Orchestrating stem cell fate:Novel tools for regenerative medicine
Sara Cruciani,Sara Santaniello,Andrea Montella,Carlo Ventura,Margherita Maioli
Sara Cruciani,Sara Santaniello,Andrea Montella,Margherita Maioli,Department of Biomedical Sciences,University of Sassari,Sassari 07100,Italy
Sara Cruciani,Sara Santaniello,Carlo Ventura,Margherita Maioli,Laboratory of Molecular Biology and Stem Cell Engineering,National Institute of Biostructures and Biosystems - Eldor Lab,Innovation Accelerator,Consiglio Nazionale delle Ricerche,Bologna 40129,Italy
Andrea Montella,Operative Unit of Clinical Genetics and Developmental Biology,Sassari 07100,Italy
Margherita Maioli,Istituto di Ricerca Genetica e Biomedica,Consiglio Nazionale delle Ricerche,Cagliari 09042,Italy
Margherita Maioli,Center for Developmental Biology and Reprogramming-CEDEBIOR,Department of Biomedical Sciences,University of Sassari,Sassari 07100,Italy
Abstract
Key words:Stem cells; Epigenetics; Self-renewal; In vitro differentiation; Physical stimuli; Stem cell fate; Clinical practice; Cell transplantation
INTRODUCTION
Stem cells are known for their self-renewal and their capability to differentiate into various lineages,participating in tissue regeneration after damage[1].Since human embryonic stem cells (ESCs) are isolated from the inner cell mass of the blastocyst[2]their applicationin vitroandin vivois burdened by ethical issues,causing researchers to turn their interests toward other sources[3,4].Mesenchymal stem cells,defined by other authors as mesenchymal stromal cells[5],have shown a high proliferative potentialin vitro,being identified as the elements that maintain the bone marrow microenvironment,improve hematopoiesis and give rise to various cell lineages[6,7].The most common source for human mesenchymal stem cells (hMSCs) is the bone marrow,usually obtained from the iliac crest of adult patients.Bone marrow-derived stem cells (BM-MSCs) can be separated from the tissue by centrifugation in a density gradient media and,once placed in culture,they can be easily induced to differentiate towards different phenotypes[8].MSCs are found in many other adult tissues,including the dental pulp[9],adipose tissue (ASCs)[10],umbilical cord blood[11]and Wharton's jelly of umbilical cord12].Despite some differences in terms of growth kinetics and pluripotency,donor age- and -gender-related features[13,14],MSCs can differentiate under a variety of external cues,acting to replace damaged cells and maintain tissue homeostasis[15].In order to reduce manipulation of the stromal fraction,minimize enzymatic digestion and ensure maximum yield in culture,the interest of researchers has turned to the optimization of MSC isolation protocols[16,17].In particular,new devices have been developed for adipose tissue,based only upon mechanical forces,thus allowing a micro-fragmented tissue fraction in one-step that is enriched in hMSCs and pericytes[18,19].Stem cells represent an important model to study the molecular pathways involved in disease onset and progression and to develop drug delivery system and differentiation processes,in view of a successful application in tissue engineering and clinical practice[20,21].In this review,we summarize the influence of specific chemical and physical agents able to affect stem cell behavior and fate,pointing out the current development of hMSCs applicationsin vivo.
EPIGENETIC REGULATION OF SELF-RENEWAL AND PLURIPOTENCY
Stem cell differentiation is an essential complex process involved in the maintenance of tissues homeostasis,being in turn orchestrated by a wide range of signaling pathways[22].In vitrodifferentiation involves different molecular mechanisms influencing the expression of the main markers of stemness:Octamer-binding transcription factor 4 (Oct-4),sex determining region Y-box 2 (Sox-2) and Homeobox protein Nanog[23,24].These transcription factors are essential for maintaining stem cell pluripotency and are also involved in adult somatic cell reprogramming[25,26].
