissue organoids constitute more than one cell type,can resemble part of the architecture of liver tissue,and possess some functions that may not exist in HLCs.Liver organoids can either be derived from adult stem cells[54,55]or hPSCs[56-59].Other than HLCs,development of protocols to obtain other cell types derived from hPSCs that constitute the liver tissue are important.To date,protocols of directed differentiation to obtain cholangiocytes[56,57],endothelia cells[60]and hepatic stellate cells[61]have been established,which may further aid the generation of functional liver tissue organoids.Other reviews discuss the generation and application of tissue organoids,which can assist in better understanding the opportunities as well as challenges in this field[62,63].
Bioartificial livers
Artificial liver support systems have been developed to provide an alternative to orthotopic liver transplantation (OLT).Artificial livers use nonbiological components to perform hepatic detoxification,removing toxins and drugs that accumulate in the blood during liver failure[64].However,artificial livers do not have the capacity to adequately replicate the physiological liver function.The incorporation of live cells harboring liver functions into these artificial liver systems,which establishes the bioartificial livers (BALs) systems,offers a solution to overcome these limitations[64].BAL support systems are extracorporeal bioreactors in which whole livers or liver cells are cultured in a 3D manner within a network of hollow fibers for blood plasma perfusion.BAL systems provide both biotransformation and hepatic synthetic functions[65].To date,different sources of liver cells have been tested in BAL devices,for example,human primary hepatocytes,immortalized human hepatoma cell lines,porcine hepatocytes[66],as well as induced human hepatocytes transdifferentiated from human fibroblasts (hiHeps)[67].While human hepatocytes are the preferred cells,obtaining sufficient human hepatocytes faces the same difficulty of organ shortage.Porcine hepatocytes are close to human hepatocytes,but have potential risk of xenozoonosis and immunological response.Hepatoma cells can provide large amounts of materials,but suffer from incompetent metabolism and ammonia clearance[68].HiHeps representing a new invaluable cell source for BAL devices,and have been successful in pigs[67]as well as in primary tests in patients.While we have not seen reports of HLCs being applied in BAL devices,we envisage that HLCs will be a potential cell source for the treatment of liver failure in BAL support systems in the future.The advantages of HLCs are obvious:human or patient-specific genetic background,normal karyotype,potentially unlimited supply,and better liver functions.However,to obtain a large amount of functional and homogeneous hepatocytes from hPSCs still depends on continuous improvement to the differentiation protocols and development of optimal large-scale culture systems.
In vivo transplantation
OLT remains the most effective treatment for end-stage liver diseases.However,liver donor shortage and life-long need for immunosuppression are the main limitations to liver transplantation.A potential alternative to liver transplantation is hepatocyte transplantation[69-71].However,cell transplantation is also limited by the availability of effective cell sources,generation of alternative hepatocytes is thus an urgent problem.The ideal cell source should at least meet the following requirements:(1) Available in large quantity.Similar to hepatocytes needed in BAL devices,a large number of cells(> 109) may be needed for transplantation to every adult patient; (2) High efficiency ofin vivohoming and repopulation.Transplanted cells can home and adapt to the microenvironment in recipient and successfully repopulate the liver; (3) Low immunogenicity.Cells have no or low immunogenic responses,which can be suppressed by low doses of immunosuppressant; (4) No tumorigenic risk.Transplanted cells should have normal karyotype and be free of potential tumorigenic modulations,such as modifications in oncogenic or tumor suppressor genes.To date,several mouse models have been adopted in testing the transplantation efficiency of human hepatocytes,which in general can be divided into two categories[72].One is a mouse model with a genetic disorder that causes depletion of the host hepatocytes,such as mice expressing urinary plasminogen activator (uPA) driven by the albumin or Mup promoter[73,74],and immunodeficient FRG [Fah(-/-)Rag2(-/-)Il2rg(-/-)] mice[75];another is a mouse model with drug- or surgery-induced liver damage,including mice receiving treatment with retrorsine[7],CCl4[24,76],diethylnitrosamine[77]or partial hepatectomy[7,78](Table 2).Transplantation using primary human hepatocytes has been successful in mouse models,for example,with the FRG mouse model,the ratio of human hepatocytes in a mouse liver can be up to 90%[75].However,there are no definitive conclusions so far regarding whether the maturity of transplanted liver cells affects the efficiency of transplantation when HLCs are used.Cells in endoderm,hepatoblasts,and mature hepatocyte stages along the HLC differentiation process all have possibilities as donor cells in cell transplantation[7,24,73,76,77](Table 2).The microenvironment in recipient liver is thought to supply necessary signals to promote further maturation of transplanted cells,although direct evidence and the underlying mechanism are lacking.However,the overall HLC transplantation efficiency is lower compared to that of human primary hepatocytes[75](Table 2).Furthermore,transplantation with HLCs may suffer tumorigenic risks due to remnant undifferentiated hPSCs,and the immunogenicity has not been addressed so far,as most studies were performed with immunocompromised animals.