Epigenetics refers to the range of heritable changes in the structure of chromatin able to affect gene expression and represents the molecular reaction to all the environmental changes[27].These chromatin modifications are orchestrated by different kind of enzymes,such as DNA methyltransferases (DNMTs),or enzymes controlling post-translational histone modification,as Histone deacetylase (HDACs)and histone acetyltransferases[28].Epigenetic mechanisms are involved in the progression from the undifferentiated to differentiated state,through silencing of selfrenewal genes and activation of differentiation markers.The onset of these specific gene expression patterns is stimulated by developmental and environmental stimuli,causing changes in the chromatin structure,thus allowing a specific transcriptional program,with a mechanism not fully clarified yet[29-31].Therefore,epigenetics has a central role not only during embryogenesis but also in maintaining tissue homeostasis and controlling the regenerative potential through adulthood[32].Wanget al[33]demonstrated that HDAC6 takes part in dental MSC differentiation and osteoblast maturation by maintaining dental and periodontal tissue homeostasis.Interactions between the HDAC Sirtuin 6 (Sirt6) and Ten-eleven translocation (Tet) enzyme are directly involved in the regulation of Oct-4,Sox-2 and Nanog genes,finely tuning pluripotency and differentiation balance in ESCs[34].Santanielloet al[35](2018)demonstrated that a combination of melatonin and vitamin D activates HDAC1 and the (NAD)-dependent deacetylases Sirtuins 1 and 2 in ASCs.The final effect was an inhibition of adipogenic differentiation,even when cells were cultured in a medium able to prime adipogenic differentiation[35].
Exposure of human amniotic fluid stem cells to DNMT inhibitors induces cardiomyogenic differentiation via chromatin remodeling,upregulation of cardiacrelated genes and repression of HDAC1 expression[36].In addition,a combination of DNMT and HDAC inhibitors counteracts cancer stem cell growth,reducing the tumor mass in mouse mammary tumor models,thus increasing mice survival,and unfolding novel epigenetic-based therapies for drug-resistant breast cancer[37].DNA methylation plays a key role in maintaining the undifferentiated state in stem cells by silencing the differentiation genes,and it is also implicated in somatic cell reprogramming[38,39].All of these classes of enzymes promote changes in chromatin structure,exerting a crucial role in regulating the balance between pluripotency and differentiation[40].On the whole,continuous efforts to unravel epigenetic regulation holds promise for continuous innovation in strategies aimed at controlling stem cell pluripotency and tissue homeostasis.MicroRNAs (miRNAs),small non-coding RNAs,have been discovered as regulators of different signaling pathways,stem cell pluripotency and somatic cell reprogramming[41].The modulation of cell differentiation by miRNAs could be used to treat various kind of diseases,including myocardial infarction,neurodegenerative and muscle diseases[42].Moreover,epigenetic mechanisms could unravel many deregulated cellular dynamics,as those involved in cancer,aging and age-related diseases[43](Figure 1).
IN VITRO MODULATION OF STEM CELL BEHAVIOR
In the last years,several molecules capable of orchestrating the multilineage repertoire of stem cells have been largely used to generate specific conditioned media[44,45].Within this context,some authors found that medium conditioned by factors such as activin A,bone morphogenetic protein 4 (BMP4),vascular endothelial growth factor (VEGF) or Dickkopf-related protein 1,can optimize cardiac development in mouse and human stem cell lines[46,47].BMP4 itself,in combination with inhibitors of the Activin/Nodal signaling pathways,induces differentiation of ESCs into trophoblastic cells,which show similar trophectoderm profile and are able to secrete placental hormones[48].Concerning the use of chemistry to push stem cells to specific phenotypes,molecules that can affect the epigenetic code to activate a molecular differentiation program have largely been used.Venturaet al[49,50]described for the first time how a hyaluronan mixed ester of butyric and retinoic acids (HBR)increases the transcription of cardiogenic genes,acting through the epigenetic regulation of a cardiogenesis programin vitro.HBR was also able to promote cardiac regeneration in infarcted rat hearts,decreasing the number of apoptotic cardiomyocytes without the need for stem cell transplantation[49-52].More recently,a mixture of HBR and melatonin was successfully employed to induce an osteogenic phenotype in dental pulp stem cells,suggesting the use of this cocktail for futurein vivoorthopedic and dental applications[53].
MODULATION OF STEM CELL COMMITMENT BY PHYSICAL STIMULI
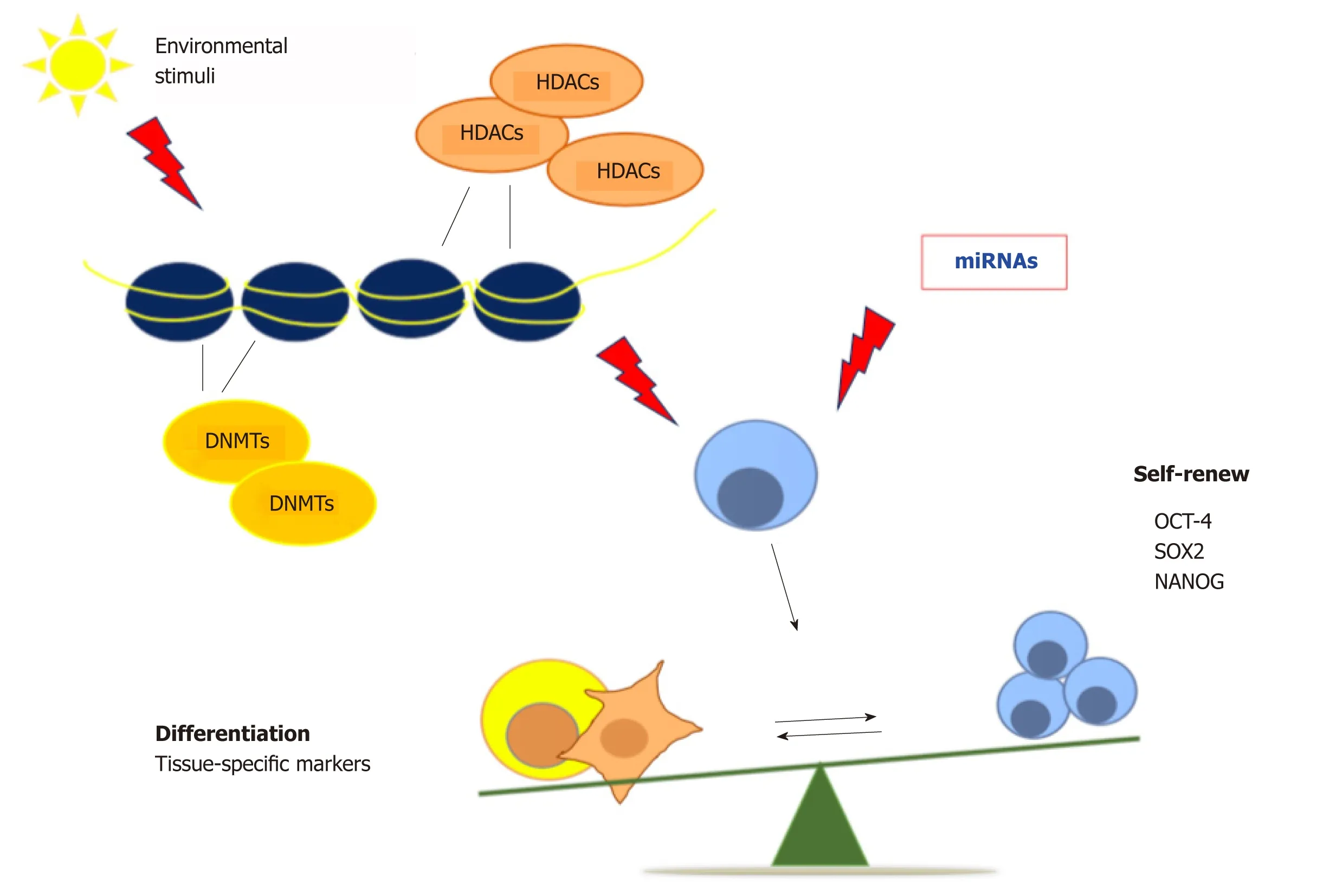
Figure1 Epigenetic regulation of stem cell fate.