To improve the transplantation efficiency,several ectopic sites have been investigated,including spleen,peritoneal cavity,kidney,lung,pancreas and fat pads.Bioengineering approaches have also been applied in cell transplantation.For example,Songet al[20]transplanted hPSC-derived HLCs in immunocompetent mice via 3D cell coaggregates with stromal cells and encapsulation.This study demonstrated an improved approach for the engraftment of hPSC-derived HLCs[79].In a different study,Nagamotoet al[78].used a cell sheet engineering technology by attaching HLC sheets onto the surface of mouse liver with acute liver failure,which showed improved hepatocyte engraftment and animal survival in contrast,genetic modification to HLCs represents another approach to improve transplantation efficiency.For example,Nagamotoet al[74]demonstrated higher transplantation efficiency using HLCs transduced with an adenovirus vector expressing FNK (Ad-FNK),by inhibiting apoptosis in the process of integration into liver.However,there is still a long way to go before HLCs can be used in clinical liver transplantation.Strenuous efforts are needed to understand the complex processes of cell transplantation,for example,the donor-host interactions,to improve the quality of HLCs and optimize the transplantation strategy.Plus,the potential tumorigenic risk of transplanted HLCs had to be carefully considered.Specifically,tumor cells can arise from cells with residual expression of factors in iPSC reprogramming process(e.g.,the myc expression),undifferentiated iPSCs remaining in the culture,and cells with mutations or karyotype abnormalities caught in the rather longin vitroculture and differentiation processes.Several approaches can be adopted to reduce the tumorigenic risk:(1) Use integrating-free viruses or small molecules for iPSC reprogramming[80,81]; (2) Improve thein vitroculture conditions and enhance the differentiation efficiency of hPSC-derived HLCs[82]; (3) Remove undifferentiated iPSCs,e.g.through treatments with small molecules or antibodies that can specifically target iPSCs[83,84]; or enrich HLCs using HLC specific surface markers before transplantation[85]; (4) Monitor the genome integrity of cells at the iPSC stage and the HLC stage,through karyotype analysis and whole-genome sequencing; (5) Engineer a self-killing circuit in cells that would allow the trigger of cell deathin vivoto remove tumorigenic cells,if necessary,to further assure safety[86].Nonetheless,hPSC-derived HLCs provide a potential valuable cell source to OLT for liver diseases that is worth pursuing.
CONCLUSION
The generation of iPSCs has revolutionized the whole field of cell biology.It is truly inspiring to imagine that we can grow any person's pluripotent cells indefinitely in a dish and turn them into any cell type.With this capability of iPSCs,the approach to the study of human biology has been profoundly changed.HLCs were among the first batch of adult cell types that have been derived from iPSCs,and have been tested ever since for disease modeling,toxicity screening,and drug discovery,and as donor cells for transplantation (Figure 1).Complexities and difficulties in the derivation and applications of these HLCs seem beyond our initial expectations.More than 10 years have passed,but HLCs derived from hPSCs remain a largely heterogeneous population with incompetent liver cell function and low transplantation efficiency.Protocols to grow HLCs from hPSCs need to be substantially and continuously improved and standardized on the basis of deeper understanding of liver development.Despite the gap between the reality and ideal conditions,efforts have paid off well and the field has made tremendous achievements in recent years,such as generation of functional liver organoids,successful modeling of certain liver diseases,identification of candidate treatments,and application of large cohorts of HLCs for human genetic studies,to name a few (Figure 1).With advances in cell culture systems including 3D culture platforms[87],coculturing conditions[88],tissue-ona-chip approaches[89],and invention of new technologies including genome editing tools and bioengineering systems,HLCs obtained from hPSCs will eventually be able to fulfill the needs in biomedical research and clinical translation.
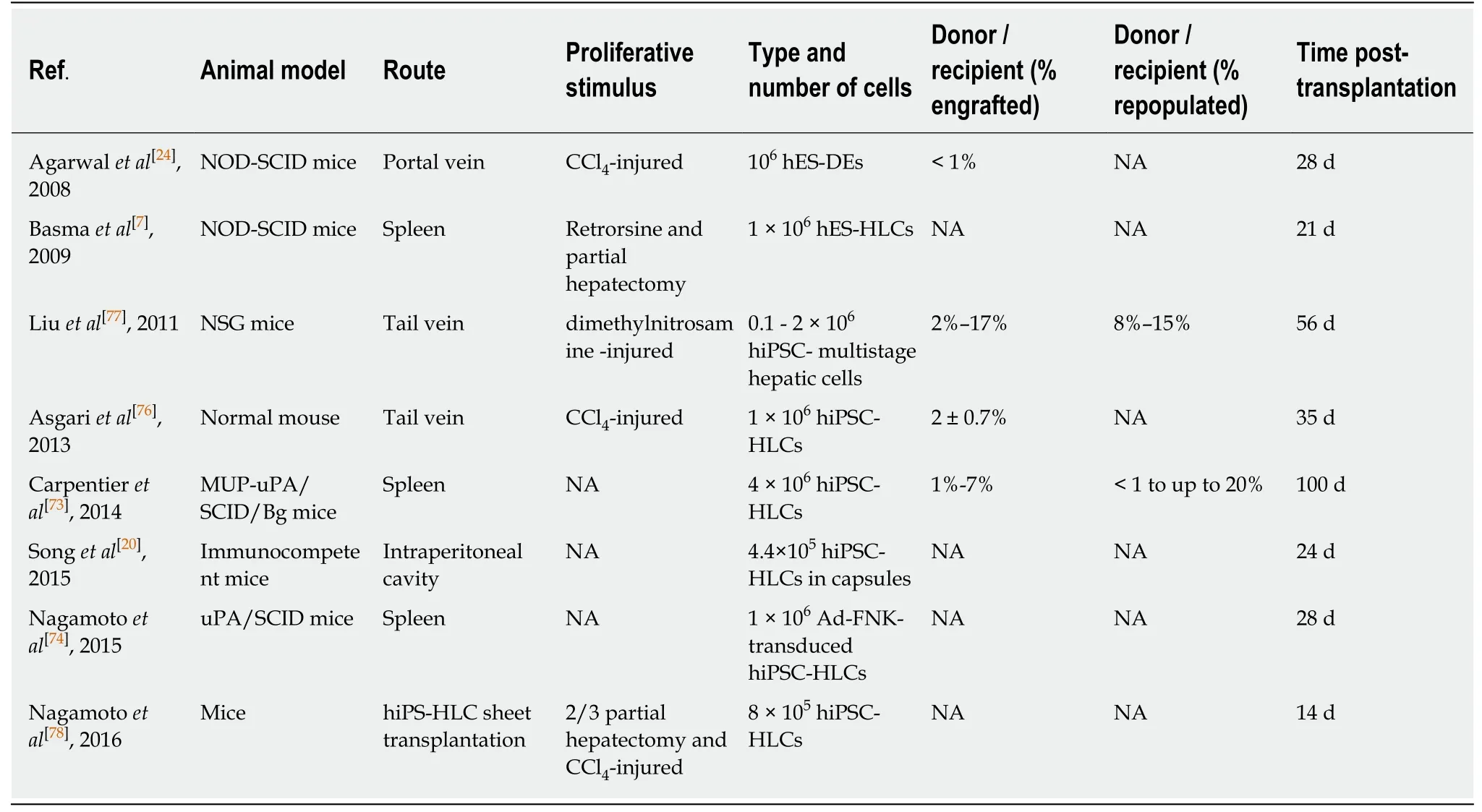
Table2 Summary of transplantation studies using hepatocyte-like cells
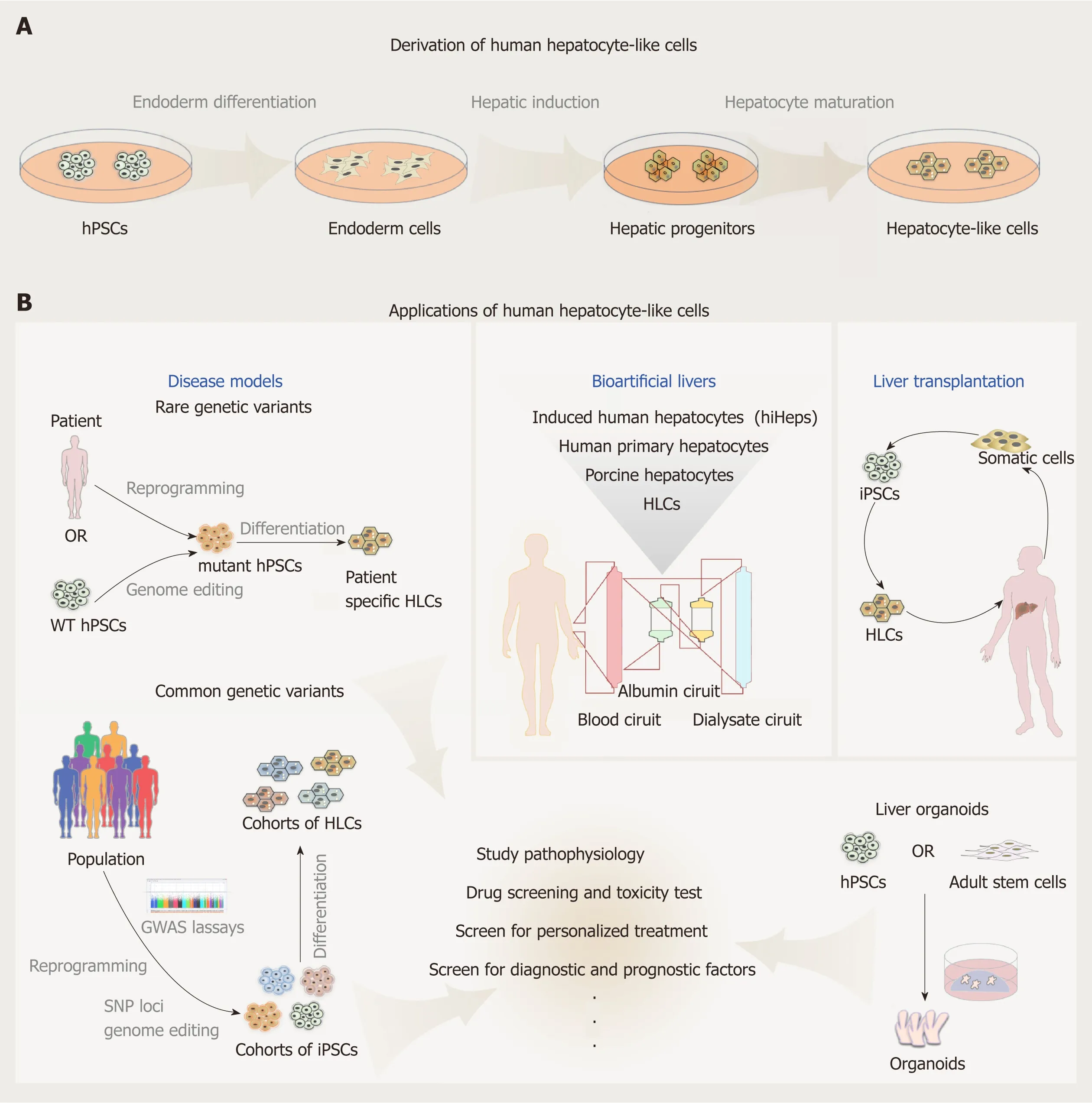
Figure1 Derivation and applications of human hepatocyte-like cells.
 World Journal of Stem Cells2019年8期
World Journal of Stem Cells2019年8期
- World Journal of Stem Cells的其它文章
- Moving forward on the pathway of cell-based therapies in ischemic heart disease and heart failure - time for new recommendations?
- Neural regeneration by regionally induced stem cells within poststroke brains:Novel therapy perspectives for stroke patients
- Orchestrating stem cell fate:Novel tools for regenerative medicine
- Bone marrow microenvironment:The guardian of leukemia stem cells
- Tonsil-derived stem cells as a new source of adult stem cells
- Linking stemness with colorectal cancer initiation,progression,and therapy