Electromagnetic fields can interact with cells,tissues and biological systems in general[54,55]and are able to influence phenotypic features,gene expression patterns and differentiation in MSCs,acting in a dose and time-dependent manner[56,57].It has been shown that 7 d of MSC growth on an electroconductive polymeric substrate was sufficient to promote Nestin and β-3 Tubulin upregulation and the appearance of neural-like morphological extensions[58].MSCs can be employed to improve cartilage regeneration[59].Synthetic scaffolds and biopolymers are incorporated in stem cell cultures to induce their growth,mimicking the stem cell niche[60].Biomaterials provide a physical environment that can control cell function.The interaction between stem cells and these surfaces modulates multiple processes such as cell migration,proliferation and differentiation,as well as extracellular matrix deposition,providing dynamic signaling able to regulate cell behavior[61,62].Non-invasive electrical stimulation therapy exerts an important role in controlling calcium channels,thus regulating the intracellular calcium concentration during chondrogenic and osteogenic stem cell differentiation,and opening novel approaches to improve tissue repairin vivo[63,64].Extracorporeal shock wave therapy (ESWT) is largely used to treat orthopedic diseases,including tendinopathies or bone disorders,as well as wound healing stimulation in radiation-damaged skin[65,66].ESWT stimulates angiogenesis,neovascularization,and recruitment of MSCs,inducing their proliferation and differentiation.These processes have been shown to involve ATP release and increased extracellular signal-regulated kinases Erk1/2 and p38 MAPK activation,which is responsible for the proliferative and reparative effects[67].Human and rat ASCs exposed to repetitive ESWT retained all cell surface markers and exhibited increased multipotency into osteogenic and adipogenic lineages[68].
Radio electric fields asymmetrically conveyed by a medical device,referred to radioelectric asymmetric conveyer (REAC),are able to induce the transcription of GATA-4,Nkx-2.5,VEGF,hepatocyte growth factor (HGF),Von Willebrand factor(vWF),neurogenin-1,and myoD,genes orchestrating different tissue lineages,both in mouse embryonic and human adult stem cells[69,70].Moreover,REAC exposure counteracted MSC senescence by downregulating the expression of p16INK4,ARF,p53,and p21,involved in cell cycle regulation,reducing the number of senescence associated-beta-galactosidase positive cells,while also preserving TERT expression and telomere length[71-74].Radio electric conveyed fields allowed for the direct reprogramming of human skin fibroblasts toward cardiac and neurogenic lineages and synergistically enhanced the cardiogenic commitment in induced pluripotent stem cells (iPSCs) cultured in cardiogenic medium[47,75].In addition,radio electric conveyed fields were sufficient to induce the neurogenic phenotype in PC12 cells,a model for dopaminergic neuron studies[76].Finally,concerning cell reprogramming,several authors have shown that mechanical stimuli such as equiaxial stretching have an important role in reprogramming somatic cells into iPSCs,with the formation of a great number of iPSC colonies without using common viral mediated gene transduction[77].These findings showed the prominence of physical stimuli in opening up new strategies for cell manipulation and regenerative medicine[78,79].
BIOACTIVE MOLECULES IN ORCHESTRATING CELL DIFFERENTIATION
The use of nutraceuticals has recently been largely employed in regenerative medicine.
A wide range of natural molecules and compounds has been described as capable to orchestrate stem cell commitment.Known as nutraceuticals or functional foods,these molecules are largely used for their therapeutic or preventive effects[80,81].Melatonin,the hormone secreted by the pineal gland,regulates many physiological functions such as circadian rhythm,hemostasis and the immune system.An alteration in its secretion is related to the onset of pathological manifestations[82,83].In vitrostudies with MSCs demonstrated that melatonin exerts anti-oxidant and antiapoptotic effects,regulating the expression of pro- and anti-apoptotic proteins,ameliorating the outcome of stem cell transplantation[84,85].Mendivil-Perezet al[86]demonstrated that melatonin in transplanted mice was able to induce proliferation and differentiation of neural stem cells into oligodendrocytes and astrocytes,reducing oxidative stress produced by mitochondrial activity.Oxidative stress has a crucial role in osteogenesis inhibition and in aging-related osteoporosis[87].MSCs exposed to melatonin exhibit increased calcium stores and osteogenic differentiation.These events include the recruitment of AMP-activated protein kinase (AMPK),Runtrelated transcription factor 2 and Forkhead box O3,with the latter usually being downregulated under stress conditions[88].AMPK activation is also involved in the regulation of adipogenesis.It regulates the expression of peroxisome proliferatoractivated receptor γ (PPARγ),the main adipogenic orchestrator gene and a molecular target of natural compounds used in obesity management[89,90].In combination with other molecules,including vitamin D,melatonin has a synergistic effect on inhibiting adipogenesis[91].The active form of Vitamin D is calcitriol,which is naturally synthesized following sun exposure or taken as dietary supplements.It controls calcium metabolism,apoptosis,and stimulates macrophages and immune responses[92,93].When ASCs are cultured in the presence of melatonin and vitamin D in adipogenic-conditioned medium,adipogenic differentiation is blocked.This inhibitory effect is through the downregulation of specific genes controlling adipogenesis,protein contents,and fat depots[91].Moreover,the synergistic effect of these two molecules epigenetically modulates ASC commitment towards osteogenic differentiation through the activation of HDAC1 and SIRT1,even in the presence of adipogenic conditions[35].Natural compounds can therefore be considered potent differentiating agents able to drive cell proliferation and apoptosis resistance by epigenetic regulations and post-transcriptional modifications[94,95].At the same time,they can act as anti-proliferative agents against many tumor cells,including hepatocarcinoma cells,without affecting the cell cycle or viability of non-cancer cells,thus representing novel specific tools for cancer prevention[96,97](Figure 2).
FROM BENCH TO BEDSIDE
MSCs have largely attracted the attention of clinicians in regenerative medicine for their easy expansion and differentiation potential,avoiding the ethical issues related to the use of ESCs[98,99].Stem cells are currently applied in gene therapy and treatment of serious pathologies,sometimes representing the only alternative to conventional treatments,to slow down the progression of the disease and improve life qualities of the patients[100,101].Moreover,when transplanted in both autologous and allogenic fashion,MSCs can migrate into the damaged tissue to control inflammation and immune responses[102].The use of stem cells represents the most frequently applied cell therapy in hematological diseases[103],although with the risk of rejection and potential failure[104].Starting with allogenic bone marrow transplantation in 1957[105],stem cell therapy nowadays represent the main actor in many different clinical trials for several diseases,such as neurological diseases like amyotrophic lateral sclerosis(commonly known as ALS)[106].
BONE MARROW HEMATOPOIETIC STEM CELLS IN

Figure2 Natural molecules and stem cell fate.
CLINICAL PRACTICE
MSCs are multipotent cells that are able to differentiate into different lineages,and they can also be easily expanded for clinical practice[107].Bone marrow is a mesenchymal specialized connective tissue composed of progenitor cells that can undergo adipogenic,osteogenic,chondrogenic and myogenic differentiation[108].Thus,bone represents a microenvironment in which hematopoietic stem cells (HSCs) can maintain their undifferentiated state and participate in hematopoiesis when exposed to different stimuli[109].Hematopoiesis is a complex process,during which HSCs undergo asymmetric division to become progenitor blood and bone marrow cells,as erythrocytes,lymphocytes and monocytes[110].HSC self-renewal potential is regulated by different signaling pathways.Among them,physiological Notch signaling is required for bone formation,regulates the HSC microenvironment and cell fate decisions,and is also associated with tumorigenic potential and leukemia when dysregulated[111].Moreover,the crosstalk between Notch and Wnt signaling is crucial for tissue development and turnover[112].Wnt/β-catenin signal is essential for HSC growth and homeostasisin vitroandin vivo,and its inhibition causes cell growth arrest with a related decline in self-renewal potential of stem cells.On the other hand,activation of Wnt patterning increases Notch expression and supports the selfrenewal potential of progenitor cells from different tissues,suppressing differentiation[113,114].Alterations in signaling pathways and normal microenvironment play a crucial role in the development of hematopoietic diseases,such as chronic and acute myeloid leukemia[115].HSCs are employed as therapeutic tools in stem cell transplantations[116]due to their immunomodulatory properties,secretion of growth factors and regeneration of injured tissues,especially in patients refractory to conventional chemotherapy[117].Autologous transplantations are used in leukemia,lymphomas,multiple myeloma and other hematological malignancies[118].There are several retrospective studies in which patients were monitored after 10-12 years from the transplant to evaluate survival and transplant-related mortality[119-121].HSC transplantation was shown to be effective in counteracting the progression of the disease,notably at the early stages of disease[122].
MSC TRANSPLANTATION FOR AMYOTROPHIC LATERAL SCLEROSIS
ALS is the most frequent neurodegenerative dysfunction of the midlife[123].ALS is characterized by progressive degeneration of spinal cord motor neurons,muscle paralysis and death in 3-5 years due to respiratory failure.Degeneration involves toxicity and inflammatory processes associated with proliferation of resident cellular populations[124].Genetic and epigenetic risk factors are certainly the main causes related to progression of the disease.Superoxide dismutase 1 (SOD1),which encodes Cu/Zn superoxide dismutase 1,was the first gene whose alteration was associated with ALS.Its mutation is related to protein misfolding and loss-of-function,and it is found in many familiar forms[125,126].Misfolded proteins have a central role in neurodegenerative disease,since in their abnormally aggregated forms,cellular proteins are prevented from exerting their essential roles in RNA binding/metabolism and cellular homeostasis[127].MicroRNAs (miRNAs) are able to regulate gene expression and promote or repress mRNA stabilization through posttranscriptional modification and by binding specific targets[128].MiRNAs are involved in different physiological mechanisms,such as cell growth and apoptotic processes,while orchestrating pluripotency and differentiation in stem cells[129].Altered miRNA expression in the skeletal muscle is related to neurological symptoms and disease progression.Somein vivoandin vitrostudies have described how MiR-206 is enrolled upon muscle denervation in the attempt to regenerate neuromuscular synapses,highlighting the role of this miRNA in different stages of ALS progression[130,131].Actually,there are no curative therapies for ALS.While drugs that suppress oxidative stress can be used to try to maintain motor neuron function[132]to slightly increase patient survival,novel compounds are now being tested[133].An alternative to conventional therapy may be autologous MSC transplantation.Stem cells,thanks to their immunomodulatory properties,secrete neurotrophic factors and other antiinflammatory cytokines,thus supporting motor neuron survival and functionality[134,135].Notwithstanding,bone marrow is the most common source for MSCs,Wharton jelly,umbilical cord blood and in particular ASCs,represent a valid alternative in ALS therapy[136],due to their efficient isolation and high toleration by the patients.
In several clinical studies,patients received intravenous injection of MSCs while being monitored at regular time intervals.In all trials,autologous cell therapy proved to be a safe procedure.The recipient tissues did not exhibit any structural changes,tumor formation or toxicity related to transplantation,while it was shown to be effective in counteracting disease progression,improving the quality of patient's life[137-139].
CONCLUSION
Epigenetic regulators were identified as new promising therapeutic targets in patients with hematological,breast cancer and other malignancies,as well as in neurodegenerative diseases[140,141].The rescuing potential of stem cells is under control of different kinds of signals,including the environment,which epigenetically regulate their differentiation processes[142].Understanding the molecular pathways involved in stem cell fate is critical to develop novel tools for both the prevention and treatment of a variety of diseases,with great impact in regenerative medicine,bioengineering and clinical transplantation.
 World Journal of Stem Cells2019年8期
World Journal of Stem Cells2019年8期
- World Journal of Stem Cells的其它文章
- Moving forward on the pathway of cell-based therapies in ischemic heart disease and heart failure - time for new recommendations?
- Neural regeneration by regionally induced stem cells within poststroke brains:Novel therapy perspectives for stroke patients
- Bone marrow microenvironment:The guardian of leukemia stem cells
- Tonsil-derived stem cells as a new source of adult stem cells
- Linking stemness with colorectal cancer initiation,progression,and therapy
- Derivation and applications of human hepatocyte-like cells
